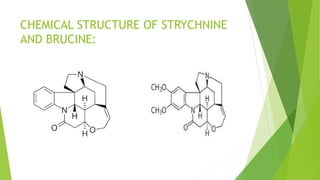
Strychnine is a toxic alkaloid found in the seeds of Strychnos nux-vomica. It acts as a central nervous system stimulant that binds to glycine receptors in the spinal cord, causing muscle contractions and ultimately death by asphyxiation. While highly toxic, strychnine was historically used as a pesticide for rodents and insects. Today its only minor medicinal use is as a stimulant and appetite enhancer in very small doses. The document provides details on the source, isolation, chemical properties, identification tests, derivatives and toxicity of strychnine.






![Characteristic Features:
1. It is obtained as brilliant, colourless cubes from a mixture of chloroform and
ether having mp 275-285°C, and d18 1.359.
2. Its specific optical rotation [α] 18 D-104.3° (C = 0.254 in ethanol); [α] 25 D-
13° (C = 0.4 in chloroform).
3. Its dissociation constant pKa (25°) 8.26.
4. It has uvmax (95% ethanol); 2550, 2800, 2900 Å (E1% 1cm 377, 130, 101).
5. Solubility Profile: 1g dissolves in 182 ml ethanol, 6.5 ml chloroform, 150 ml
benzene, 250 ml methanol, 83 ml pyridine; and very slightly soluble in water and
ether.
6. A solution of strychnine containing 1 part in 700,000 parts of water gives a
distinct bitter taste.](https://image.slidesharecdn.com/mohanppt-230309144312-37b6ca64/85/Strychnine-PPT-pptx-8-320.jpg)


![Identification Tests:
Strychnine may be identified either by specific colour tests or by specific derivatives:
(a) Colour Tests
1. Sulphuric Acid-Dichromate Test: Strychnine (5-10 mg) when dissolved in a few drops of
concentrated sulphuric acid and stirred with a crystal of pure potassium dichromate [K2 Cr2 O7]
it gives an instant reddish-violet to purple colouration. Note: Strychnine derivatives will also give
this test except strychnine nitrate.
2. Mandelin’s Reagent Test: Strychnine or its corresponding salt when treated with Mandelin’s
Reagent* it gives rise to a violet to blue colouration.
3. Ammonium Vanadate (V) Test: Strychnine or its salt when treated with a saturated solution
of ammonium vanadate, it produces a violet to blue colouration.
4. Nitric Acid Test: Strychnine on being treated with a trace of HNO3 (conc.) yields an instant
yellow colouration.
Note: A similar test with Brucine gives an intense orange-red colouration. It may be used to
differentiate between strychnine and brucine.](https://image.slidesharecdn.com/mohanppt-230309144312-37b6ca64/85/Strychnine-PPT-pptx-12-320.jpg)

![4. Strychnine Sulphate (C42H46N4O8S): It normally crystallizes as pentahydrate
[2C21H22N2O2.H2SO4.5H2O]. It is colourless, odourless, very bitter crystals or white
crystalline powder. It effloresces in dry air and loses all its water of crystallization at 100°C.
It shows mp * Mandelin's Reagent Dissolve 0.1g of ammonium vanadate in 10 ml of hot water
and add to it 1-2 ml of-conc. H2SO4. Filter and preserve the solution. when anhydrous ~
200°C with decomposition. 1g dissolves in 35 ml water, 7 ml boiling water, 81 ml ethanol, 26
ml ethanol at 60°C, 220 ml chloroform, 6 ml glycerol, and insoluble in ether. A 1 : 100
solution shows pH 5.5.
5. Strychnine Gluconate Pentahydrate (C27H34N2O9.5H2O): Its crystals darken above
80°C. It is soluble in 2 parts water ~ 40 parts ethanol. The aqueous solution is found to be
neutral.
6. Strychnine Glycerophosphate Hexahydrate (C45H53N4O10P.6H2O): 1g dissolves in ~
350 ml water, ~ 310 ml ethanol; slightly soluble in chloroform; and very slightly soluble in
ether.
7. Strychnine Hydrochloride Dihydrate (C21H23ClN2O2.2H2O): It is obtained as trimetric
prisms which are efflorescent in nature. 1g dissolves in ~ 40 ml water, ~ 80 ml ethanol, and
insoluble in ether. The pH of a 0.01 M solution is 5.4.](https://image.slidesharecdn.com/mohanppt-230309144312-37b6ca64/85/Strychnine-PPT-pptx-14-320.jpg)



