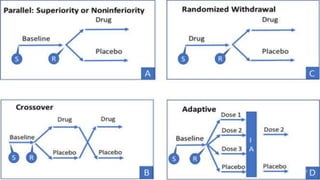
This document discusses study designs for bioequivalence studies. It describes the three main study designs: crossover design, parallel design, and multiple-dose design. The crossover design involves each subject receiving both the test and reference treatments in random order separated by a washout period. The parallel design assigns different groups of subjects to either the test or reference treatment. The multiple-dose design gives multiple doses of the same drug to reach steady state levels. Key elements of bioequivalence study design include selection of subjects, determining sample size, defining the washout period, randomization, data collection, statistical analysis, and ethical and regulatory considerations.