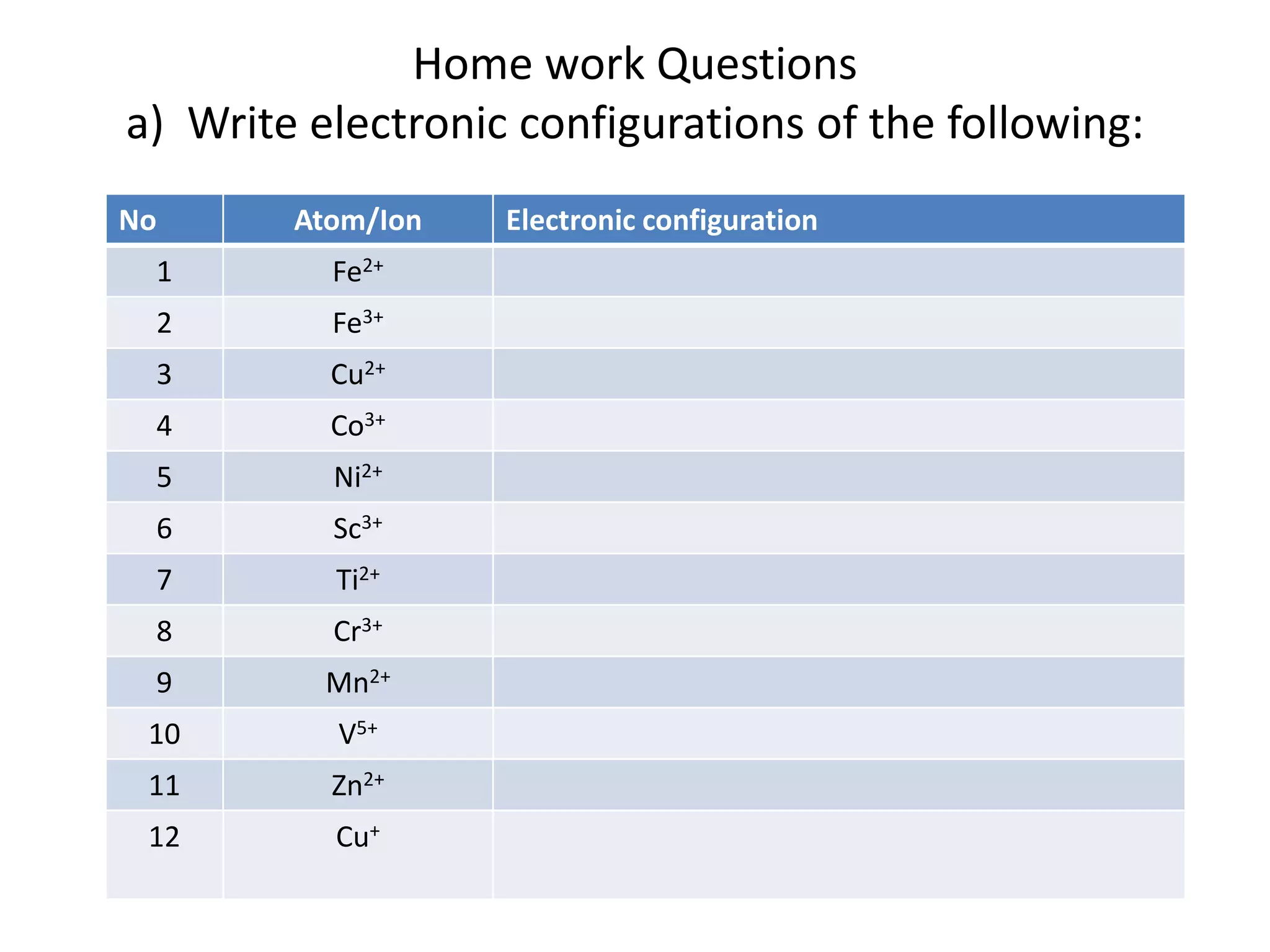
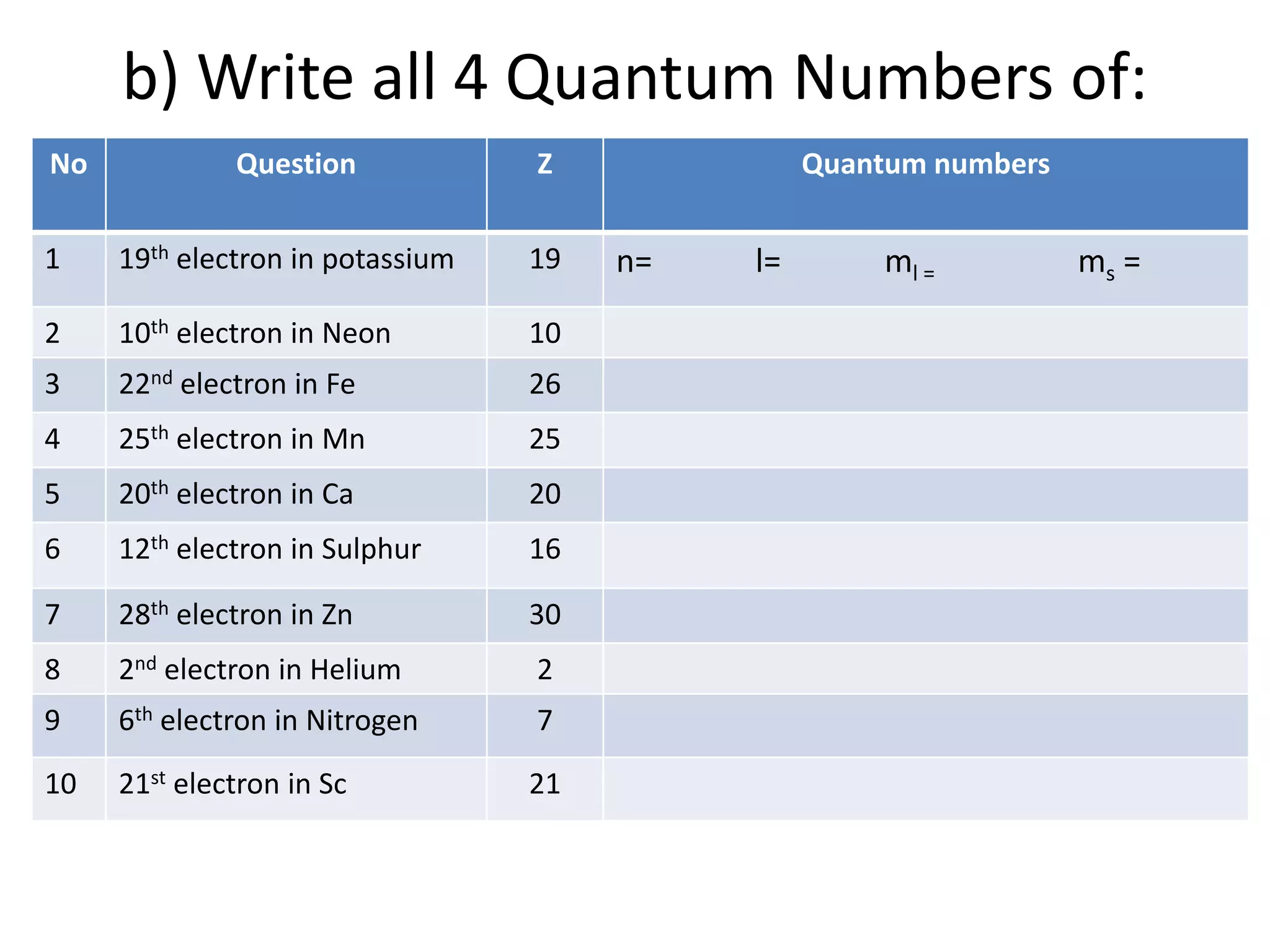
The document provides a comprehensive list of elements along with their atomic numbers, names, and electronic configurations. It includes detailed configurations for various atoms and their ions, along with questions related to electronic configurations and quantum numbers. Additionally, the text outlines tasks to derive quantum numbers for specified electrons in given elements.
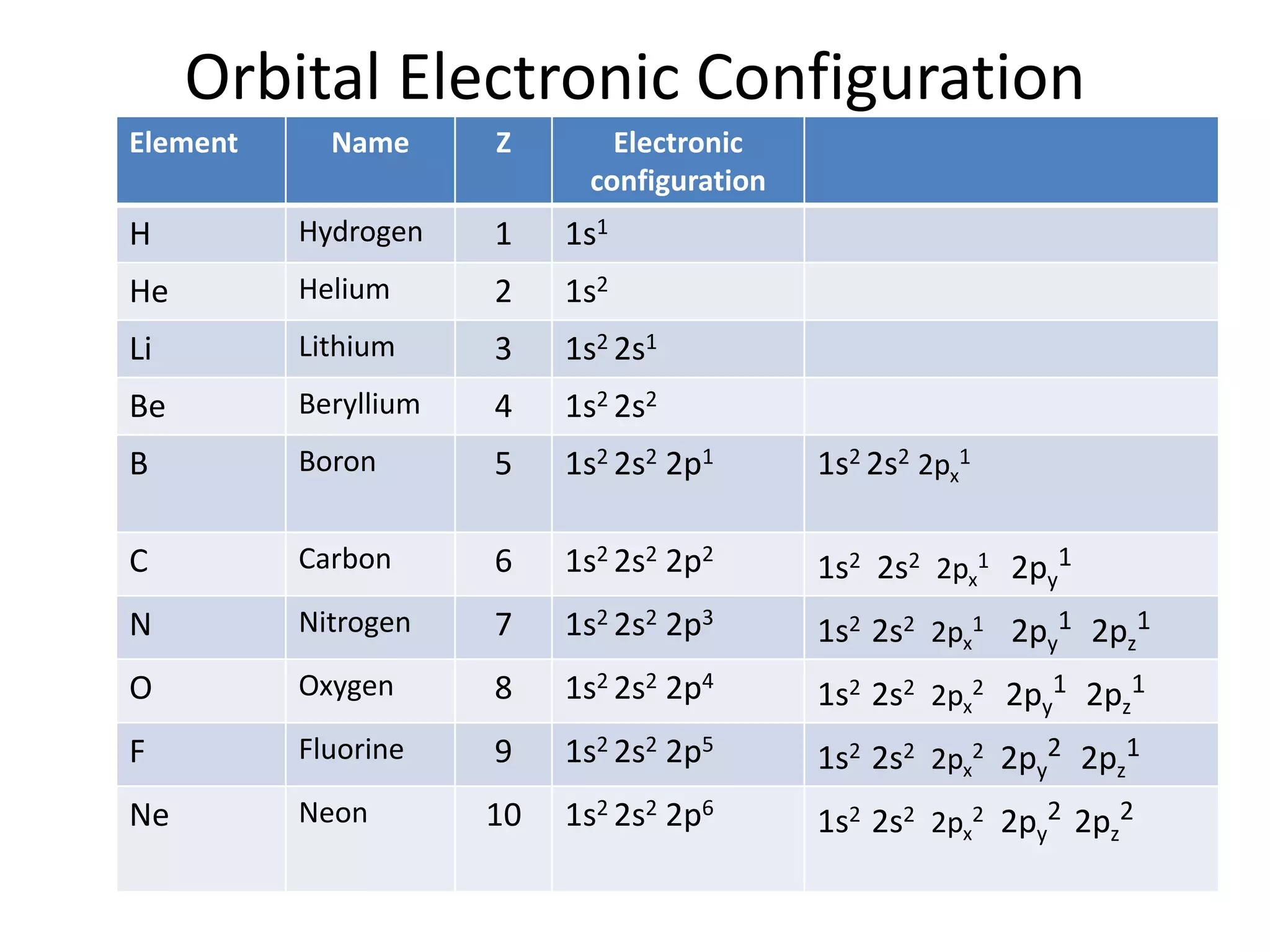
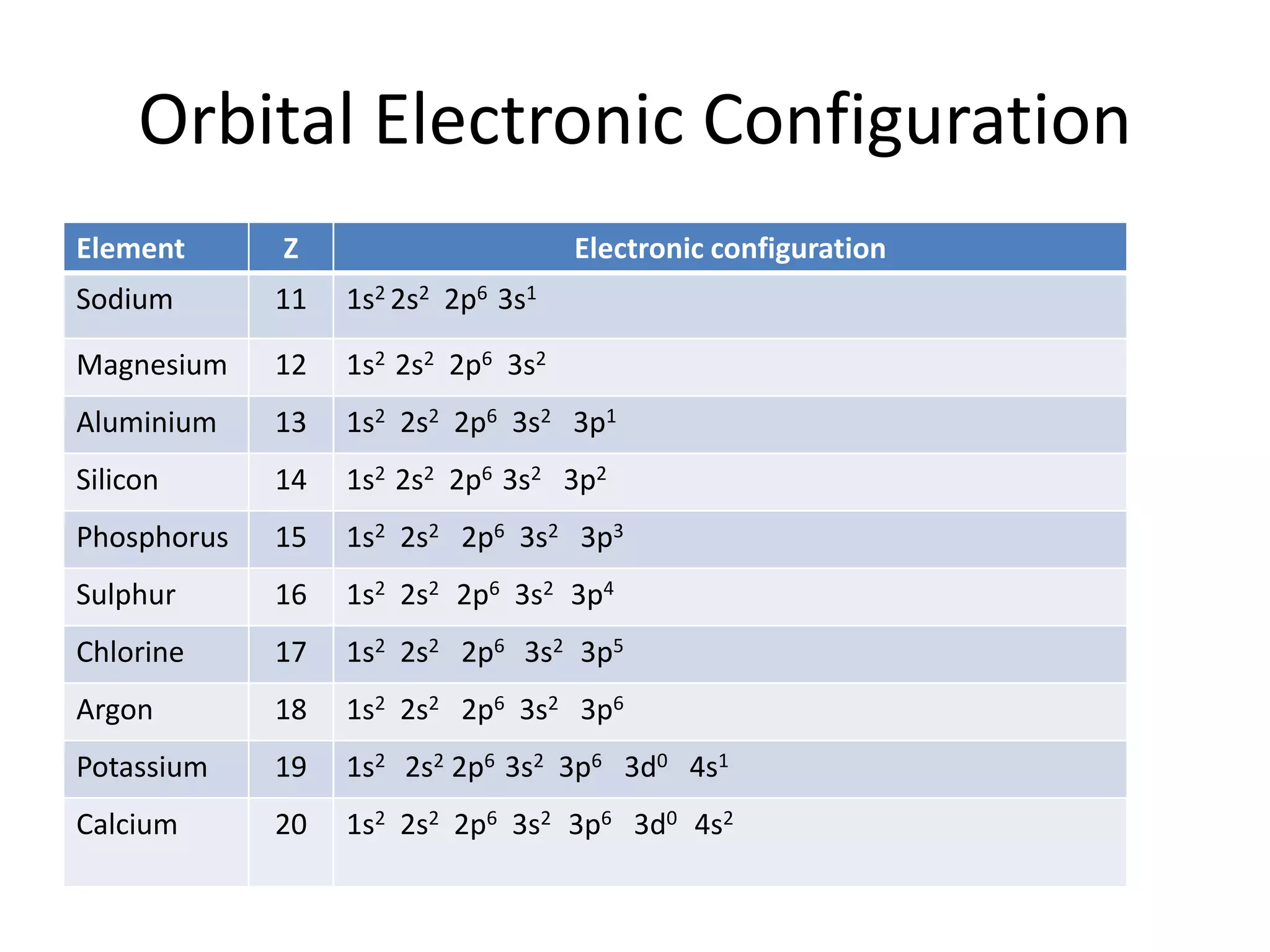
![Orbital Electronic Configuration
Element Z Symbol Electronic configuration
Scandium 21 Sc 1s2 2s2 2p6 3s2 3p6 3d1 4s2 [Ar] 3d1 4s2
Titanium 22 Ti 1s2 2s2 2p6 3s2 3p6 3d2 4s2 [Ar] 3d2 4s2
Vanadium 23 V 1s2 2s2 2p6 3s2 3p6 3d3 4s2 [Ar] 3d3 4s2
Chromium 24 Cr 1s2 2s2 2p6 3s2 3p6 3d5 4s1 [Ar] 3d5 4s1
Manganese 25 Mn 1s2 2s2 2p6 3s2 3p6 3d5 4s2 [Ar] 3d5 4s2
Iron 26 Fe 1s2 2s2 2p6 3s2 3p6 3d6 4s2 [Ar] 3d6 4s2
Cobalt 27 Co 1s2 2s2 2p6 3s2 3p6 3d7 4s2 [Ar] 3d7 4s2
Nickel 28 Ni 1s2 2s2 2p6 3s2 3p6 3d8 4s2 [Ar] 3d8 4s2
Copper 29 Cu 1s2 2s2 2p6 3s2 3p6 3d10 4s1 [Ar] 3d10 4s1
Zinc 30 Zn 1s2 2s2 2p6 3s2 3p6 3d10 4s2 [Ar] 3d10 4s2](https://image.slidesharecdn.com/electronicconfiguration-230420102017-06988010/75/Electronic-configuration-pptx-3-2048.jpg)