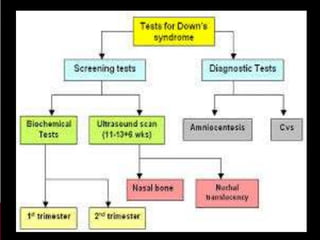
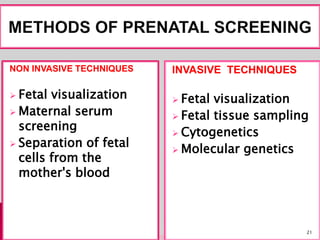
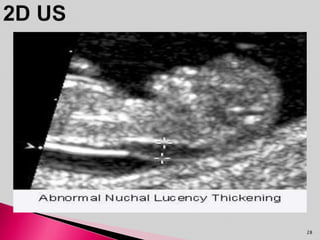
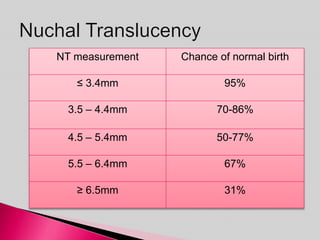
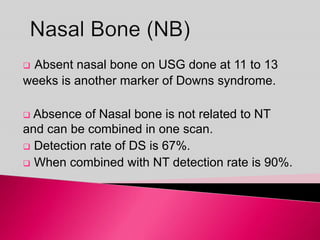
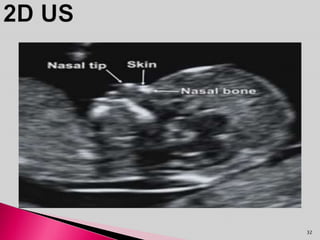
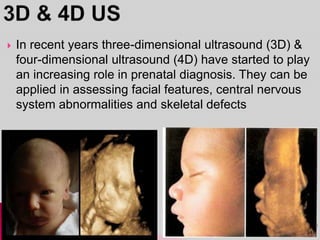
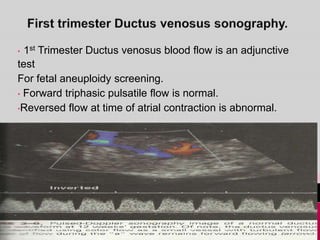
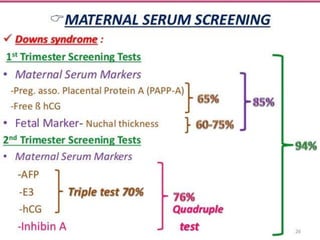
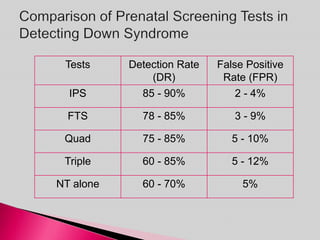
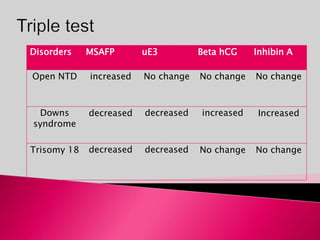
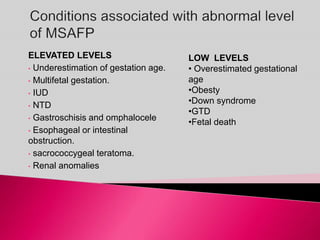
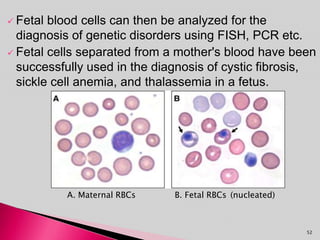
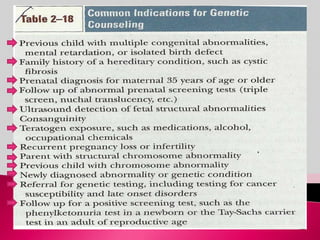
Prenatal screening and diagnosis allows for the detection of fetal abnormalities before birth. It involves various screening techniques in the first and second trimester such as nuchal translucency measurement, maternal serum markers, and ultrasound exams. Invasive diagnostic tests such as amniocentesis and chorionic villus sampling allow for the analysis of fetal cells to check for genetic abnormalities. The goal of prenatal screening and diagnosis is to provide information to parents about the health of the fetus and allow for informed decision making and management planning.