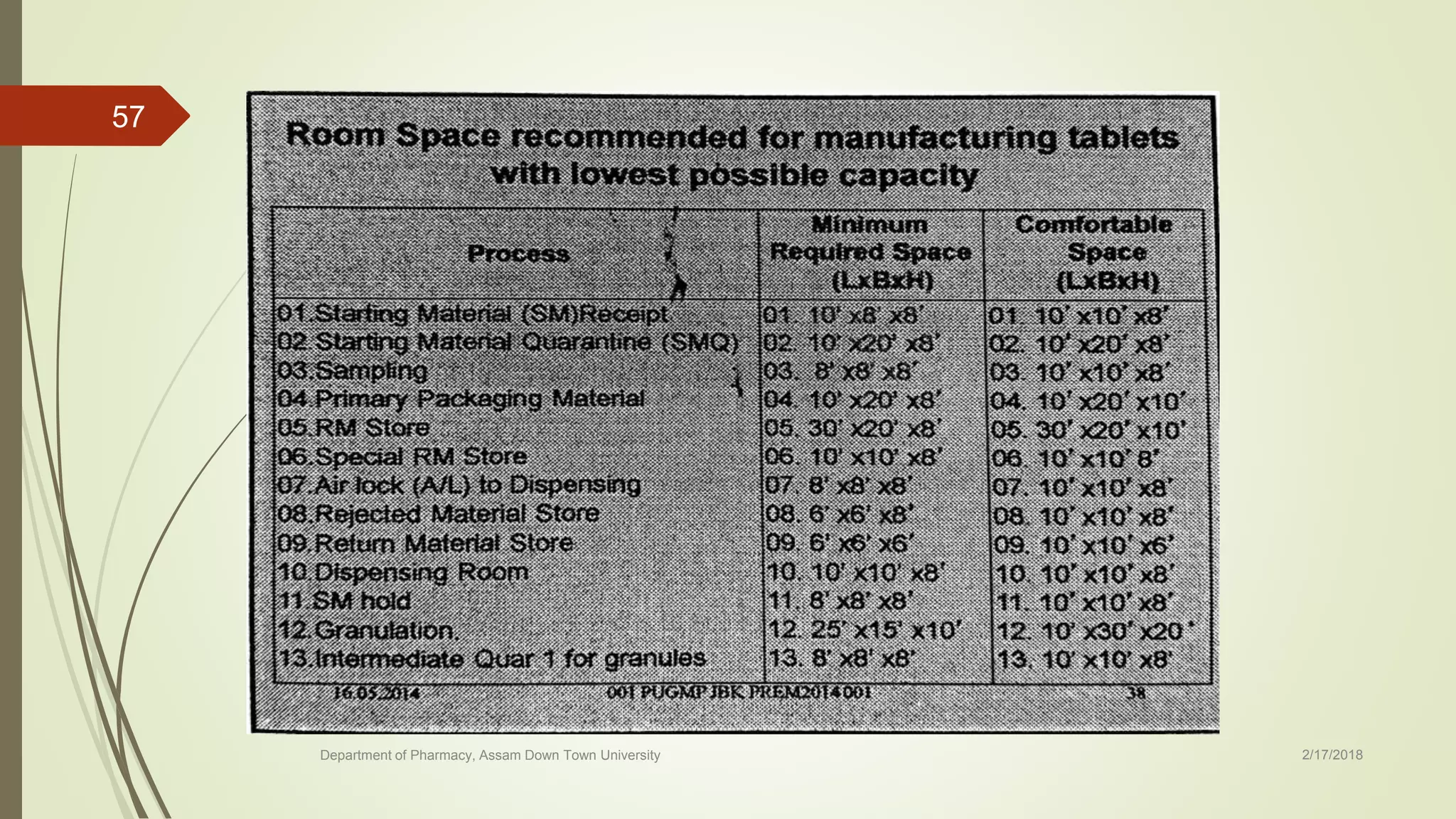
The document discusses the layout of pharmaceutical buildings and services. It begins with an introduction to good manufacturing practices (GMP) and their importance. It then covers the basic requirements for building layout, including material and personnel flows, equipment layout, and ensuring proper process flow. Specific areas of the building are also addressed, including ancillary, storage, weighing, production, and quality control areas. Proper lighting, electricity, and environmental controls are emphasized throughout.