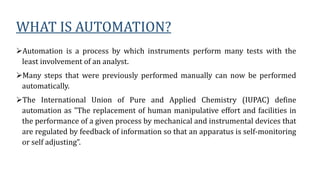
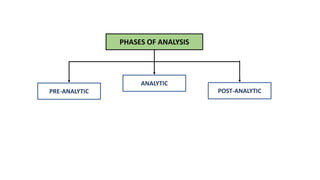
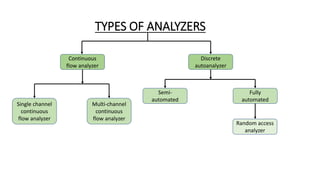
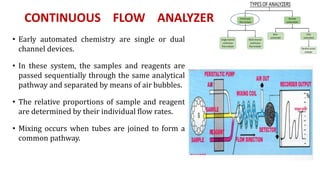
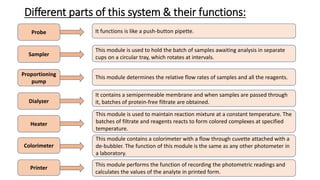
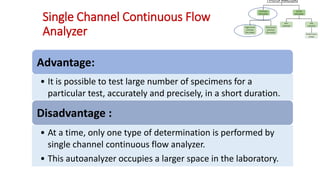
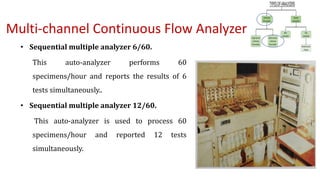
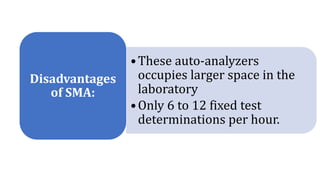
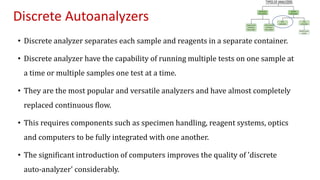
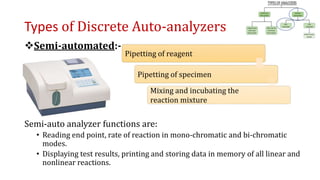
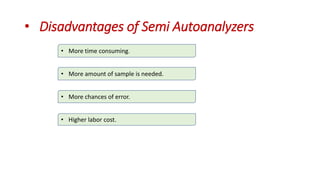
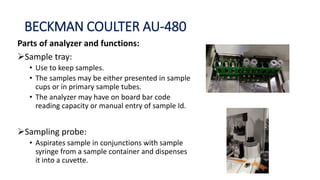
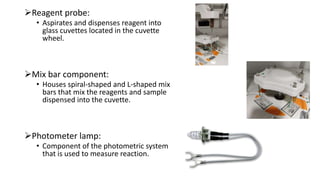
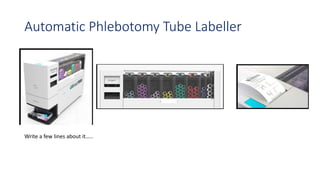
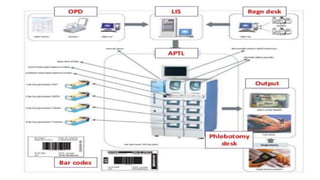
Automation in biochemistry refers to using instruments to perform biochemical tests with minimal human involvement. Automated systems can perform many steps like sample handling, reagent addition, reaction incubation, and measurement that were previously done manually. The main types of automated analyzers are continuous flow analyzers, discrete autoanalyzers, and random access analyzers. Continuous flow analyzers pass samples and reagents sequentially through a single analytical pathway. Discrete autoanalyzers separate each sample and reagent in individual containers, allowing multiple tests per sample. Random access analyzers perform tests on batches of samples, selecting tests for each sample. Automated systems provide benefits like higher throughput, reduced variability, and less manual labor, but also have high initial costs.