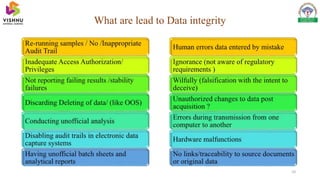
Data integrity refers to the completeness, consistency, and accuracy of data. The data should follow the ALCOA principles: Attributable, Legible, Contemporaneous, Original, and Accurate. A lack of data integrity can lead to warning letters from regulators, import alerts, and no further product approvals. Typical contents of warning letters include failing to investigate complaints, falsifying documentation, and improperly recording batch information. Maintaining data integrity is important to avoid regulatory consequences.











![References
13
1. Under the spotlight: Data Integrity in life sciences [Internet]. Deloitte LLP. 2017. [Cited: 4 March 2020].
Available at: https://www2.deloitte.com/content/dam/Deloitte/uk/Documents/life-sciences-health-
care/deloitte-uk-data-integrity-report.pdf
2. Guideline on Data Integrity [Internet]. World Health Organisation. October 2019. [Cited: 4 March 2020].
Available
at: https://www.who.int/medicines/areas/quality_safety/quality_assurance/QAS19_819_data_integrity.pdf?
ua=1
3. FDA ALCOA Guidance [Internet]. Beckman Coulter Life Sciences. Undated. [Cited: 5 March 2020].
Available at: https://www.mybeckman.uk/resources/industry-standards/alcoa
4. [Cited: 3 March 2020]. Available at: https://www.news-medical.net/news/20190729/Data-Integrity-in-the-
Pharmaceutical-Industry.aspx
5. Data Integrity in the Pharmaceutical Industry [Internet]. News-medical.net. 29 July 2019. [Cited: 5 March
2020]. Available at: https://www.forbes.com/sites/groupthink/2015/08/30/why-your-employee-training-is-
a-waste-of-time-and-money-and-what-to-do-about-it/#788f044028cf
6. Data Integrity and Compliance With Drug CGMP: Questions and Answers Guidance for
Industry [Internet]. US Food and Drug Administration. December 2018. [Cited: 6 March 2020]. Available
at: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/data-integrity-and-
compliance-drug-cgmp-questions-and-answers-guidance-industry](https://image.slidesharecdn.com/dataintegrityinpharmaceuticalindustries-210803044638/85/Data-integrity-in-Pharmaceutical-Industries-13-320.jpg)
