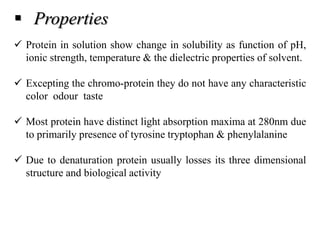
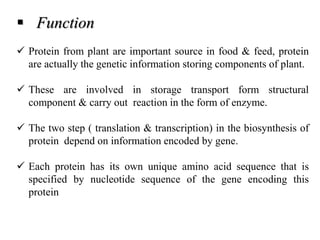
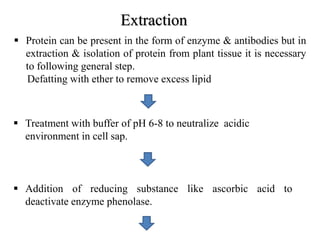
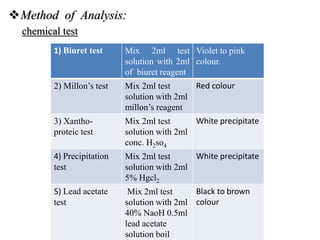
1) The document discusses techniques for isolating and characterizing various compounds from plants including proteins, lipids, ephedrine, piperine, quinine, and caffeine. Extraction methods and analytical techniques like TLC and HPLC are described for each compound.
2) Isolation generally involves extraction with organic solvents followed by precipitation or recrystallization. Characterization uses chemical tests and chromatography.
3) The document provides information on the isolation, extraction, purification, and analytical characterization methods for several important pharmaceutical compounds obtained from plants.


![classification
Based on composition protein can be categorized into general
classes.
1] Simple protein :
These proteins yield only alpha-amino acid on complete hydrolysis.
2] complex or conjugated protein:
These protein give an addition to amino acid an organic or
inorganic non-protein moiety called prosthetic group on hydrolysis.
Example ,
Conjugated
protein
• lipoprotein
• glycoprotein
Prosthetic
group
• lipid
• carbohydrate
Example
• Plasma
lipoprotein
• Immunoglobuli
n](https://image.slidesharecdn.com/seminar04-181120115508/85/isolation-technique-and-characterization-6-320.jpg)







![Stationary phase – silica gel
Mobile phase – chloroform: methanol : acetic acid :water
(170:25:25:1)
Detection – 50% sulphuric acid.
1] TLC for total lipids :
2] Argentation TLC for triglycerides :
stationary phase - silica gel impregnated with 5% AgNo3
Mobile phase - isopropanol : chloroform (3:197)
Detection - 50 % sulphuric acid.
Identification test :](https://image.slidesharecdn.com/seminar04-181120115508/85/isolation-technique-and-characterization-17-320.jpg)
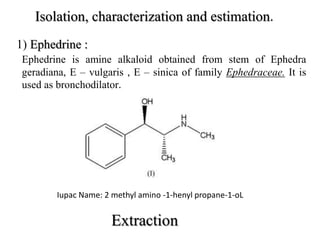


![2) Piperine :
Piperine is the major constituent of piper nigrum, piper longum of
family Piperaceae.
It is used as a food flavouring agent and bioavailability enhancer.
IUPAC Name: 1- [ 5-1,3 ( Benzodioxol 5-yl)-
1- oxo -2,4 pentadienyl ] piperidine.](https://image.slidesharecdn.com/seminar04-181120115508/85/isolation-technique-and-characterization-21-320.jpg)