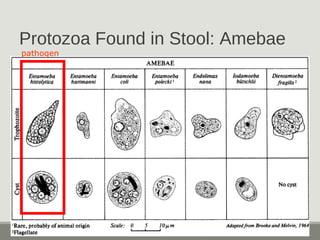
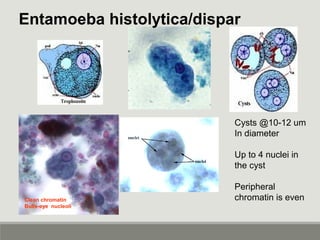
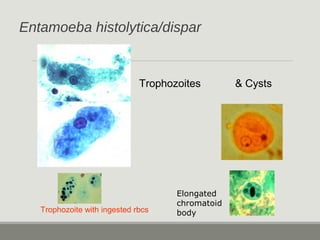
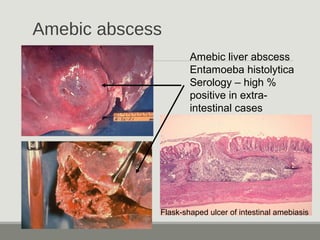
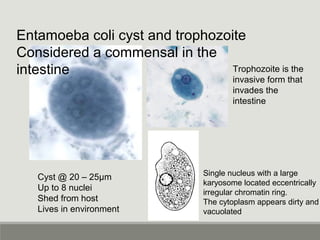
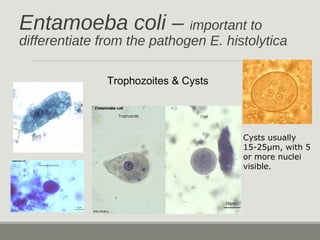
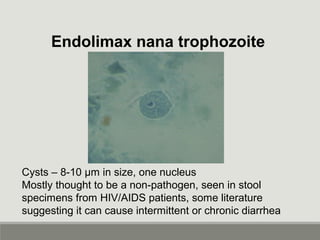
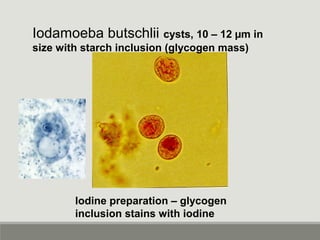
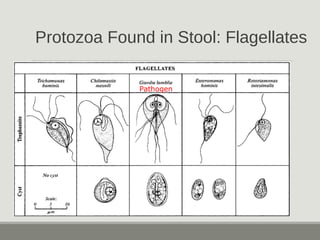
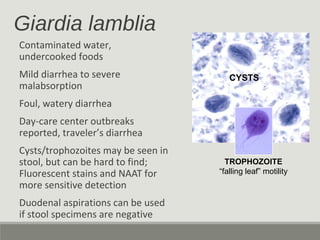
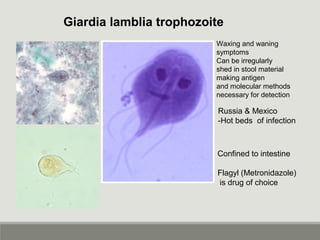
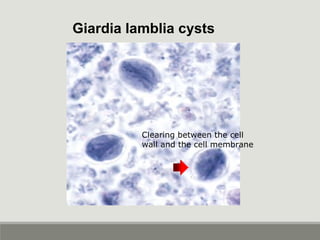
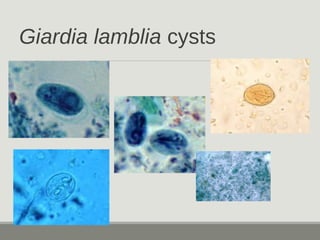
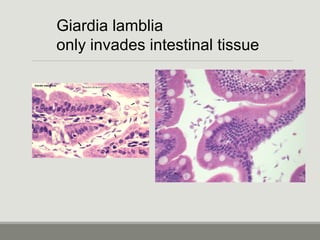
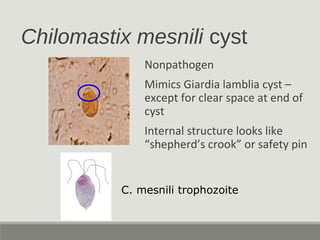
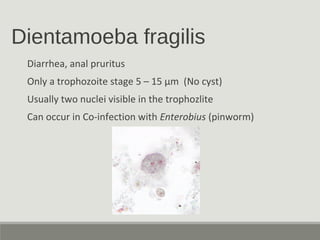
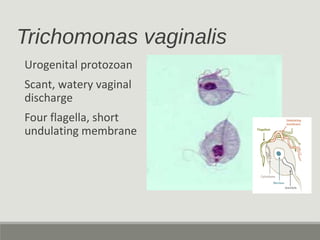
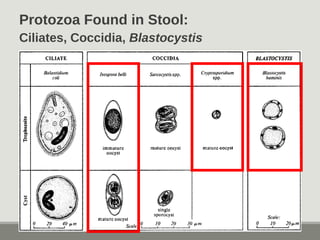
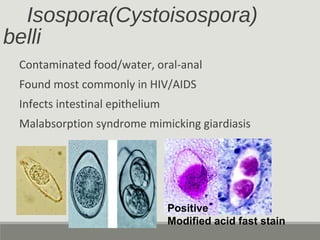
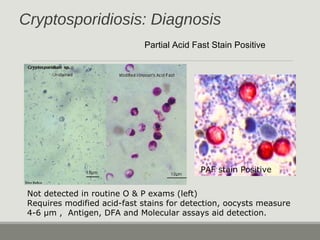
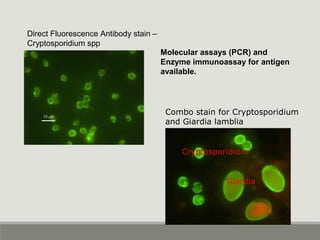
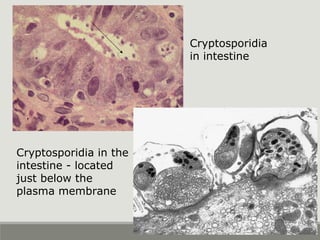
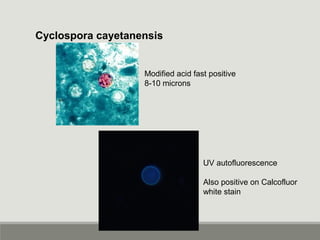
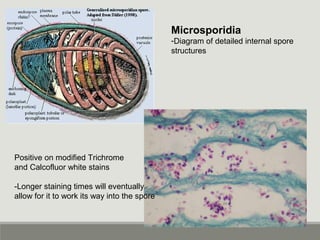
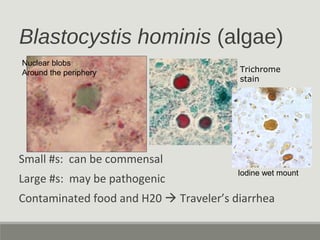
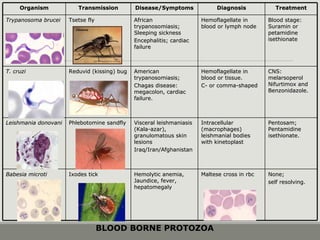
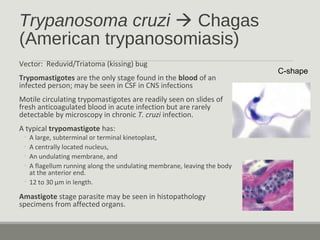
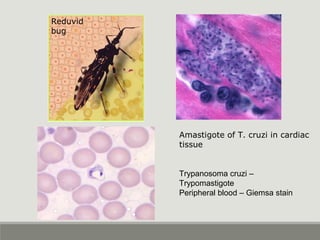
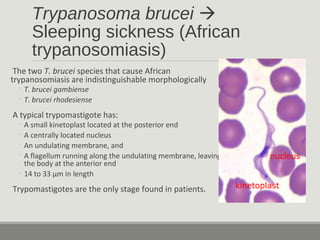
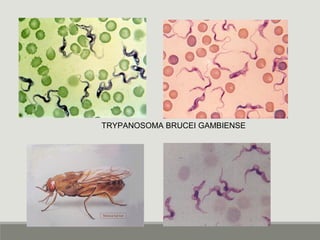
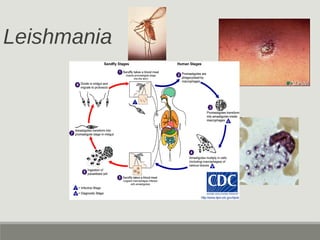
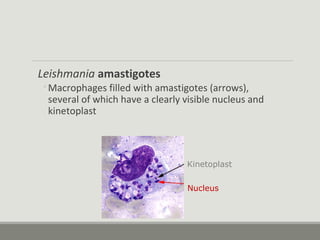
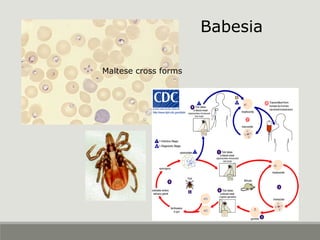
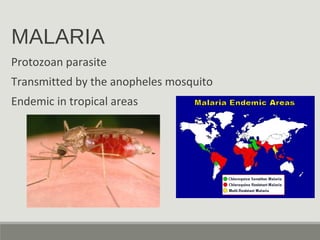
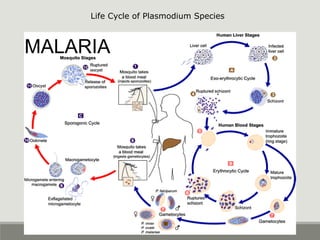
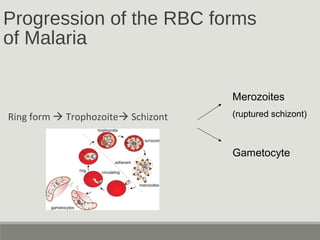
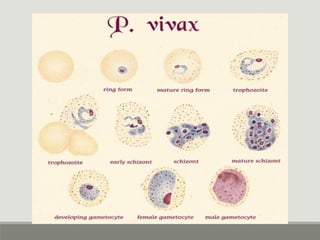
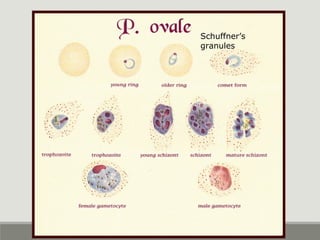
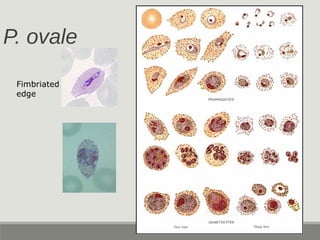
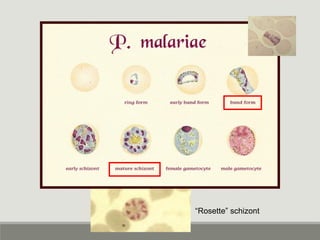
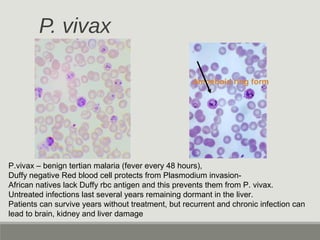
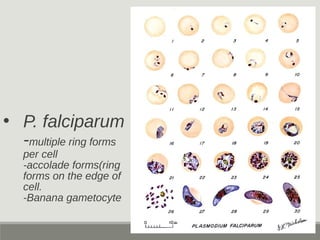
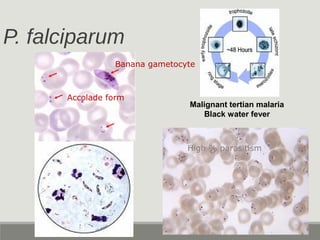
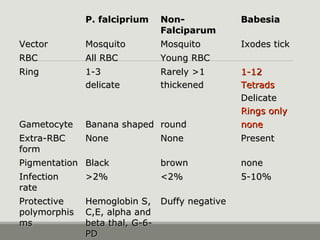
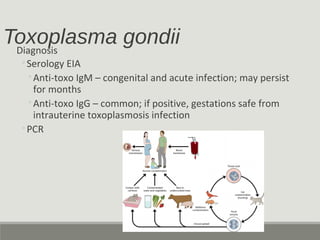
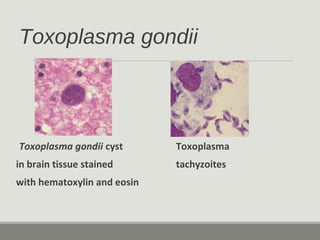
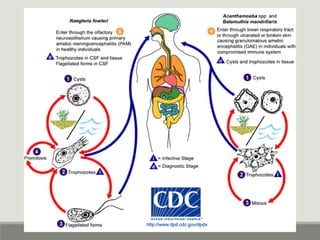
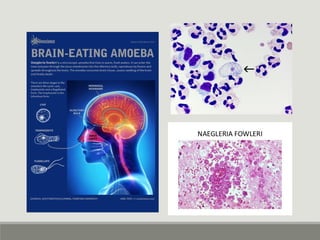
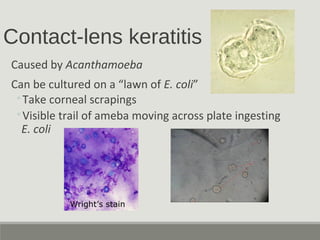
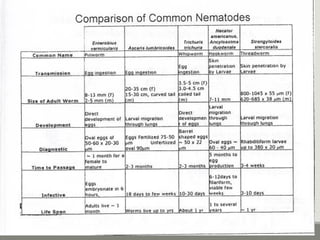
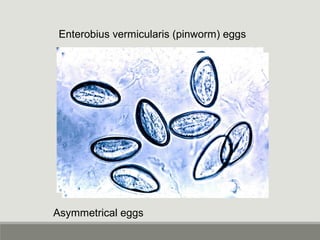
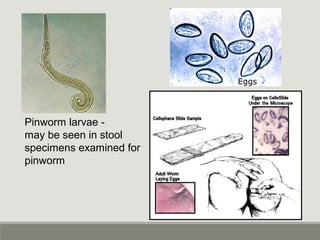
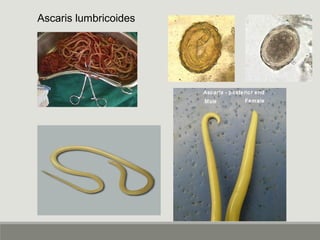
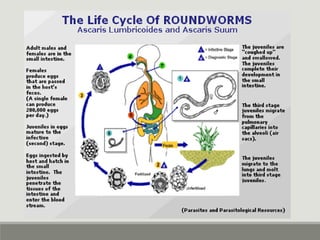
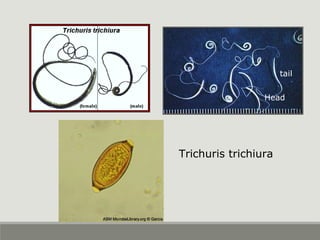
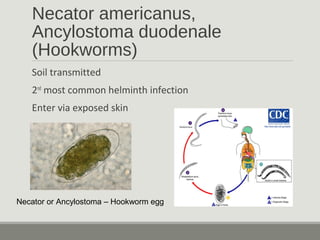
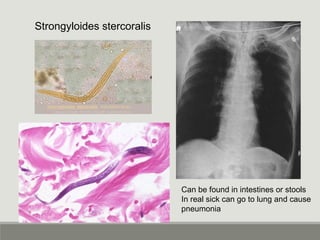
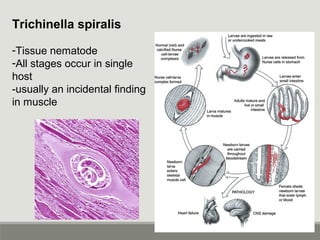
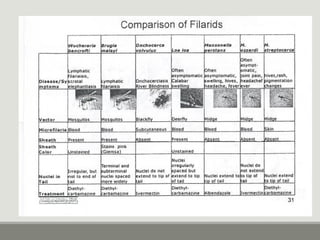
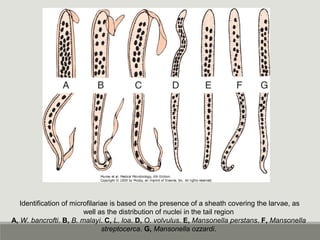
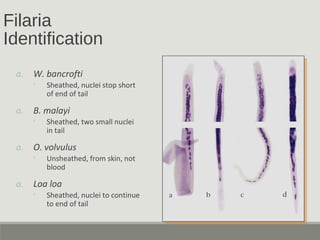
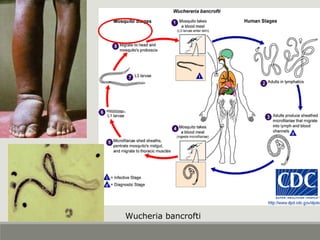
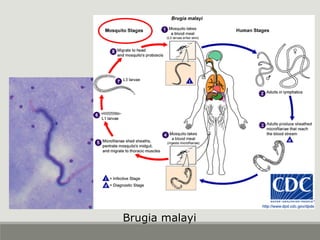
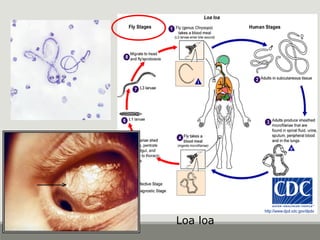
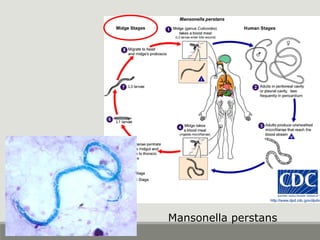
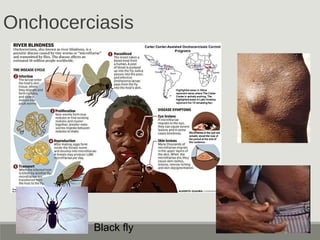
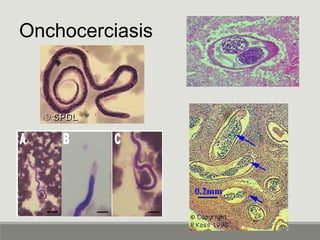
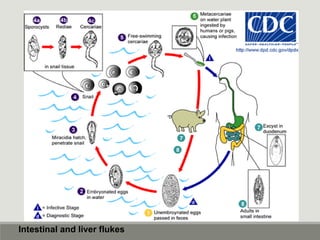
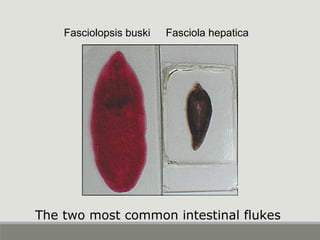
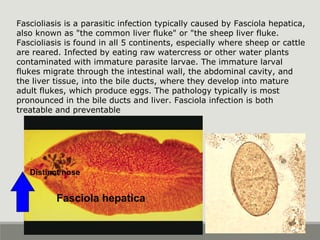
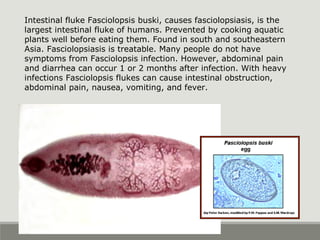
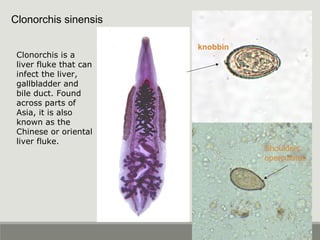
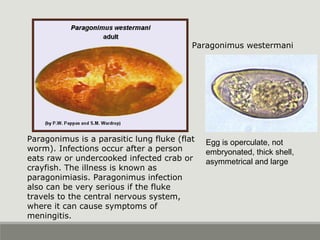
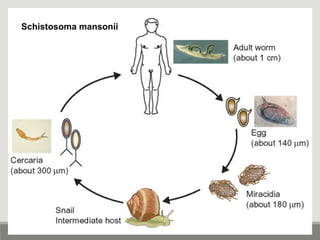
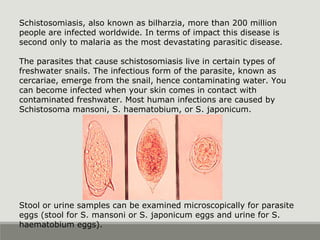
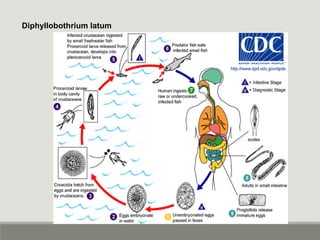
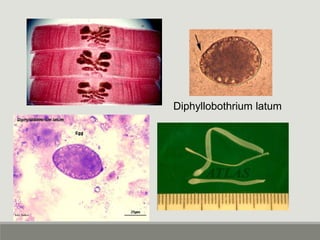
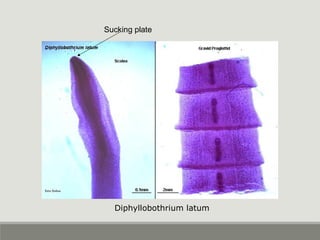
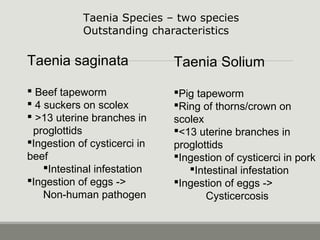
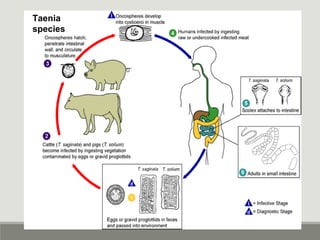
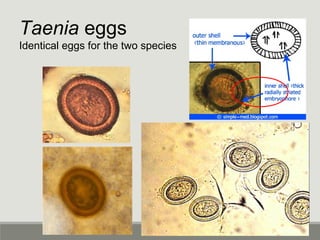
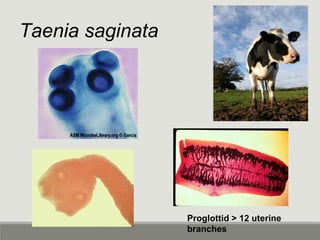
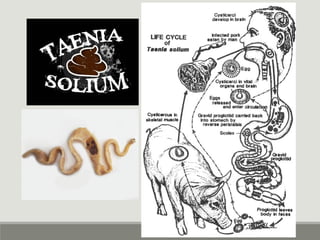
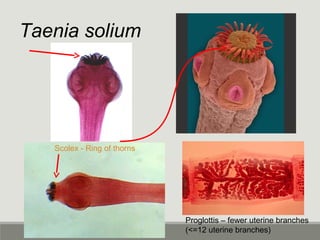
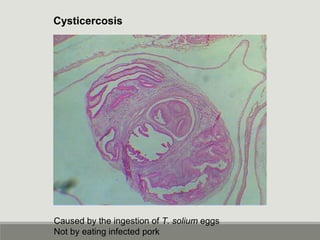
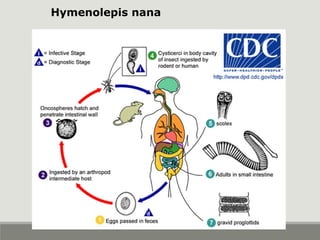
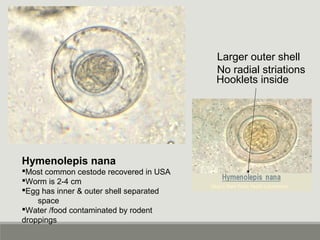
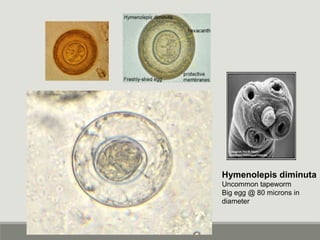
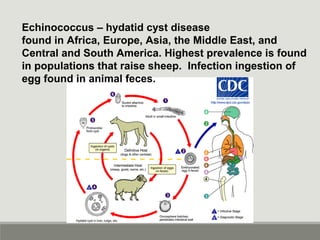
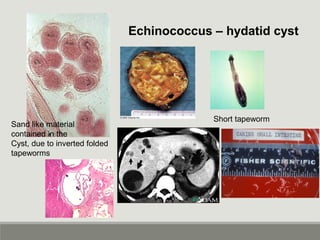
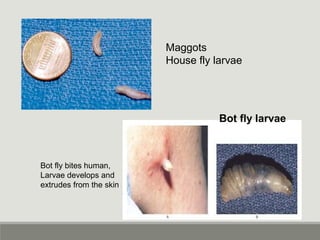
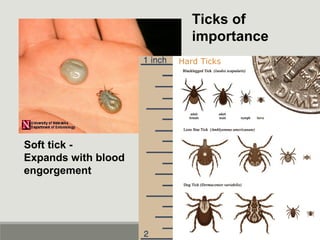
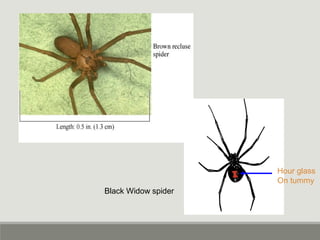
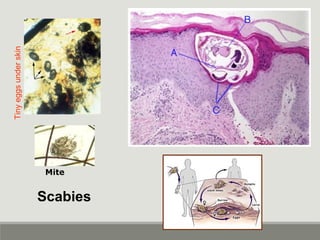
This document provides an overview of parasitic infections and their diagnosis. It discusses the most common intestinal protozoa and helminths seen in stool, including Entamoeba histolytica, Giardia lamblia, Cryptosporidium, and hookworm. It also covers blood-borne protozoa like Plasmodium, Babesia, Trypanosomes, and Leishmania. Diagnosis is based on microscopic examination of stool, tissue, or blood samples, as well as molecular testing. Symptoms vary by parasite but often include abdominal pain, diarrhea, and fatigue. Travel history and immune status factor into risk of infection.