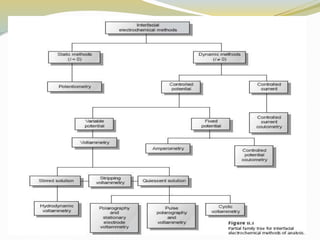
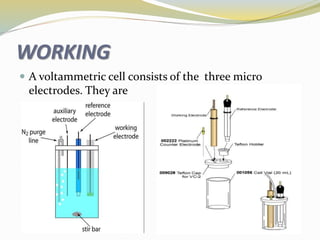
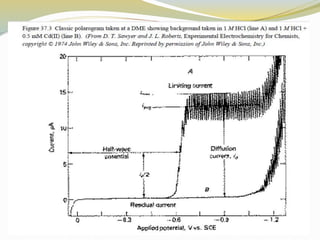
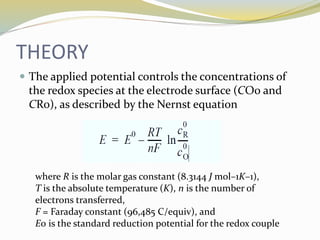
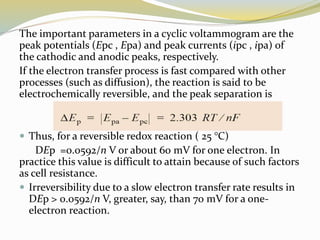
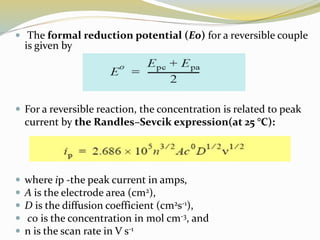
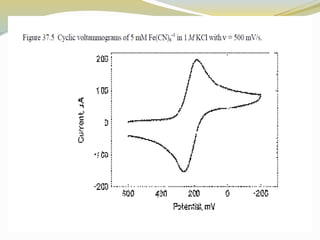
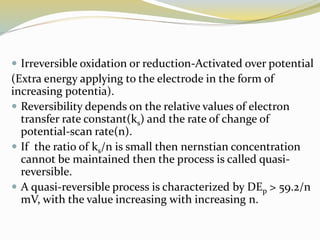
Voltammetry involves applying a potential to a working electrode and measuring the resulting current. It can characterize redox reactions through parameters like peak potentials and currents in cyclic voltammetry. Cyclic voltammetry cycles the potential of a working electrode versus a reference electrode and measures the current. It is used to study redox processes and obtain information about reaction kinetics and mechanisms. The peak separation and shapes of cyclic voltammograms provide information about whether redox processes are reversible or irreversible.