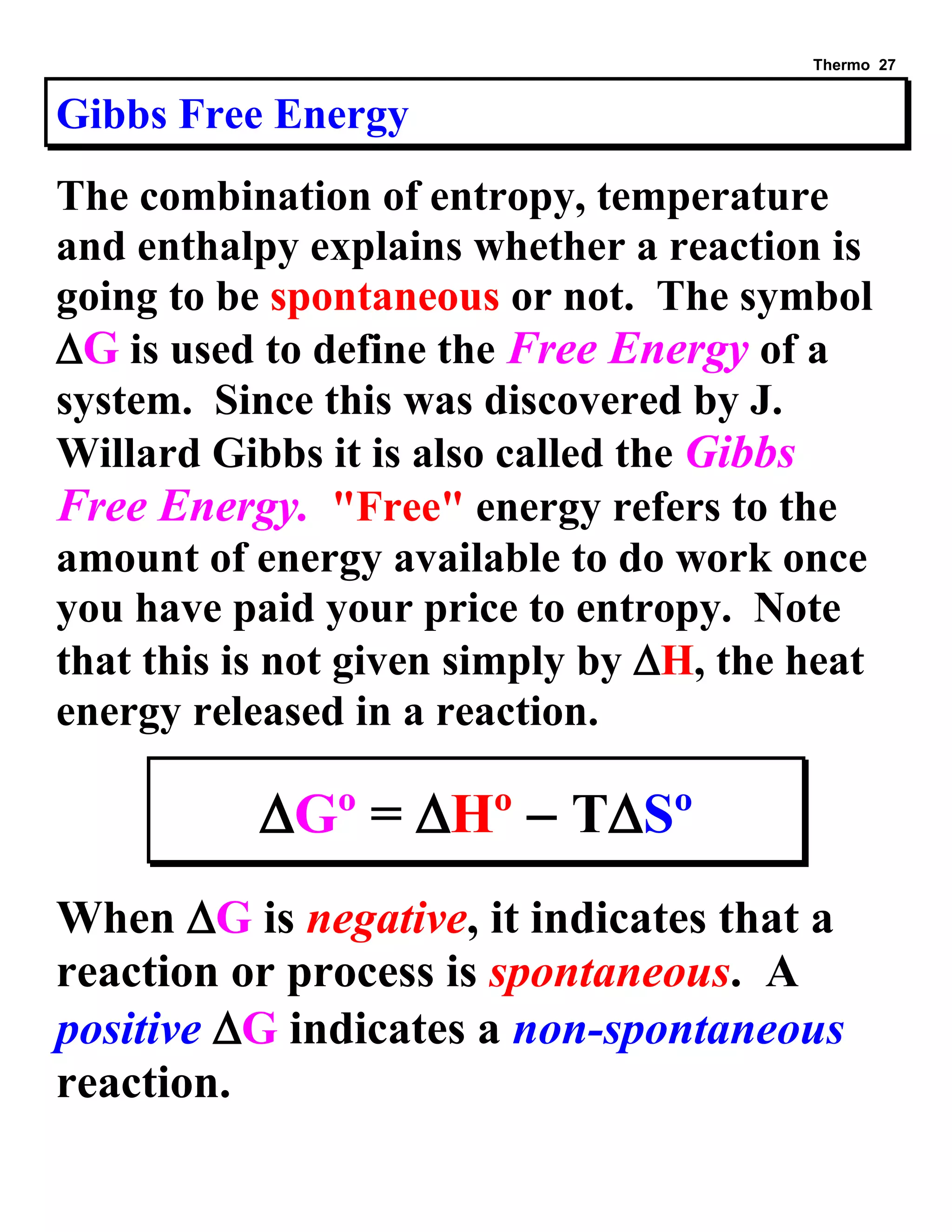
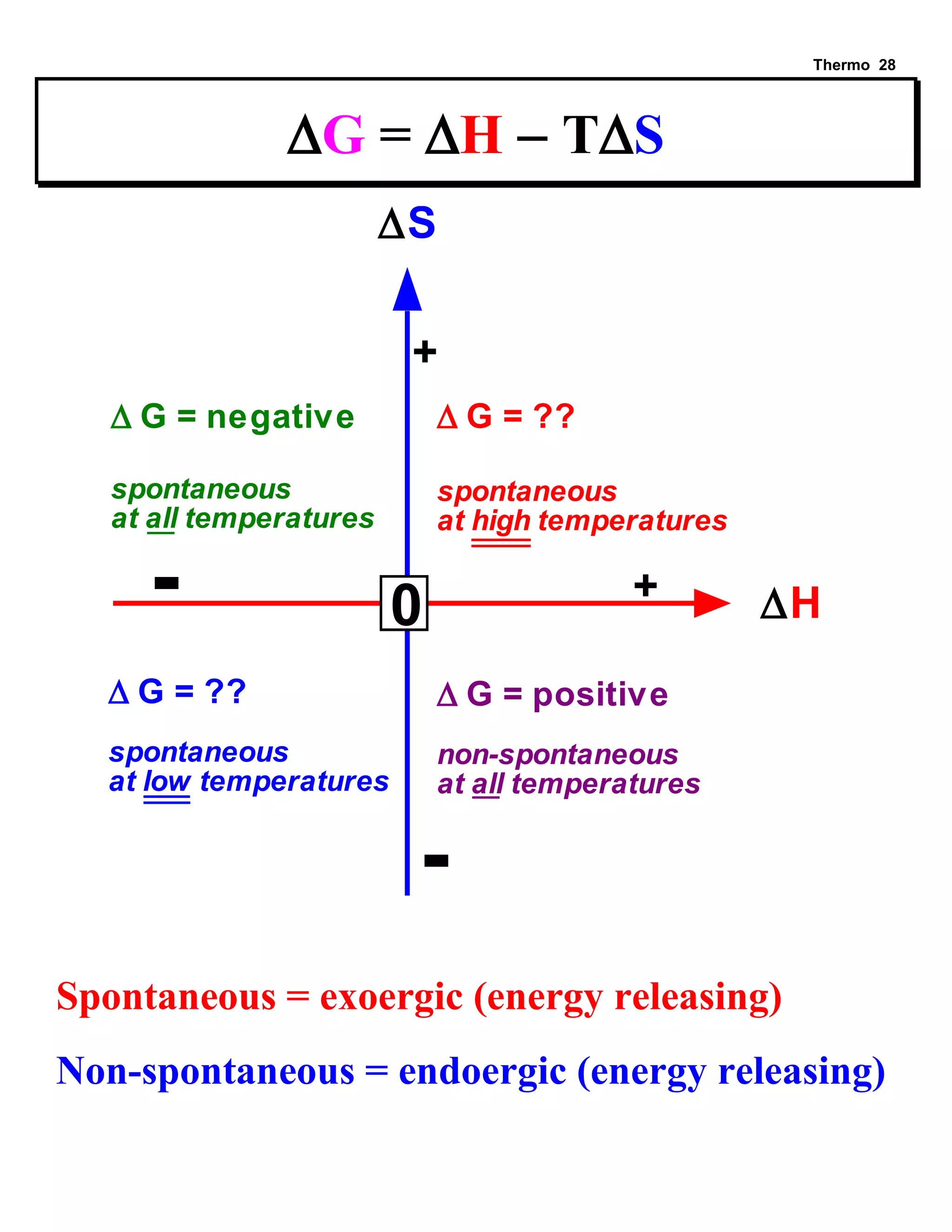
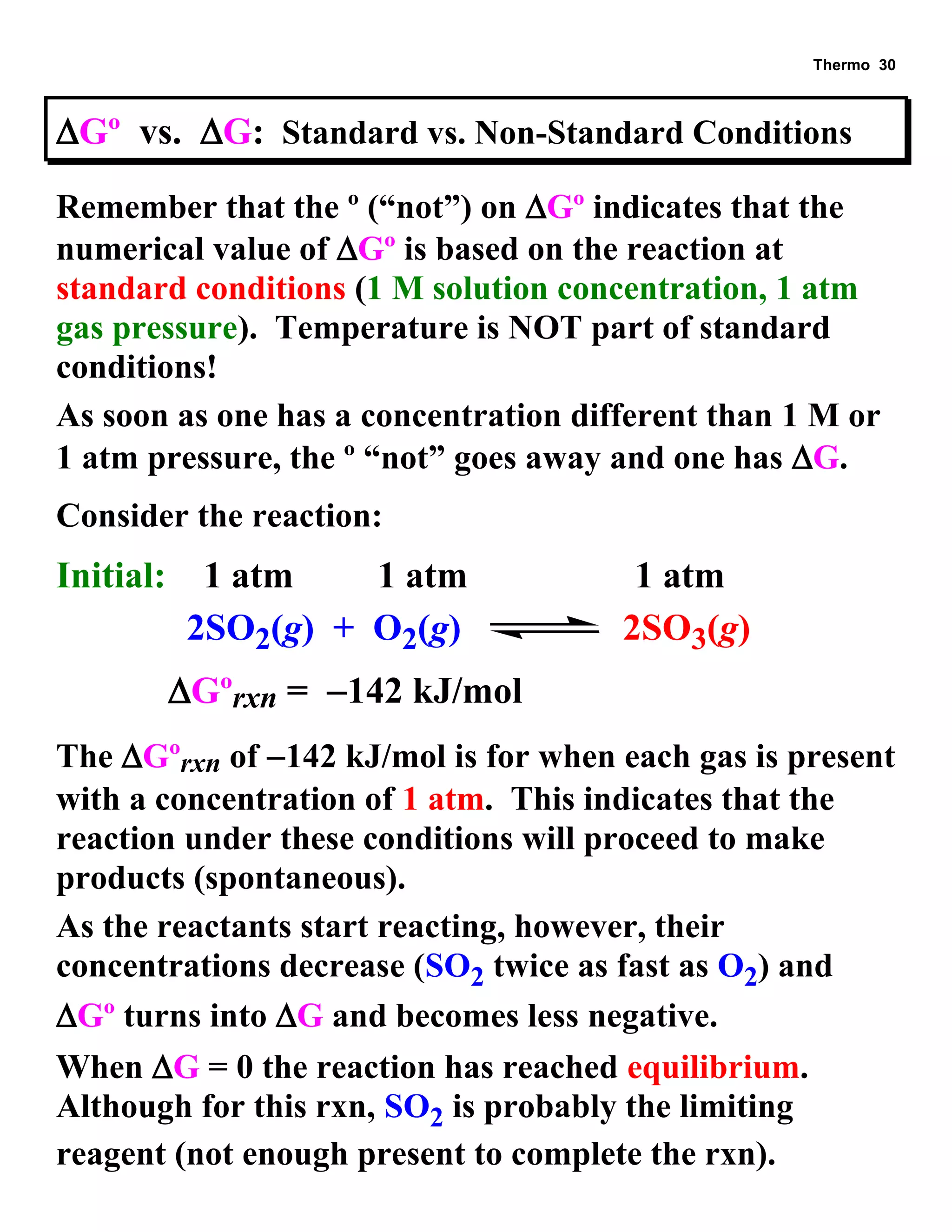
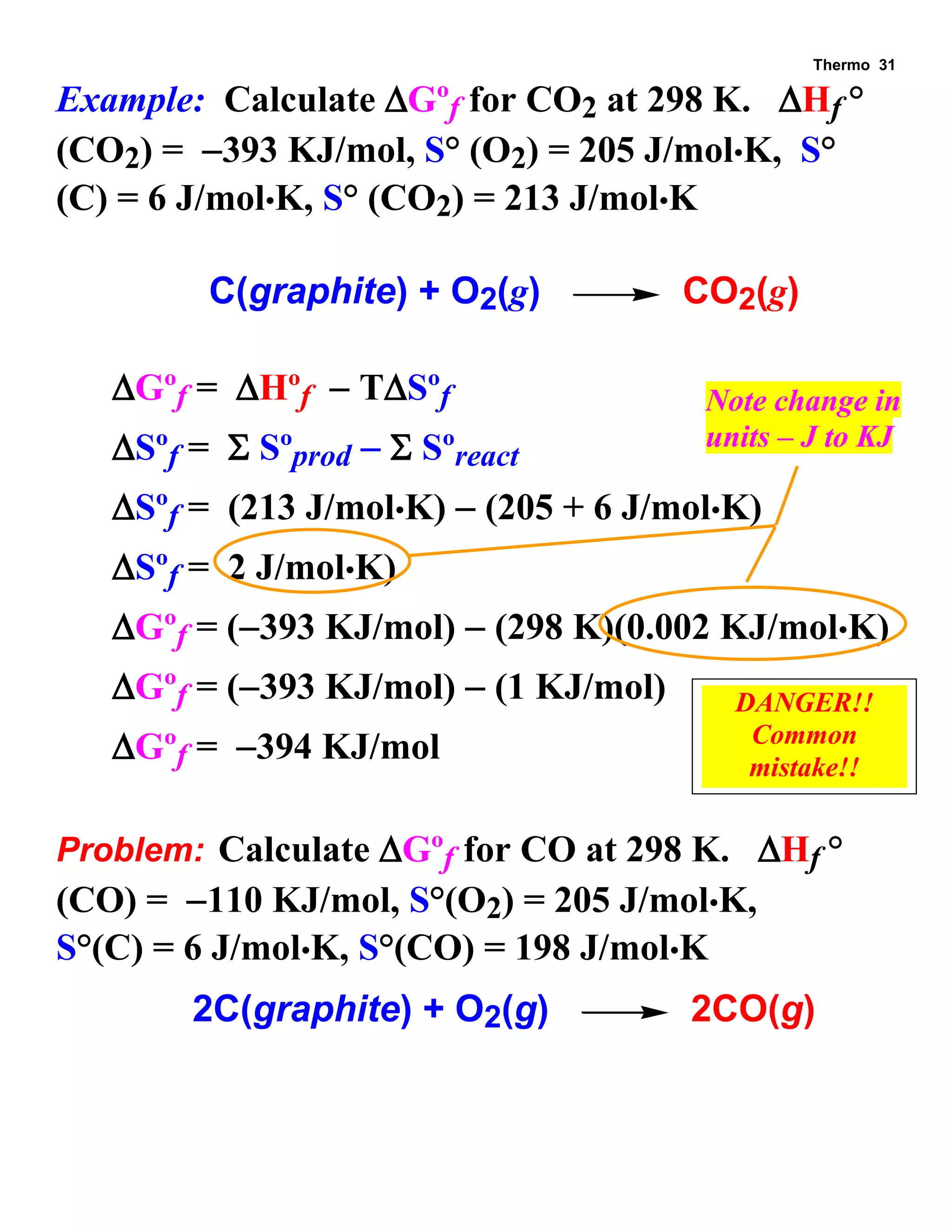
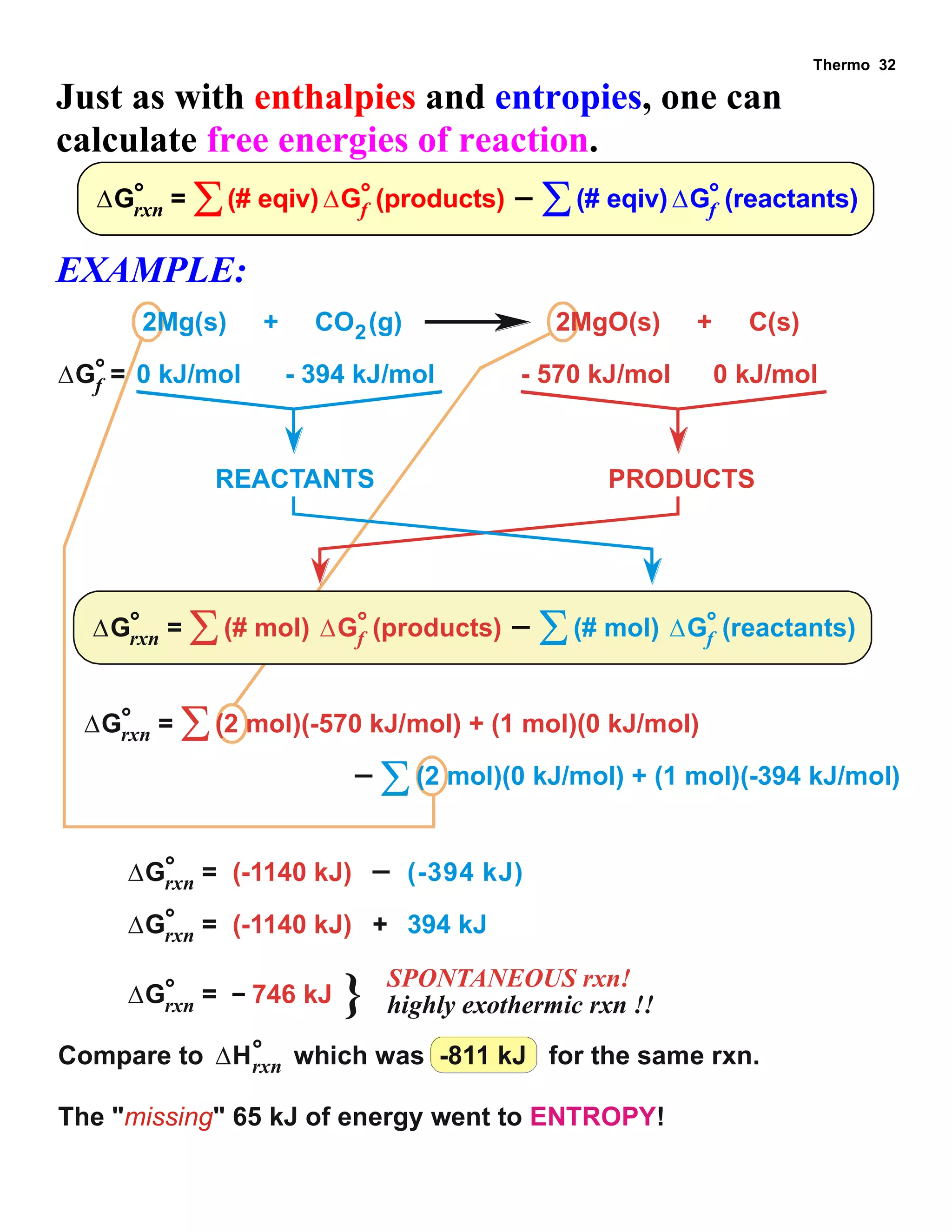
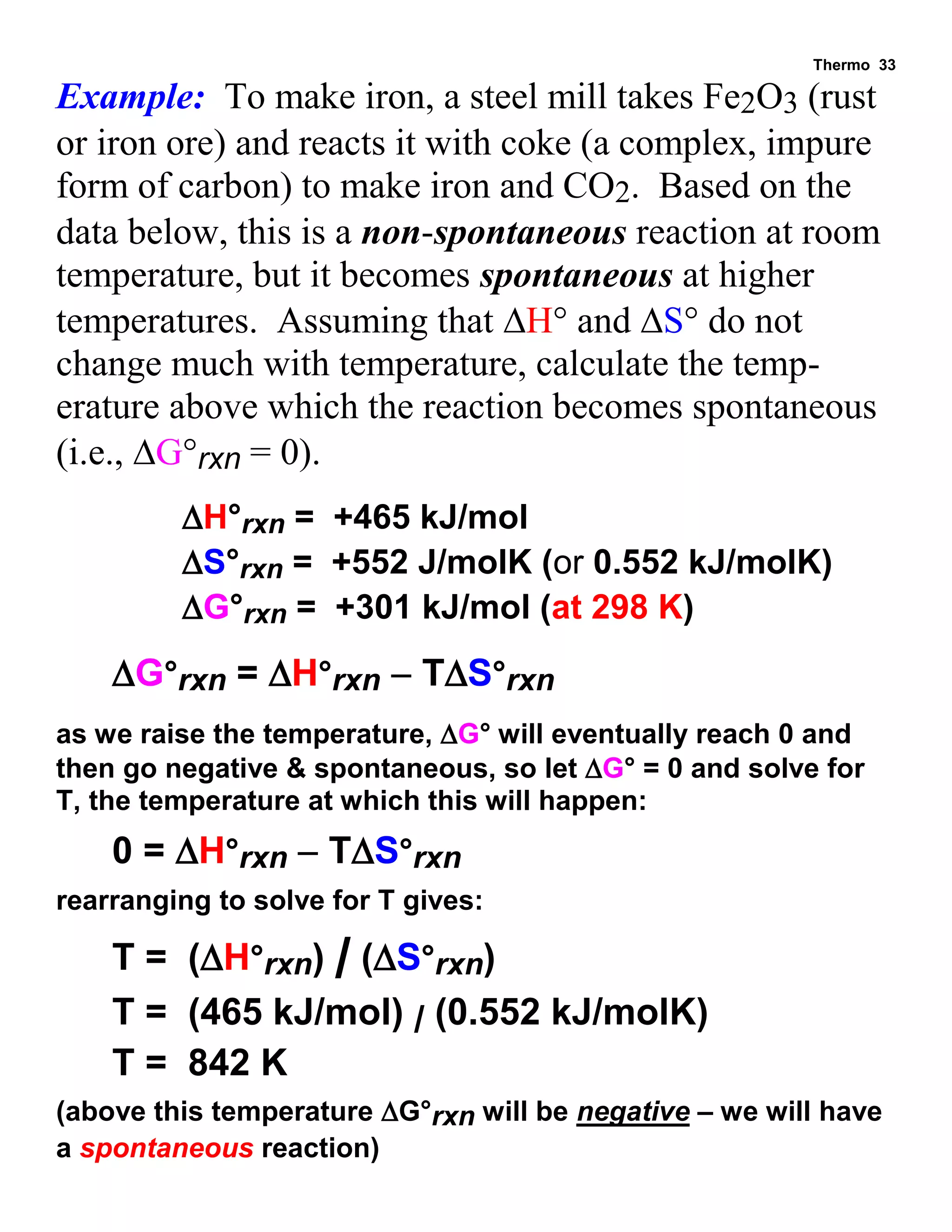
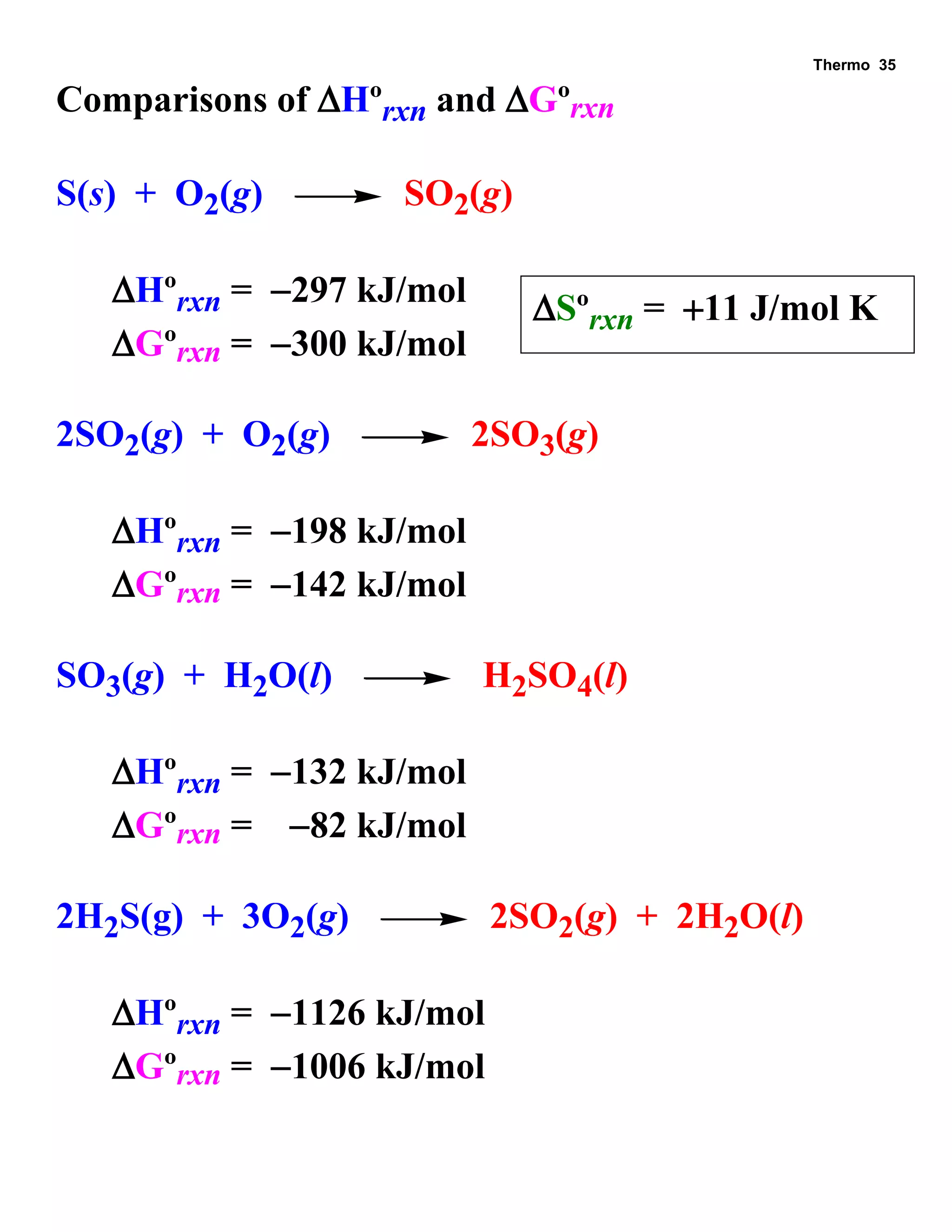
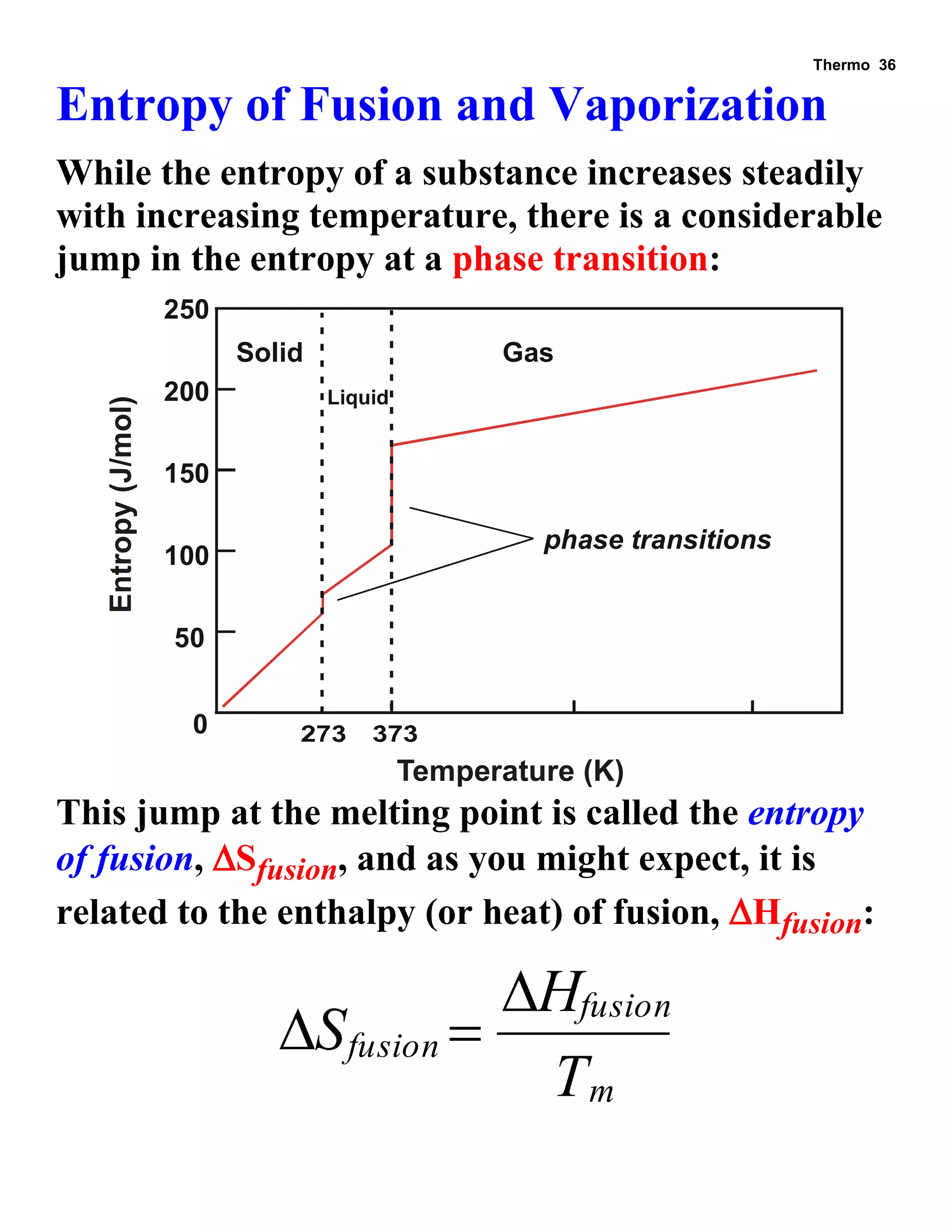
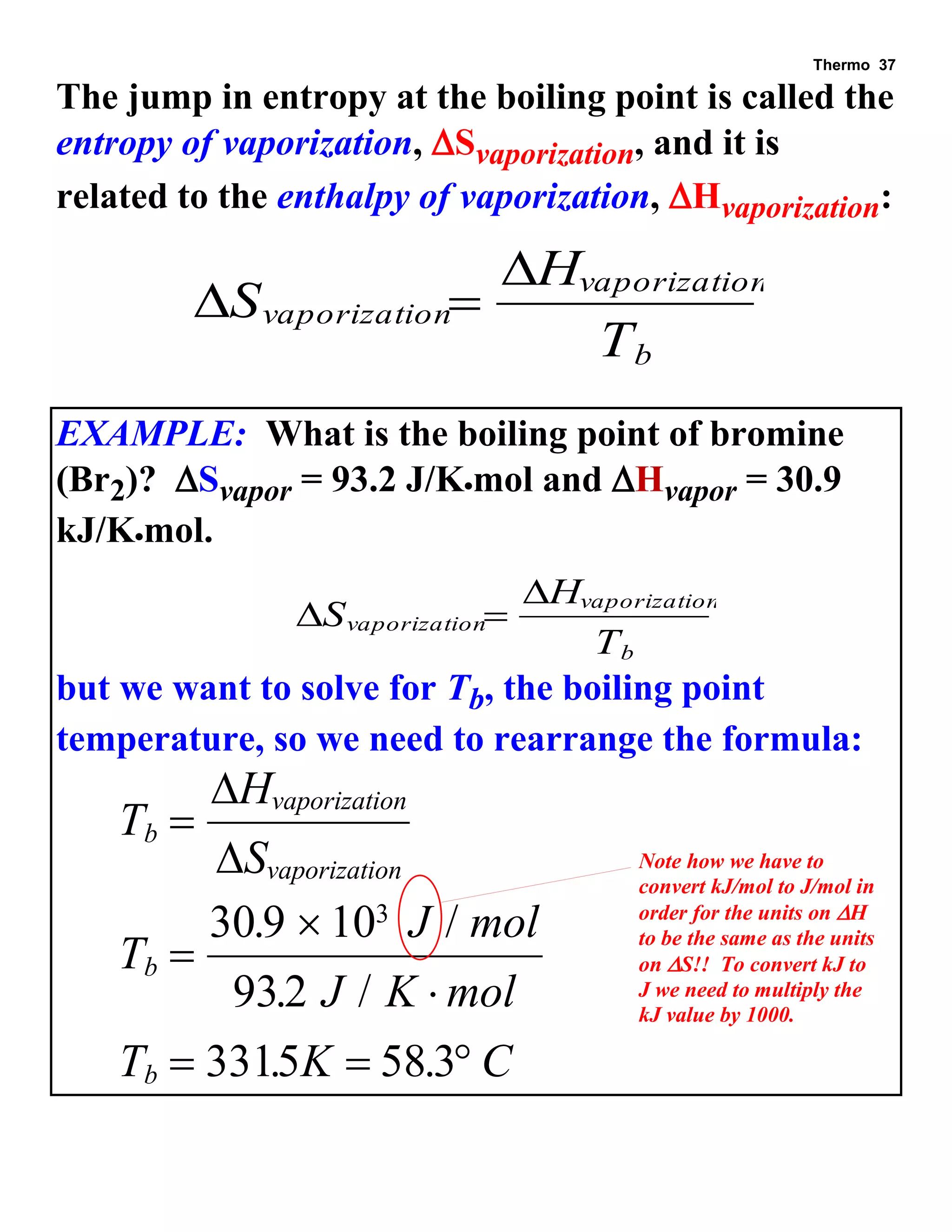
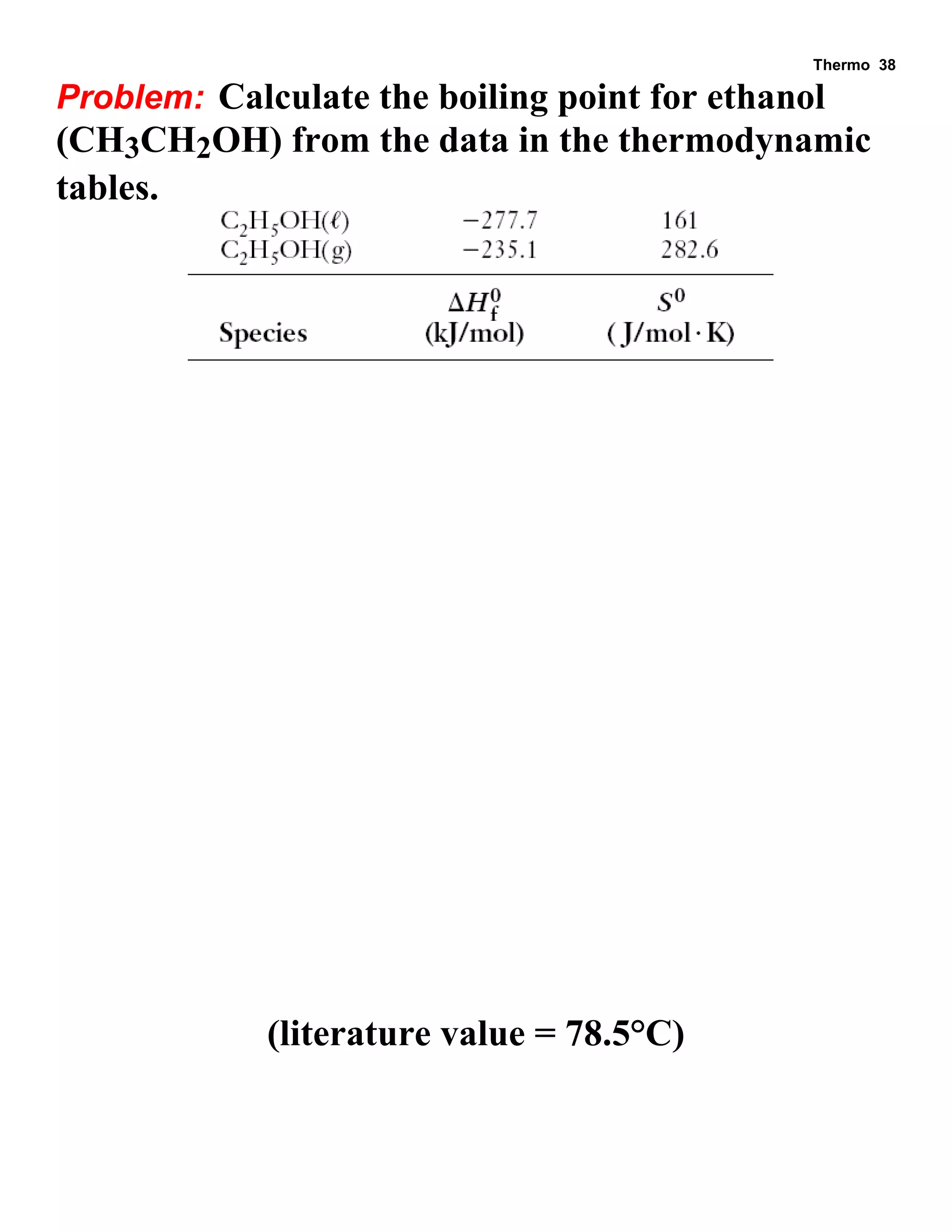
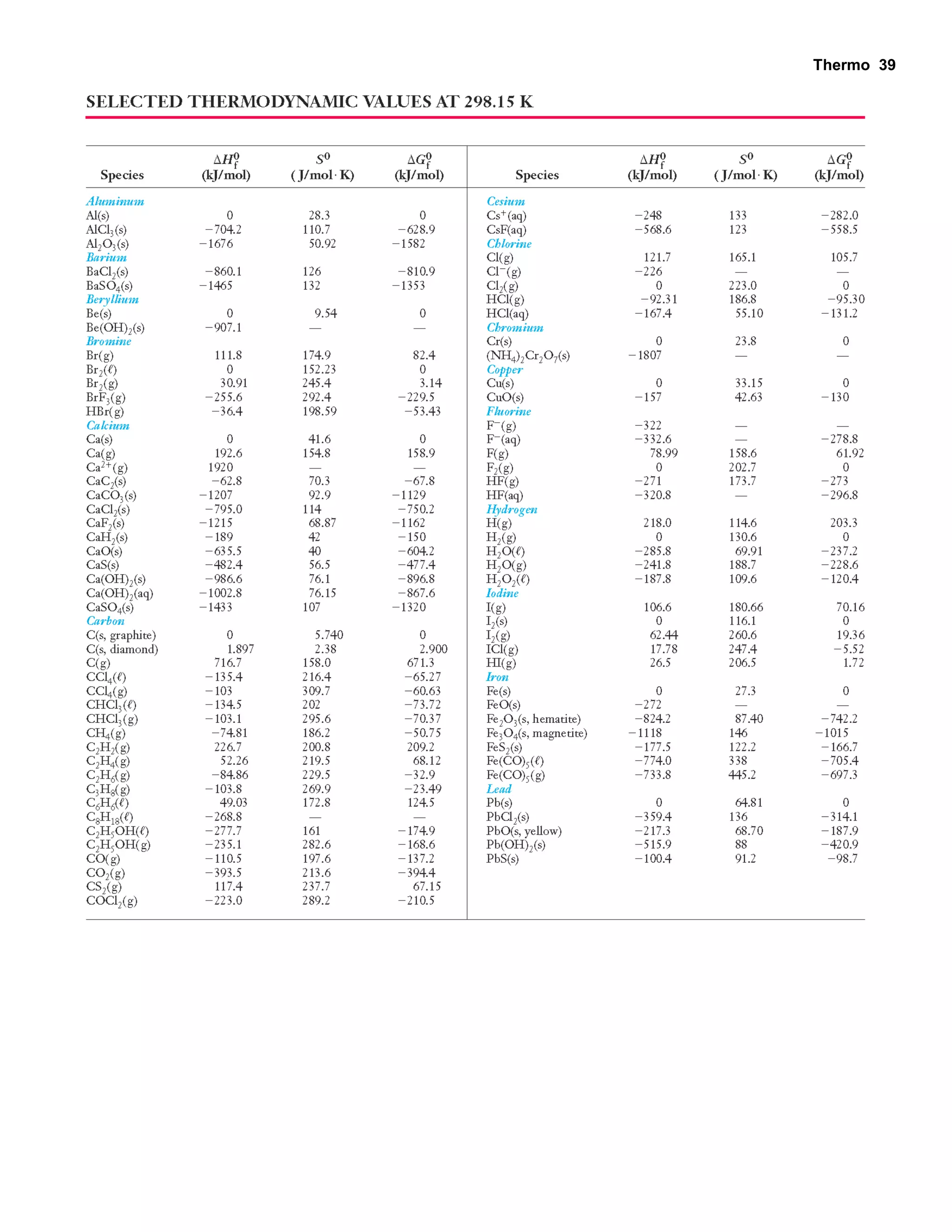
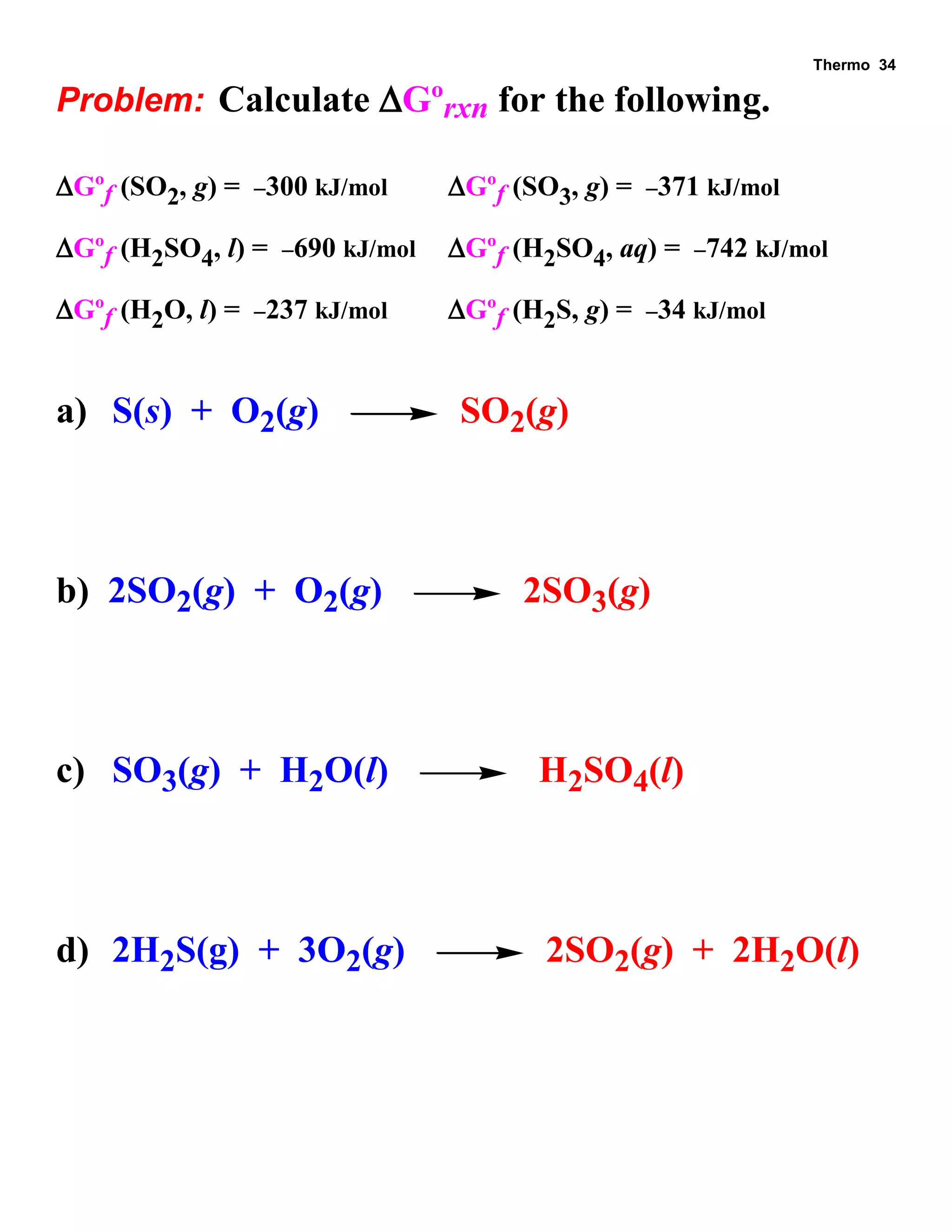This document provides an overview of thermodynamic concepts including:
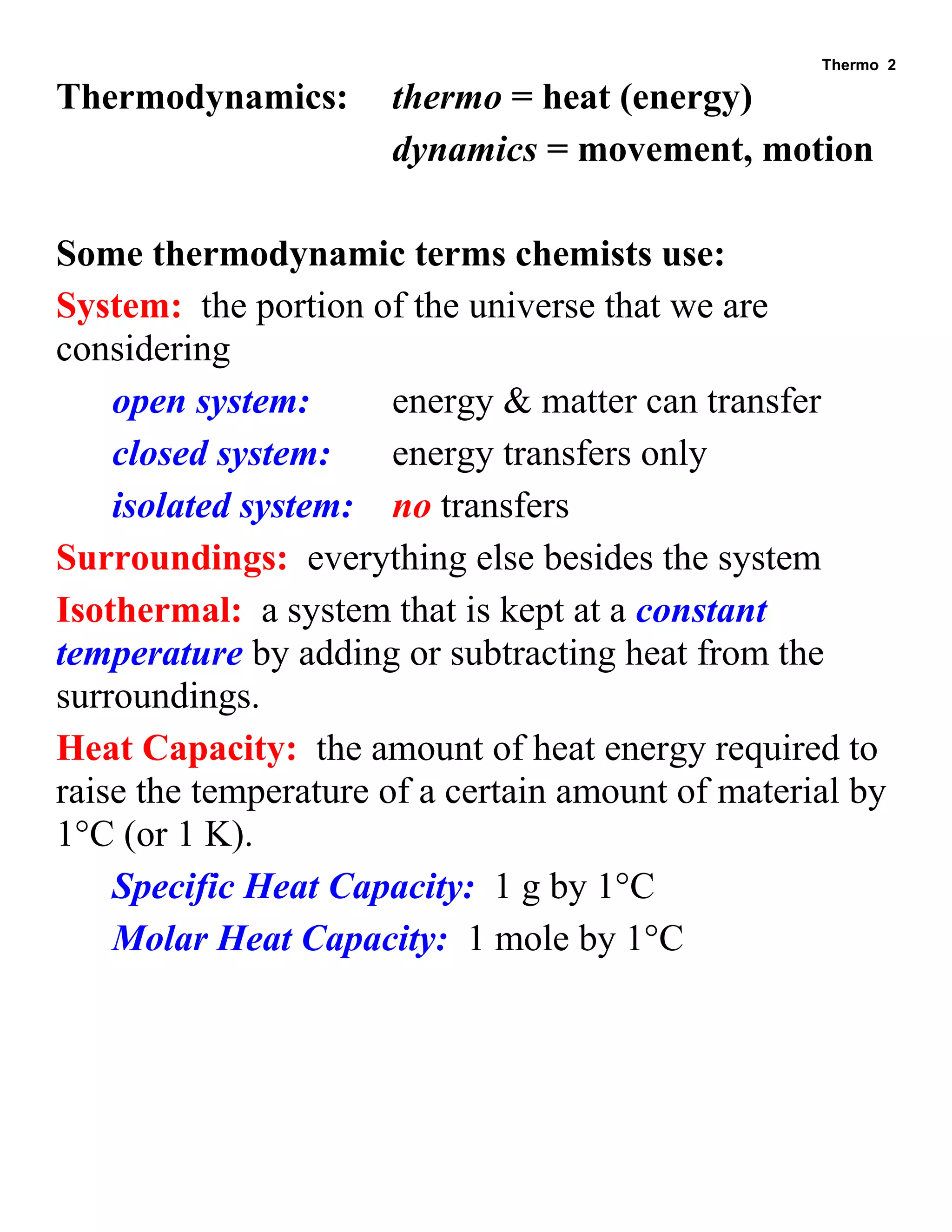
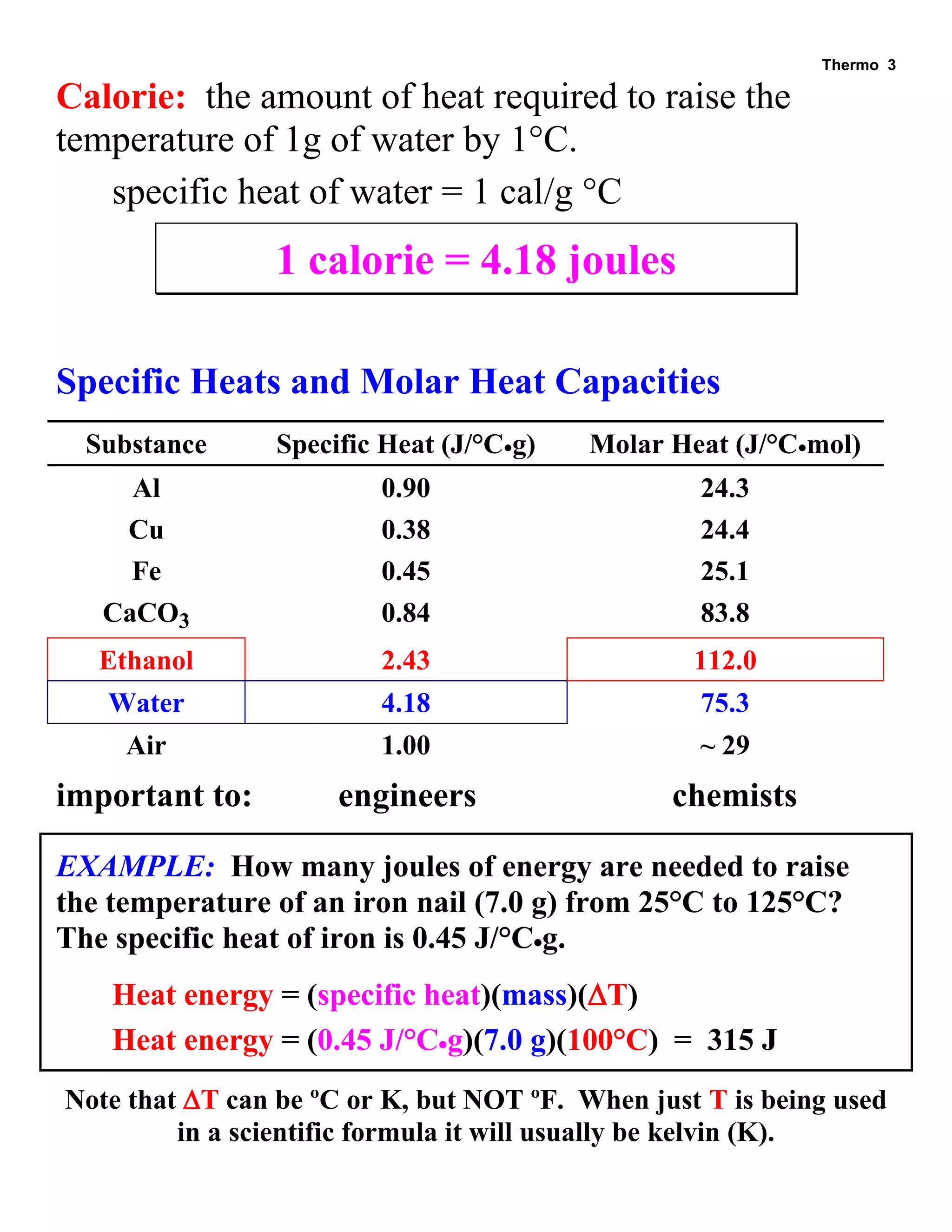
- Definitions of system, surroundings, isothermal process, heat capacity, calorie, and specific heats
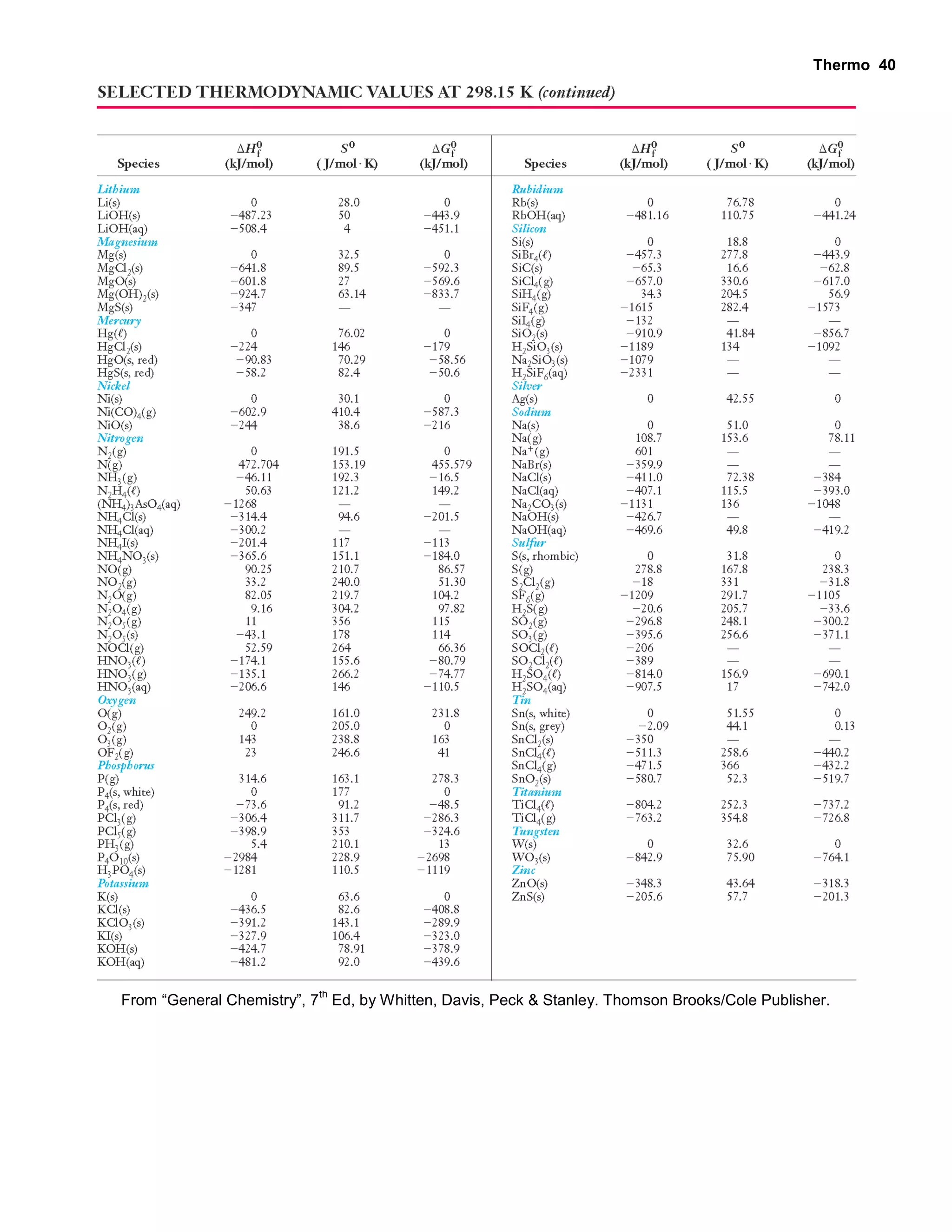
- Tables listing specific heats and molar heat capacities of various substances
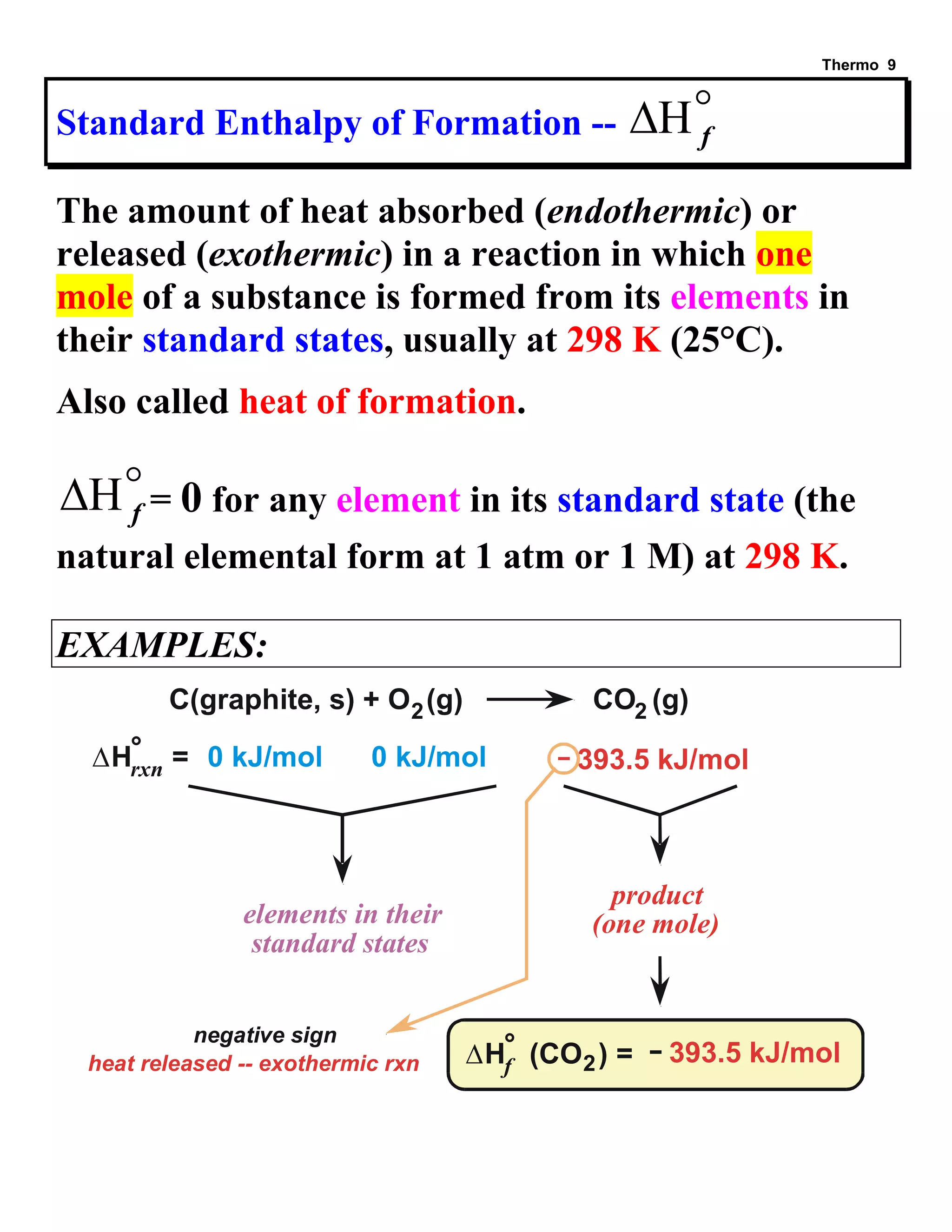
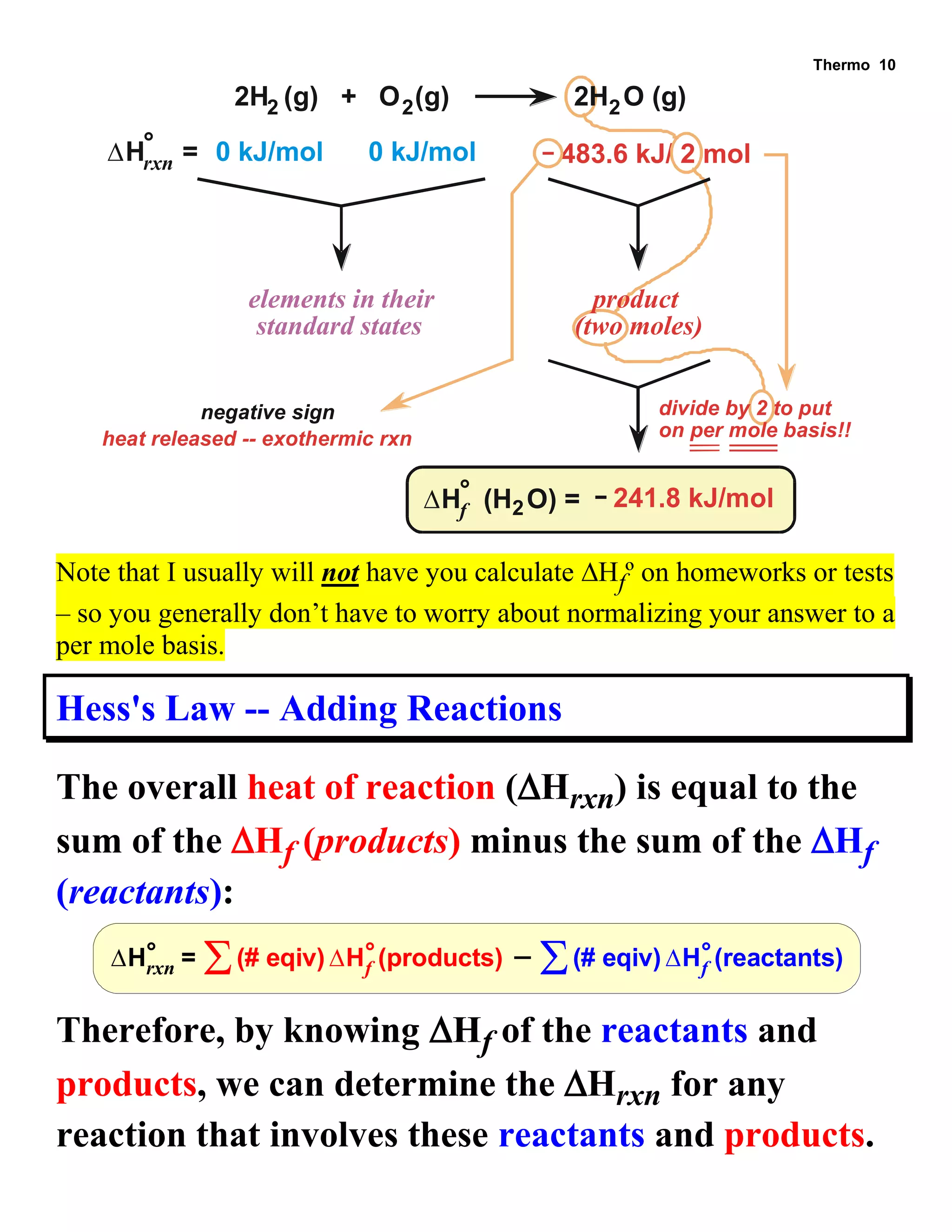
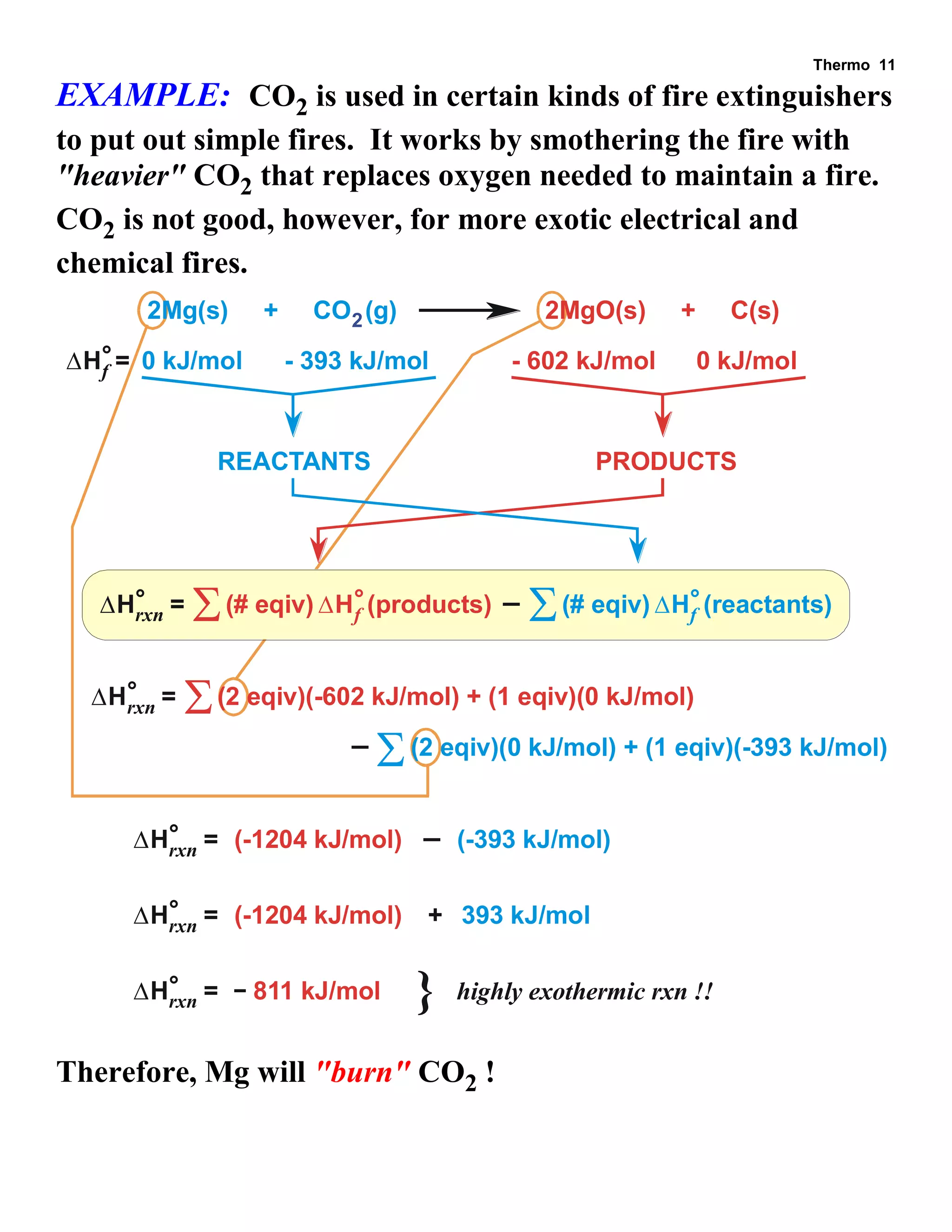
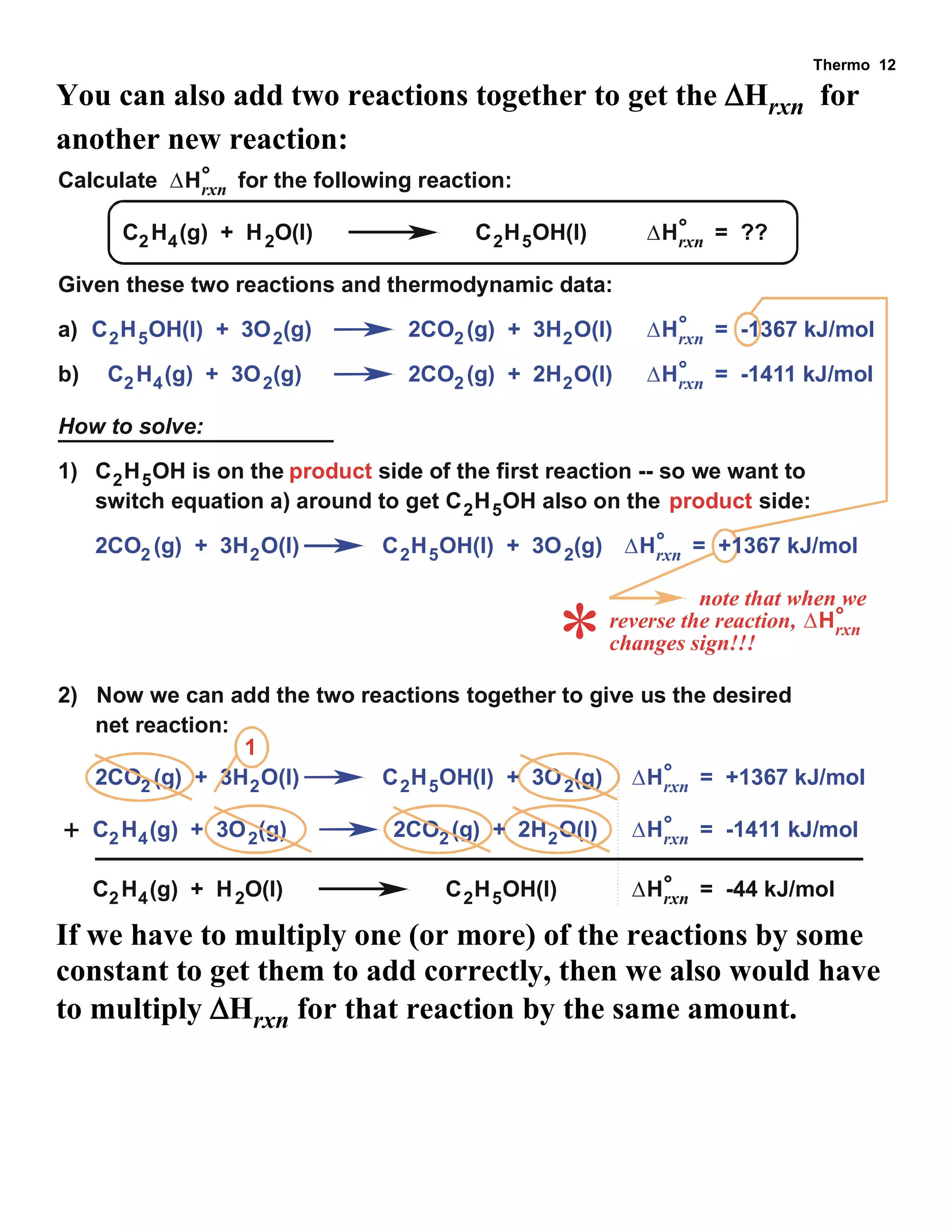
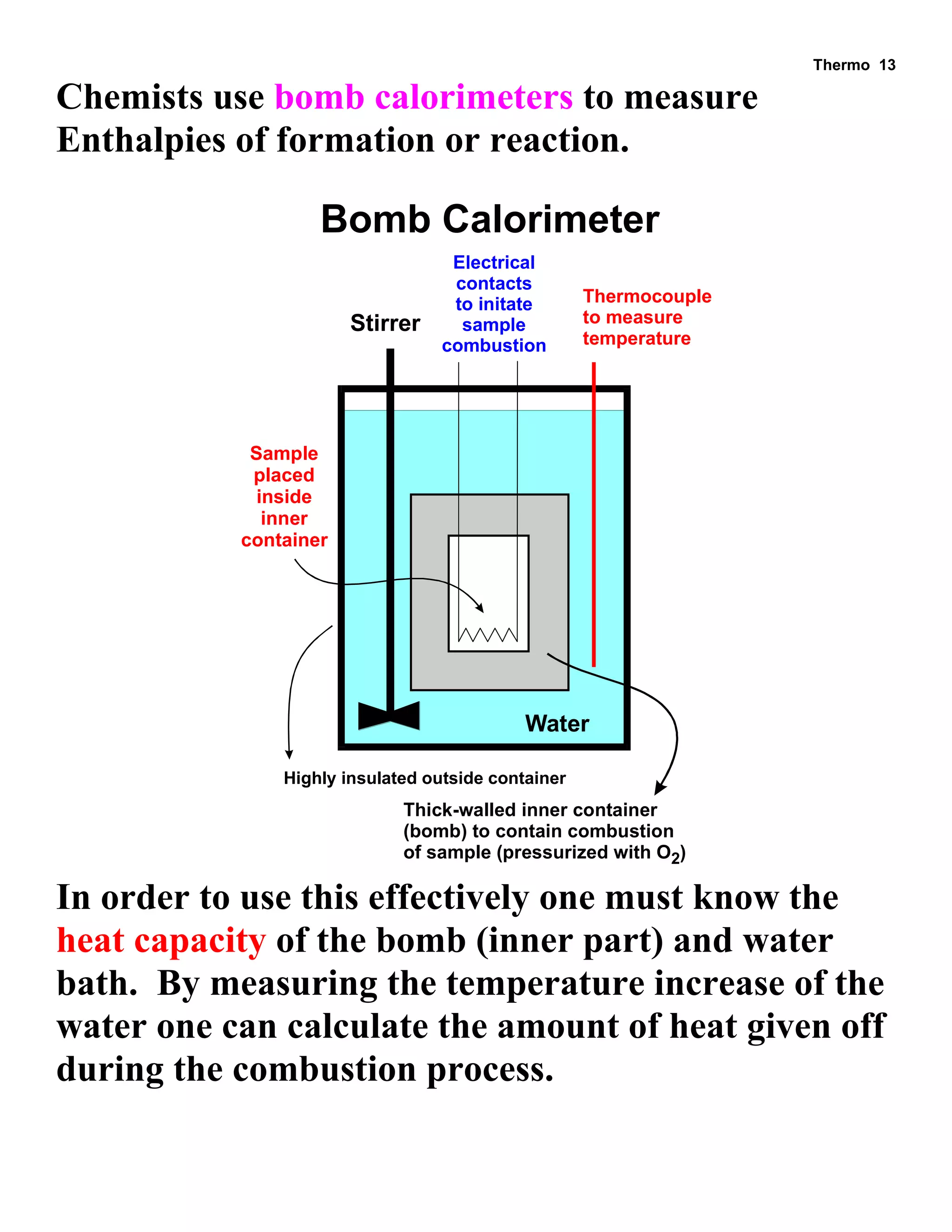
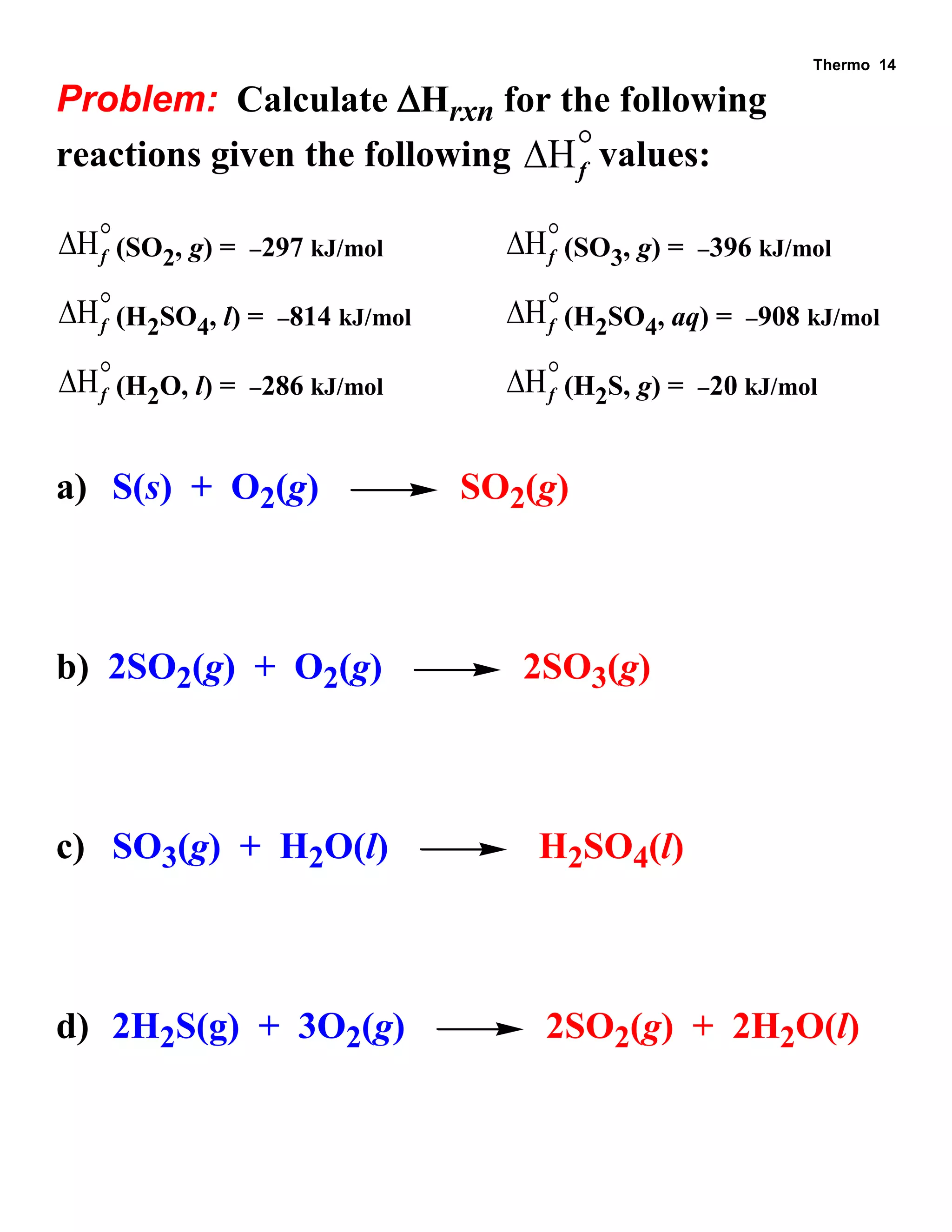
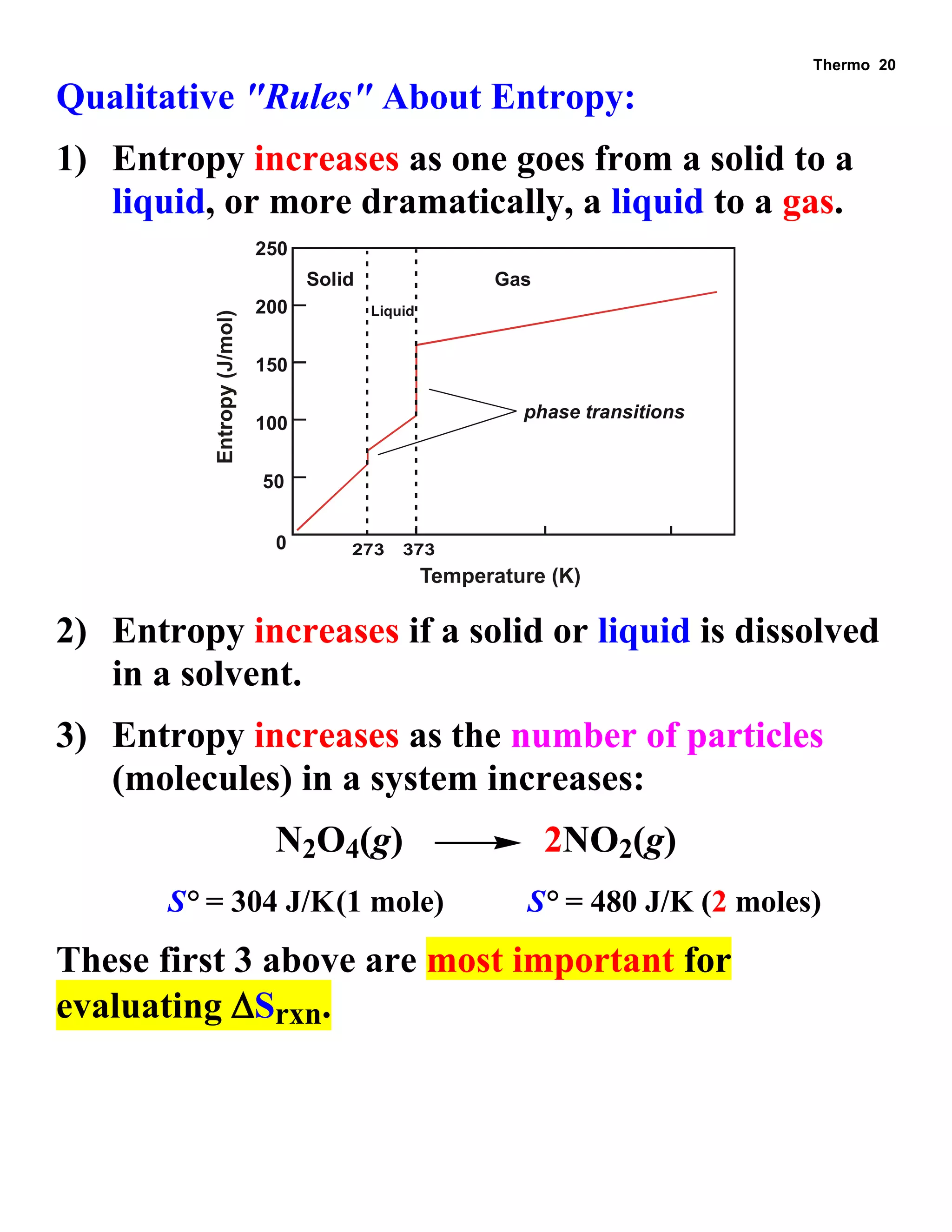
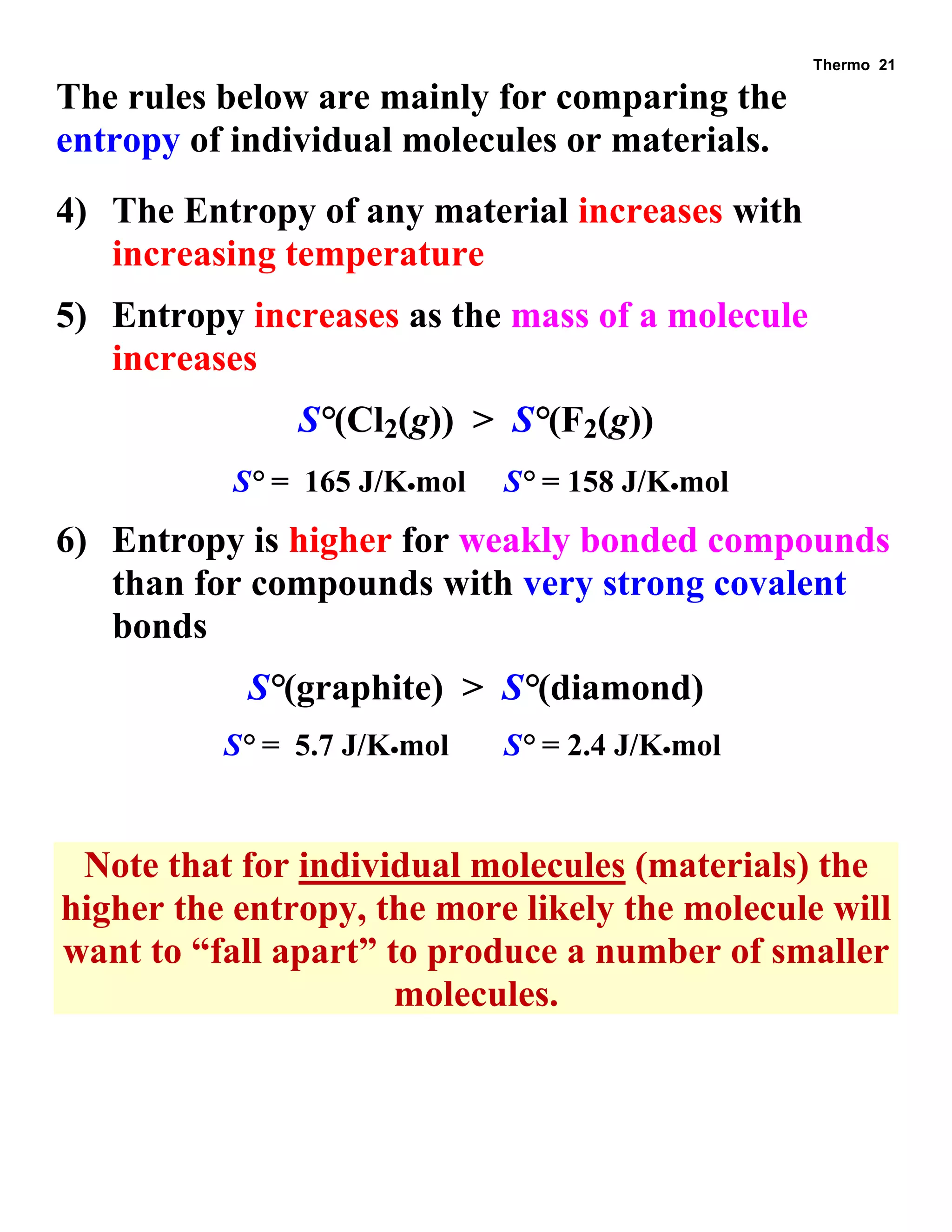
- Explanations and examples of standard enthalpy of formation (ΔH°f), Hess's law, and bomb calorimetry
- Relationships between internal energy (ΔE), enthalpy (ΔH), heat (q), and work (w)