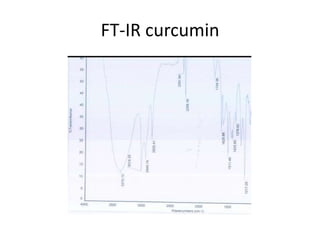
This document discusses various NMR experiments performed on curcumin and carboxylated curcumin, including 1H NMR, 13C NMR, 2D 1H-13C HSQC, 2D 1H COSY, and FT-IR. Key proton and carbon shifts are reported from the 1D and 2D NMR experiments for both curcumin and carboxylated curcumin. The COSY experiment is also introduced, explaining how it provides information on spin-spin coupling between protons through cross peaks.