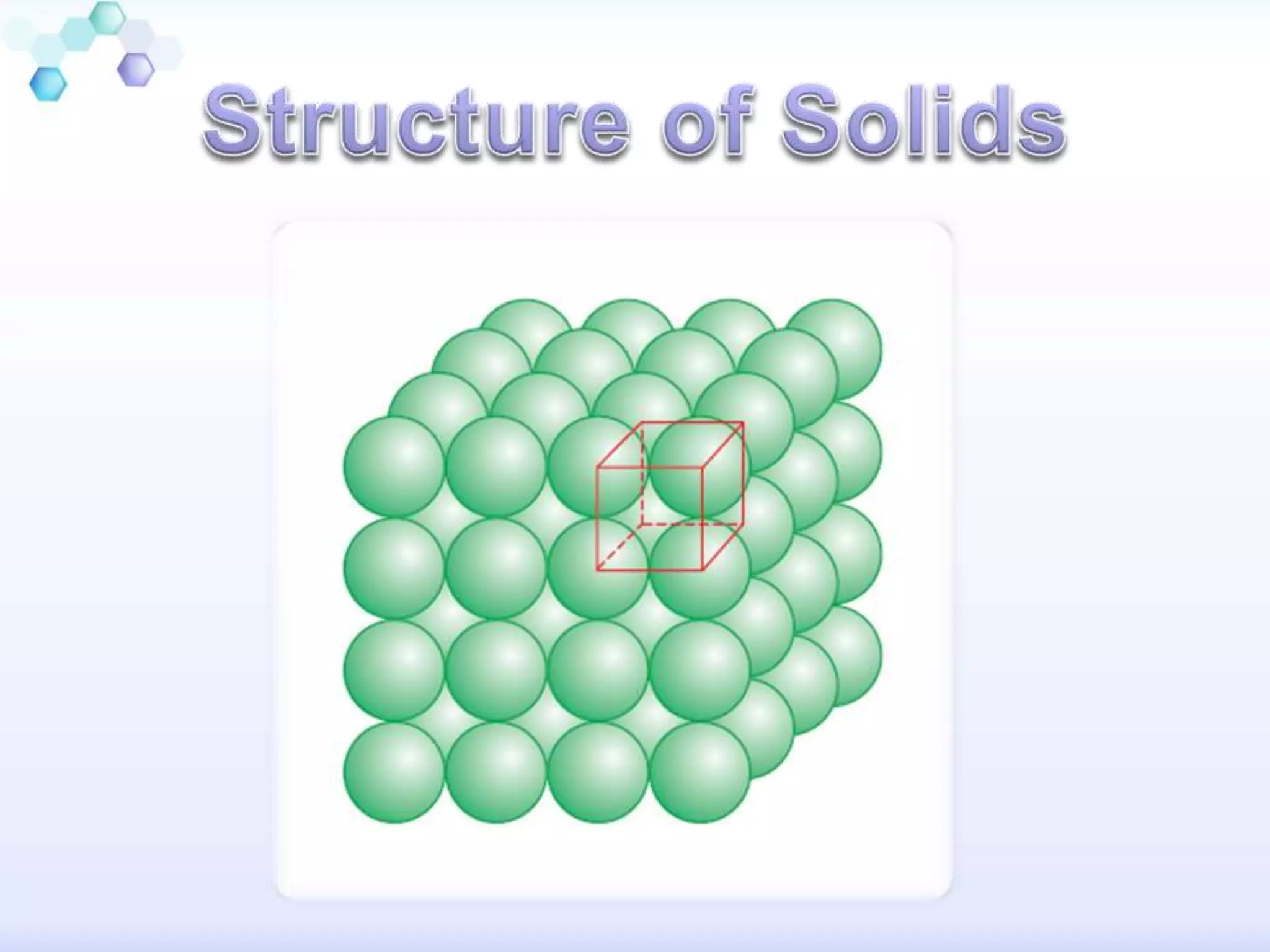
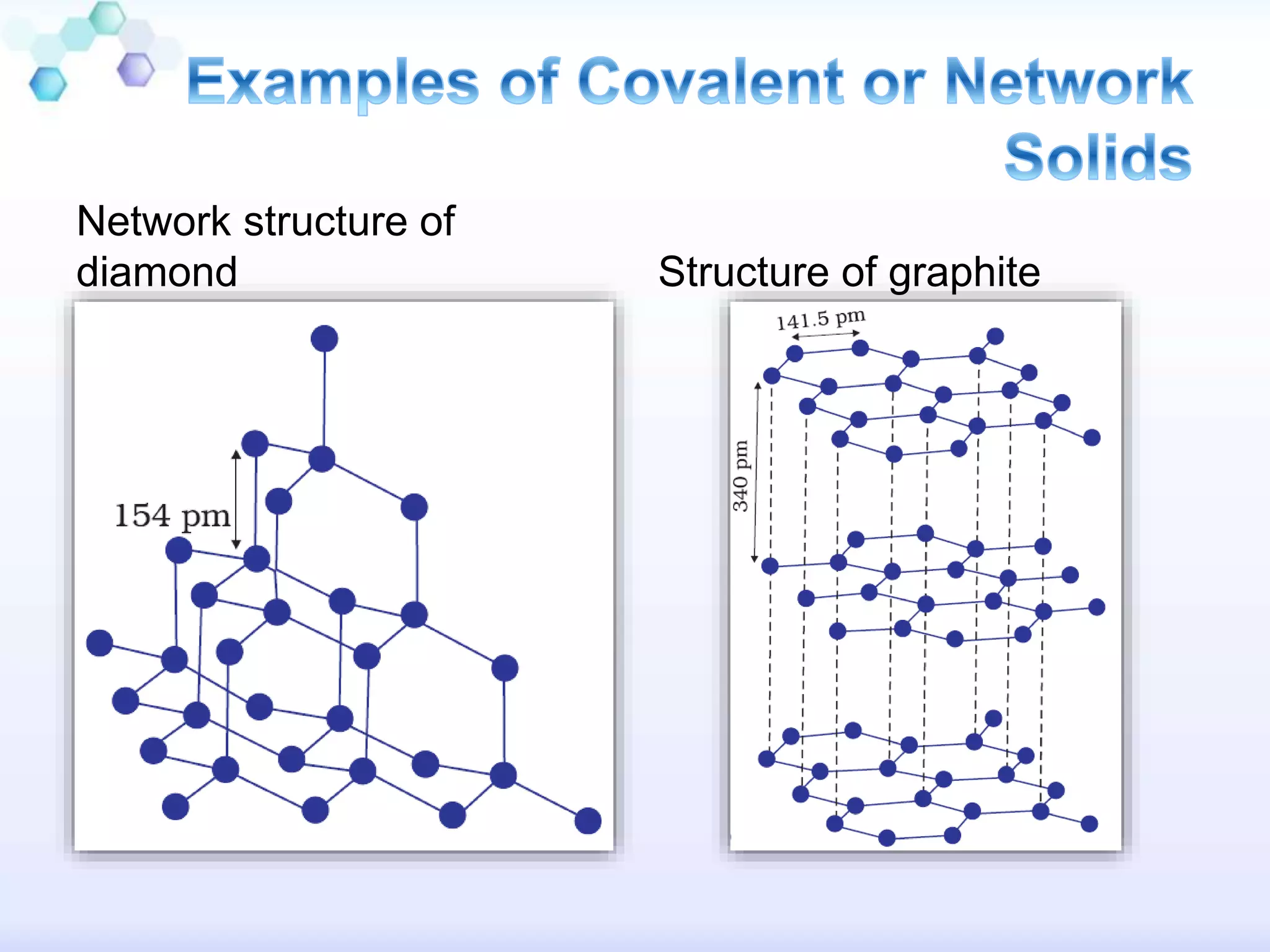
This document summarizes different types of solids. Crystalline solids have long-range order between particles which gives them distinct properties like sharp melting points and anisotropy. Amorphous solids only have short-range order, making them similar to supercooled liquids with no distinct melting point. Crystalline solids can be classified as molecular, ionic, metallic or covalent based on the interactions between particles. Each type of solid has distinct physical properties depending on these interactions.