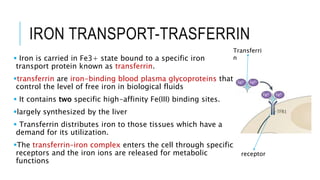
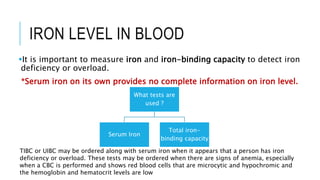
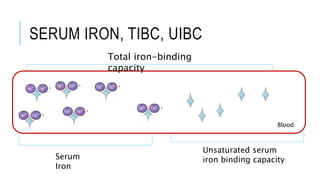
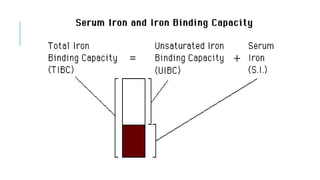
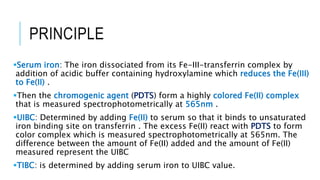
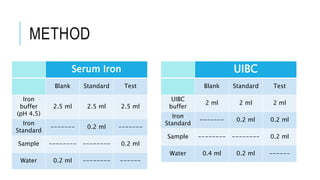
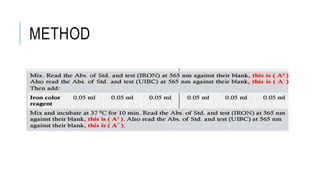
This document discusses the quantitative determination of serum iron, unsaturated iron binding capacity (UIBC), and total iron binding capacity (TIBC). It describes how iron is transported and stored in the body, bound to transferrin, and the principle and method for measuring serum iron, UIBC, and TIBC levels. Elevated or decreased levels of these tests can indicate conditions like iron deficiency anemia or hemochromatosis. Measuring these values together provides information about a person's iron status.