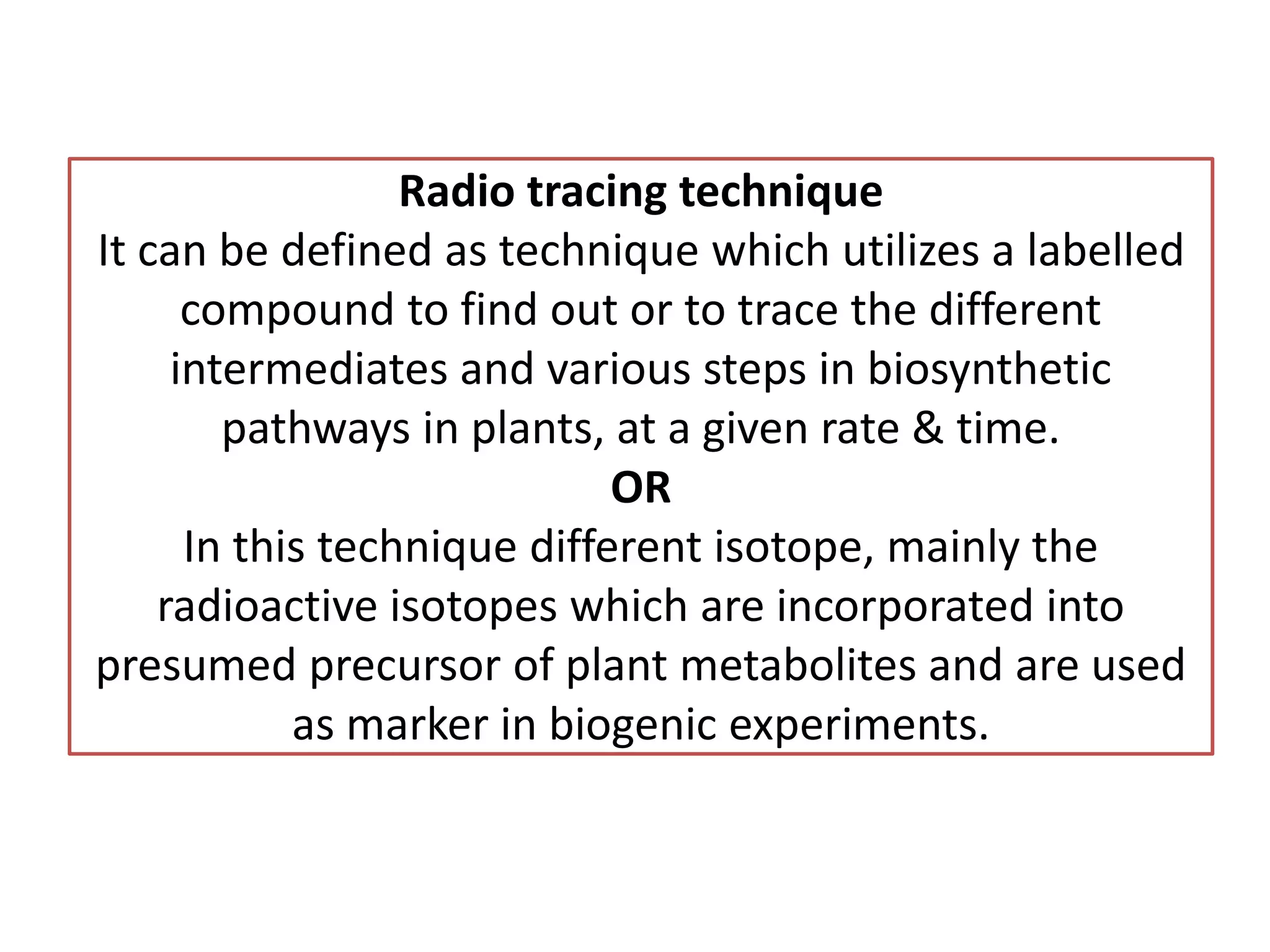
This document discusses radio tracing techniques used to trace biosynthetic pathways in plants. Radio tracing involves incorporating radioactive isotopes, like carbon-14 or tritium, into presumed precursors of plant metabolites. This allows researchers to track the intermediates and steps in pathways over time. Key aspects covered include selecting isotopes with appropriate half-lives, preparing labeled compounds, introducing the compounds into plants, and detecting the labeled molecules as they move through pathways. The document also lists several specific techniques used in radio tracing experiments, like precursor-product sequencing and competitive feeding studies.

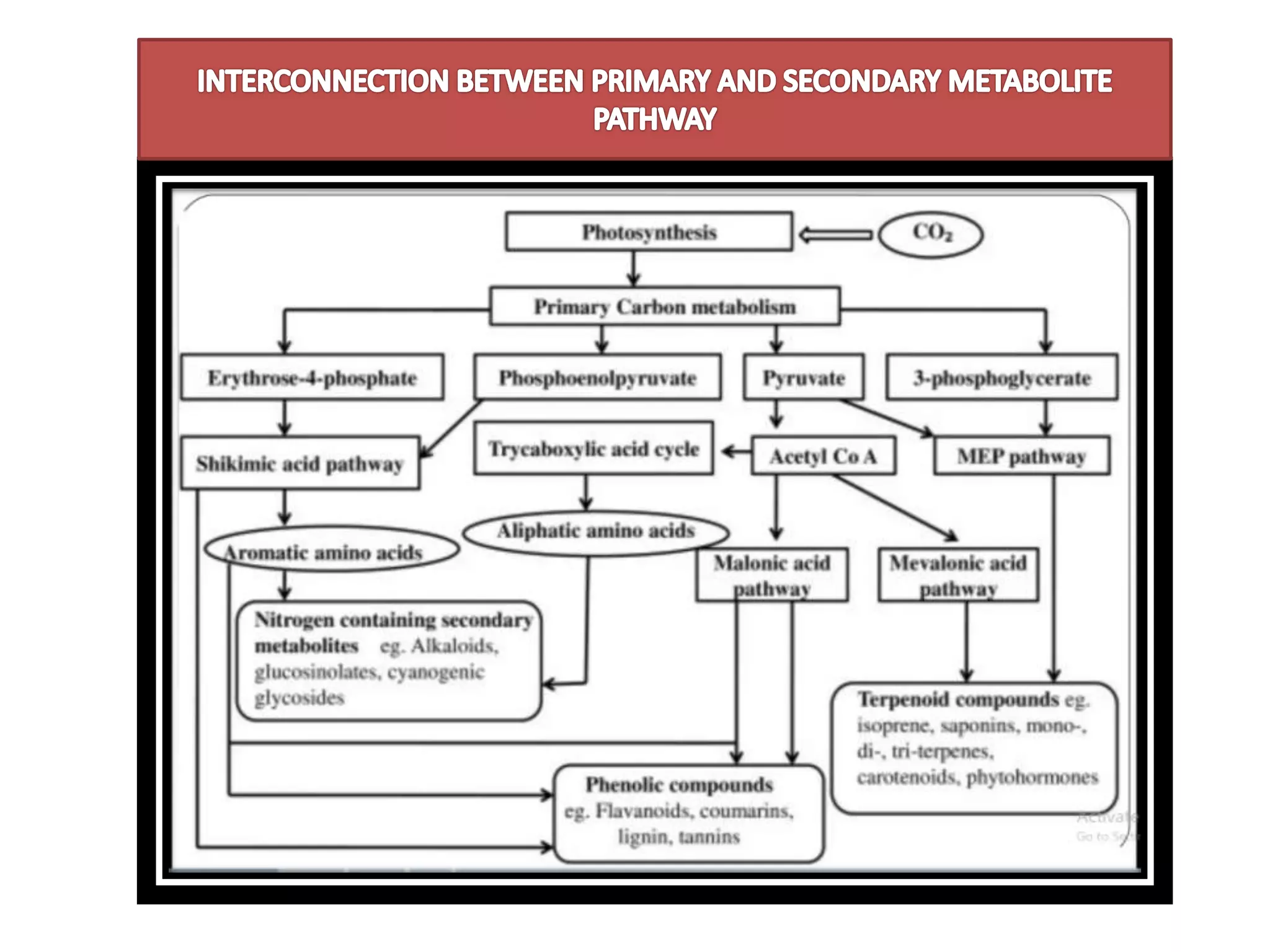
![• Primary metabolite[FATS,
CARBOHYDRATES,PROTEINS]
1. C3 CYCLE [CARBON FIXATION]
2. GLYCOLYSIS
3. KERBS CYCLE
• Secondary metabolite
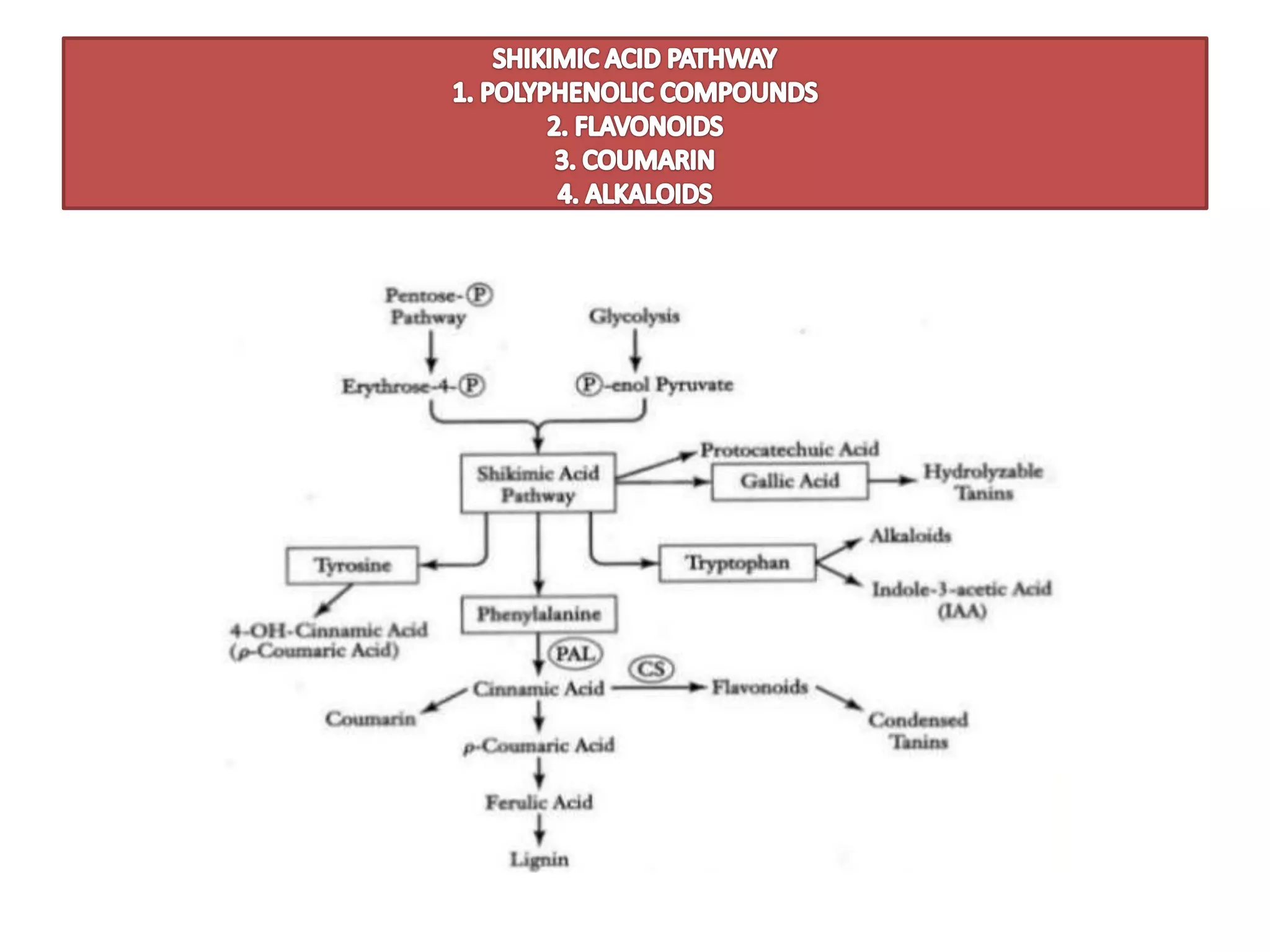
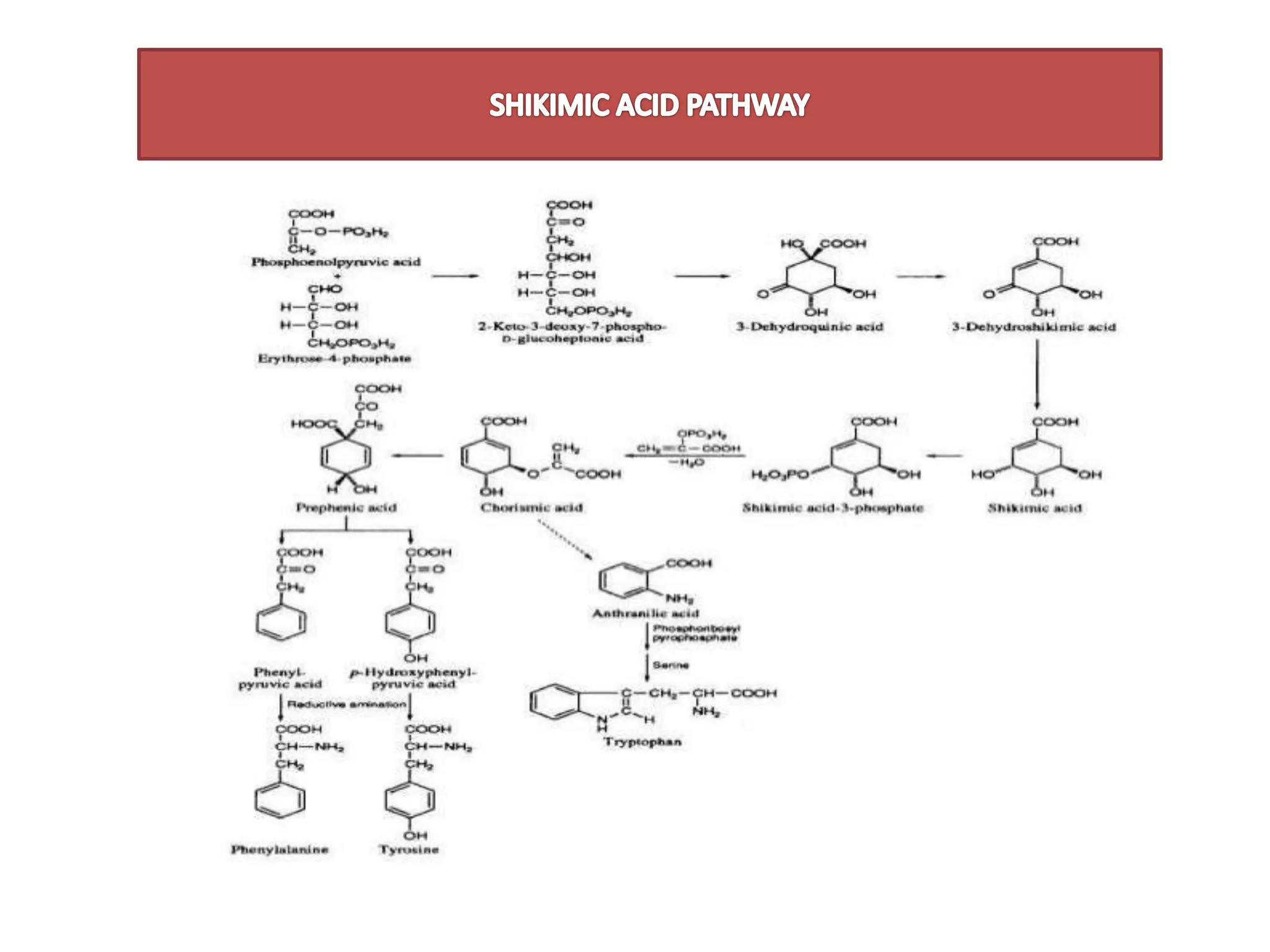
1. SHIKIMIC ACID PATHWAY
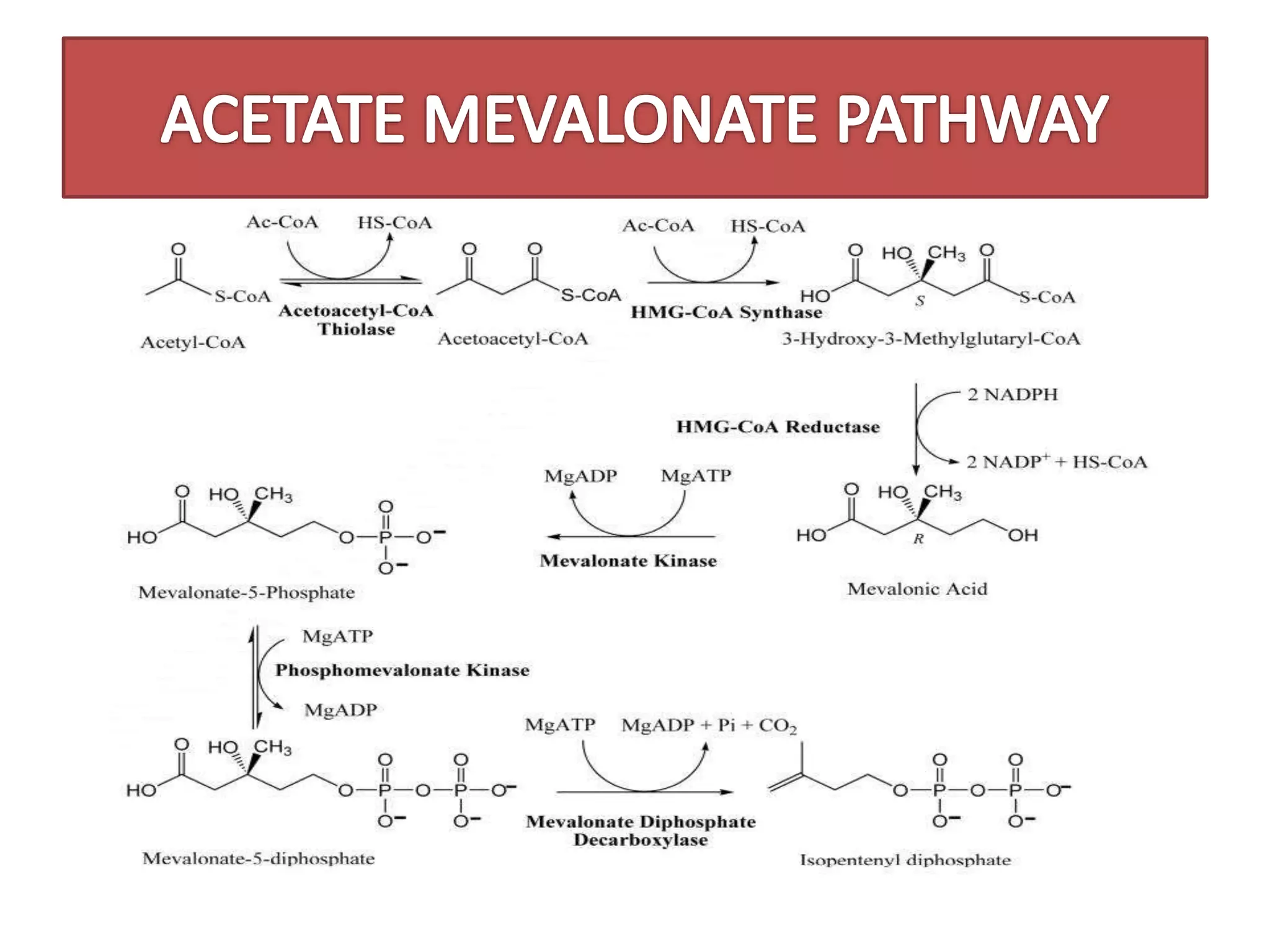
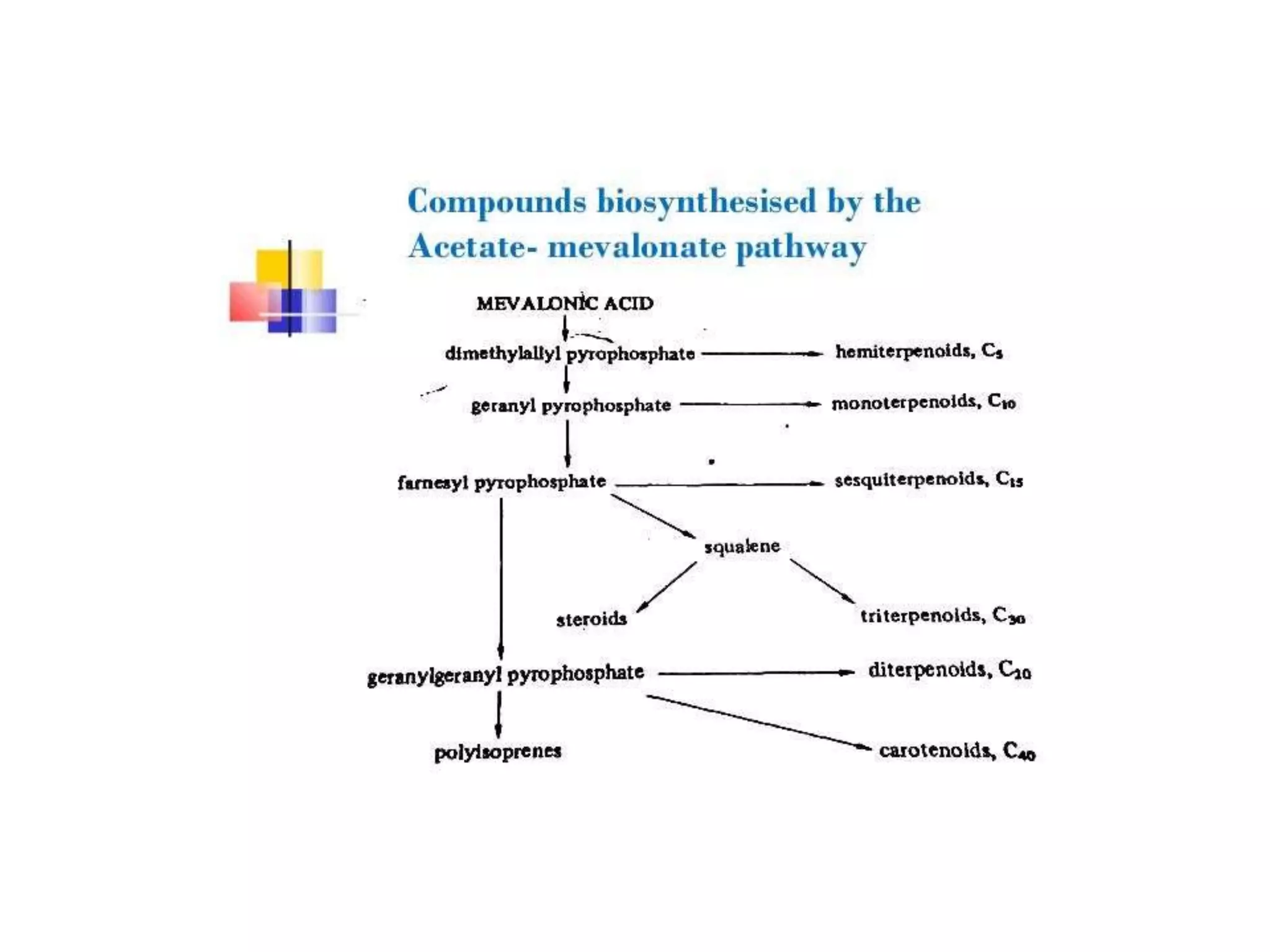
2. ACETATE MEVALONATE PATHWAY
END PRODUCTS WILL
ACT AS PRECURSORS
FOR THE SECONDARY
METABOLITES
PATHWAY](https://image.slidesharecdn.com/biosynthesispathwayandradiotracingtechnique-220426145803/75/Biosynthesis-Pathway-And-Radio-Tracing-Technique-pptx-2-2048.jpg)
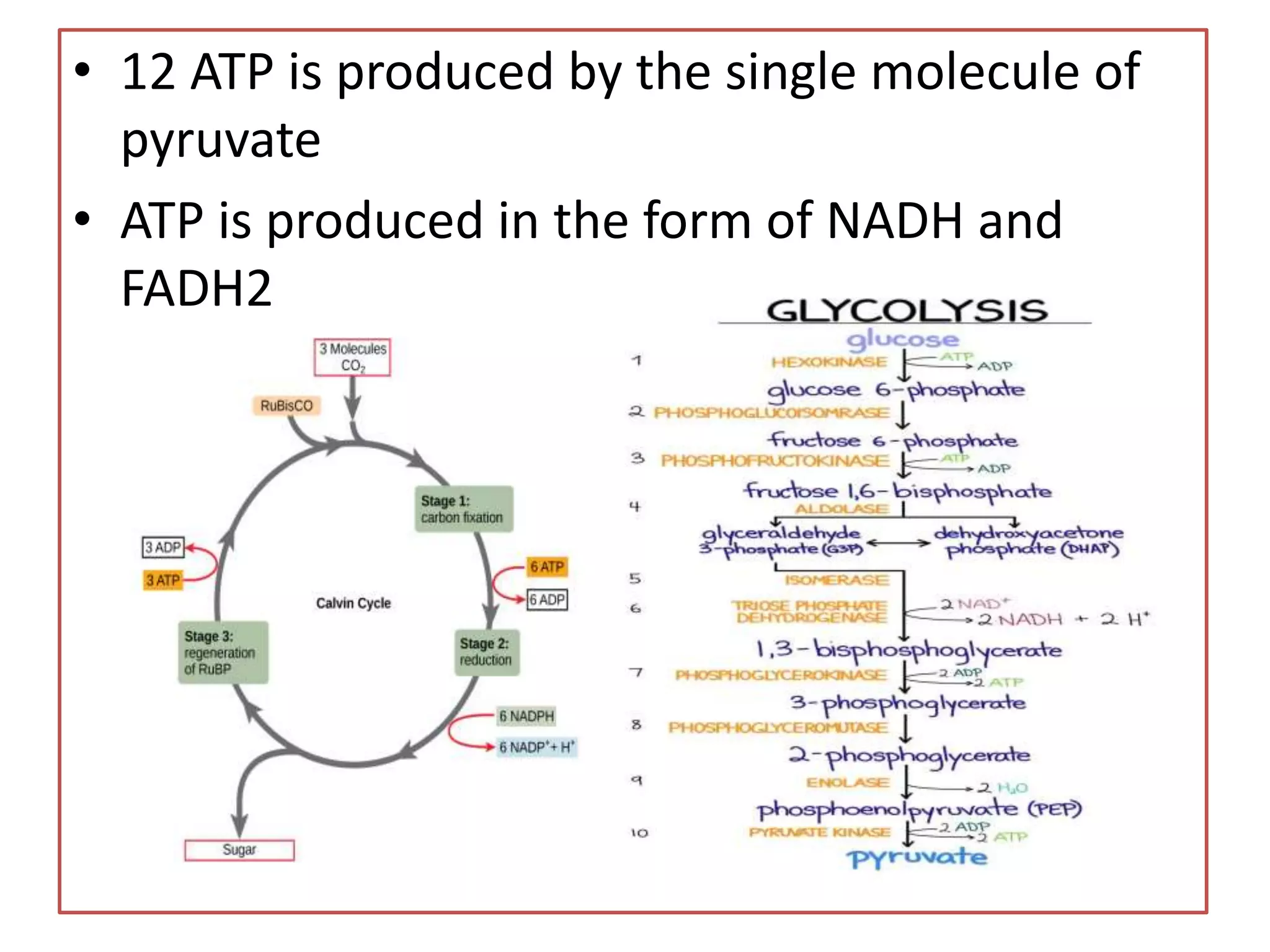
![• Photosynthesis [light dependent reaction]
H2O+LIGHT+ADP=ATP+NADPH+O2
• C3 pathway [Light independent reaction]
• Glycolysis [break down of glucose]
1. End product- pyruvate
2. Net ATP production- 2 ATP
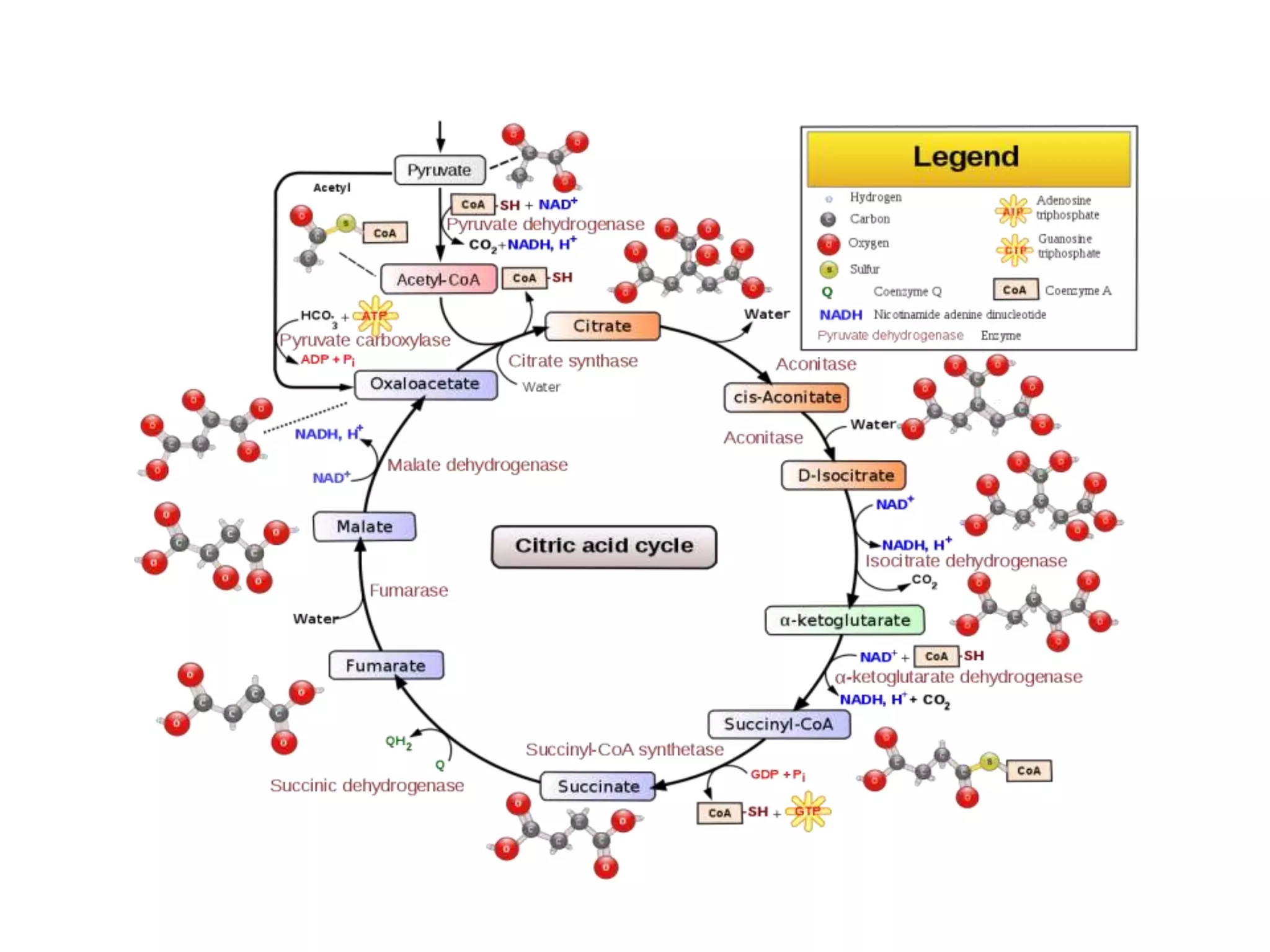
• Krebs cycle [generation of energy and
intermediates that act as a precursors for various
biosynthesis pathway]](https://image.slidesharecdn.com/biosynthesispathwayandradiotracingtechnique-220426145803/75/Biosynthesis-Pathway-And-Radio-Tracing-Technique-pptx-3-2048.jpg)

![Introduction
• Isotopes- placed at same position in periodic table
• Same atomic number but differ in the atomic mass [formula of atomic
mass is = no of proton+ no of neutron]. Posses same chemical property
but differ in the physical property
• Example :12
6C:13C, 14C
• 1H, 2H, 3H
• Types of isotopes
1. Radio isotopes- emits the radiation in the form of alpha, beta and
gamma. In the nuclei of the radio atom the no of neutron and proton are
unequal and larger amount of the energy is stored in their nuclei which
makes the radioatom unstable therefore In ordered to get stablized they
emit those energy in the form radiation. Examples: 3H, 14C, 35S, 131I,
24Na.
2. Stable isotopes- does not emit any radition
Example:2H, 13C, 15N](https://image.slidesharecdn.com/biosynthesispathwayandradiotracingtechnique-220426145803/75/Biosynthesis-Pathway-And-Radio-Tracing-Technique-pptx-13-2048.jpg)

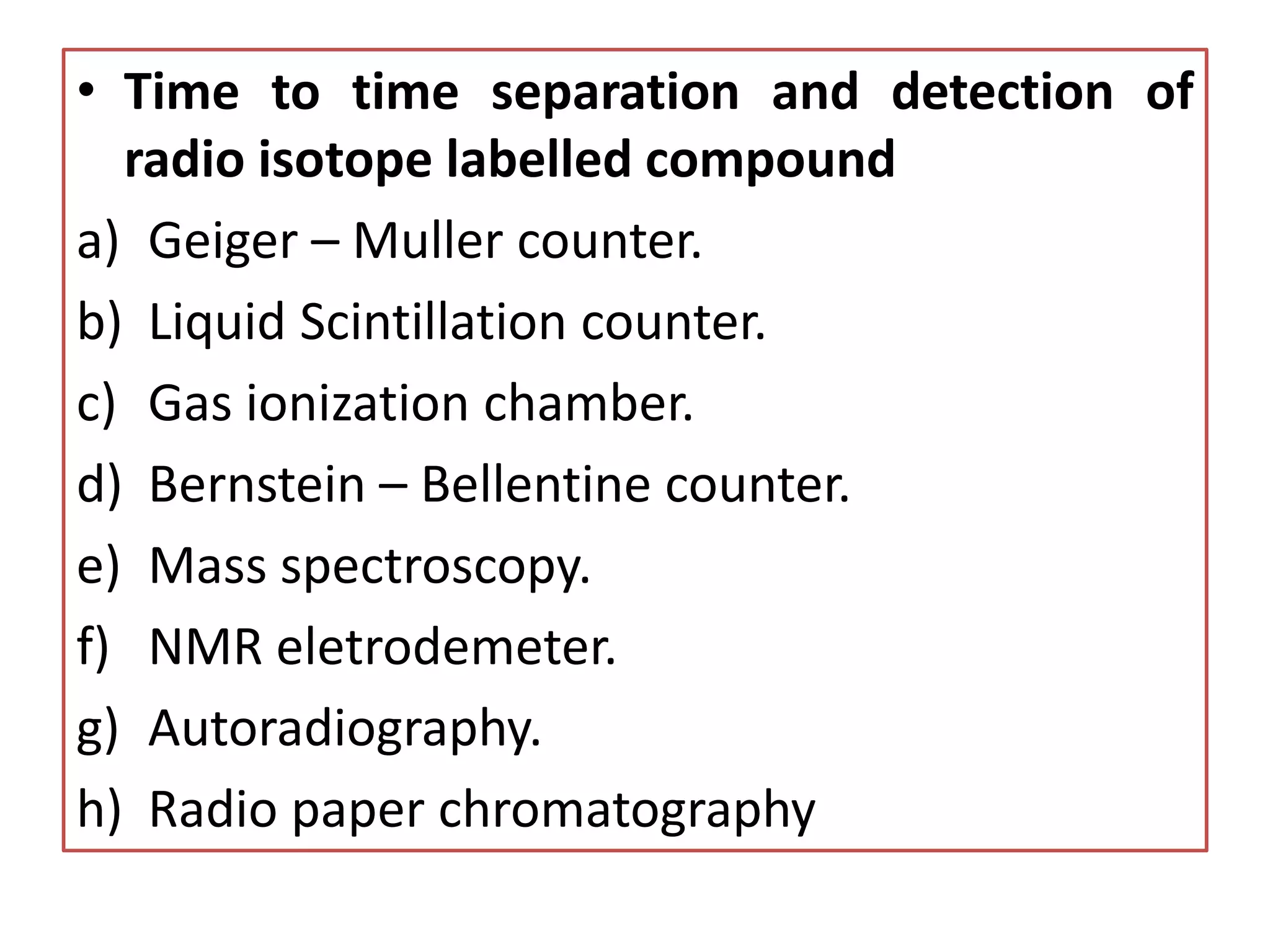
![STEPS IN TRACER TECHNIQUE
• SELECTION OF RADIOISOTOPES: Two criteria need to be
considered while selecting the radioisotopes i.e. half life and
reactivity [longer half life for example 14C radioisotope have longer
wavelength upto 6000 years and tritium roton half life is around 12
year and these isotopes does not effect the physical and chemical
properties]
• PREPARATION OF LABELLED COMPOUND
a) Growing chlorella in atmosphere of 14carbondioxide
b) Nuclear reactor/ accelarator
c) The 3H (tritium) labelled compound are commercially available.
Tritium labelling is effected by catalytic exchange in aqueous
media by hydrogenation of unsaturated compound with tritium
gas. Tritium is pure β – emitter of low intensity & its radiation
energy is lower than 14C
d) Organic synthesis](https://image.slidesharecdn.com/biosynthesispathwayandradiotracingtechnique-220426145803/75/Biosynthesis-Pathway-And-Radio-Tracing-Technique-pptx-15-2048.jpg)
![• Introduction of radiolabelled compoud[specific
site and correct time]
1. Root feeding- plants are grown under
hydroponic solution ex- tabacco and datura
alkaloids
2. Stem feeding- cut end of stem immersed in
water, nutrient and labelled compound
3. Direct injection- suitable hollow stem, capsular
fruit[opium]
4. Infiltration
5. Floating method
6. Spraying technique – sprayed over leaves and
get absorbed- steroids[non aqueous solution]](https://image.slidesharecdn.com/biosynthesispathwayandradiotracingtechnique-220426145803/75/Biosynthesis-Pathway-And-Radio-Tracing-Technique-pptx-16-2048.jpg)