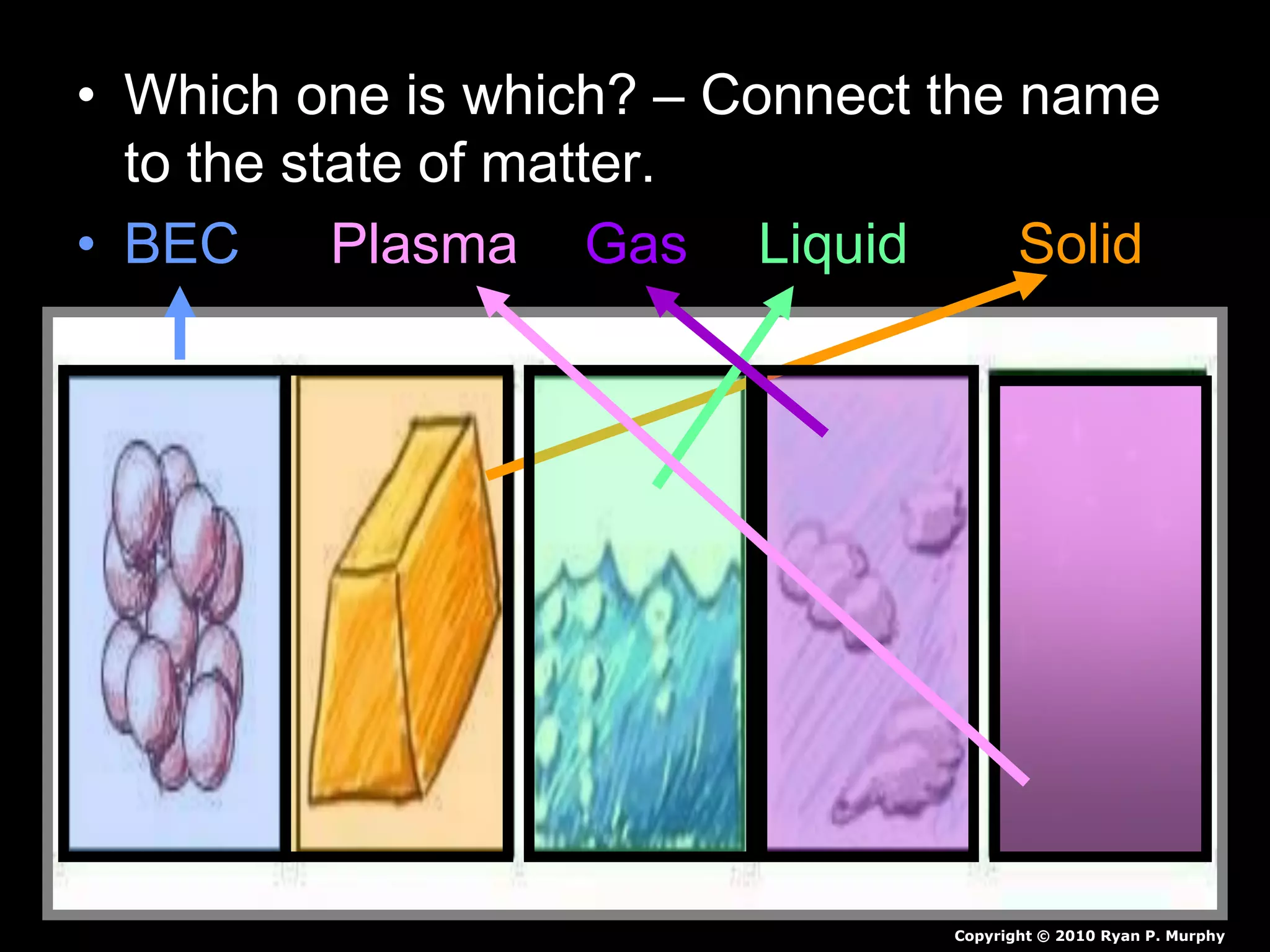
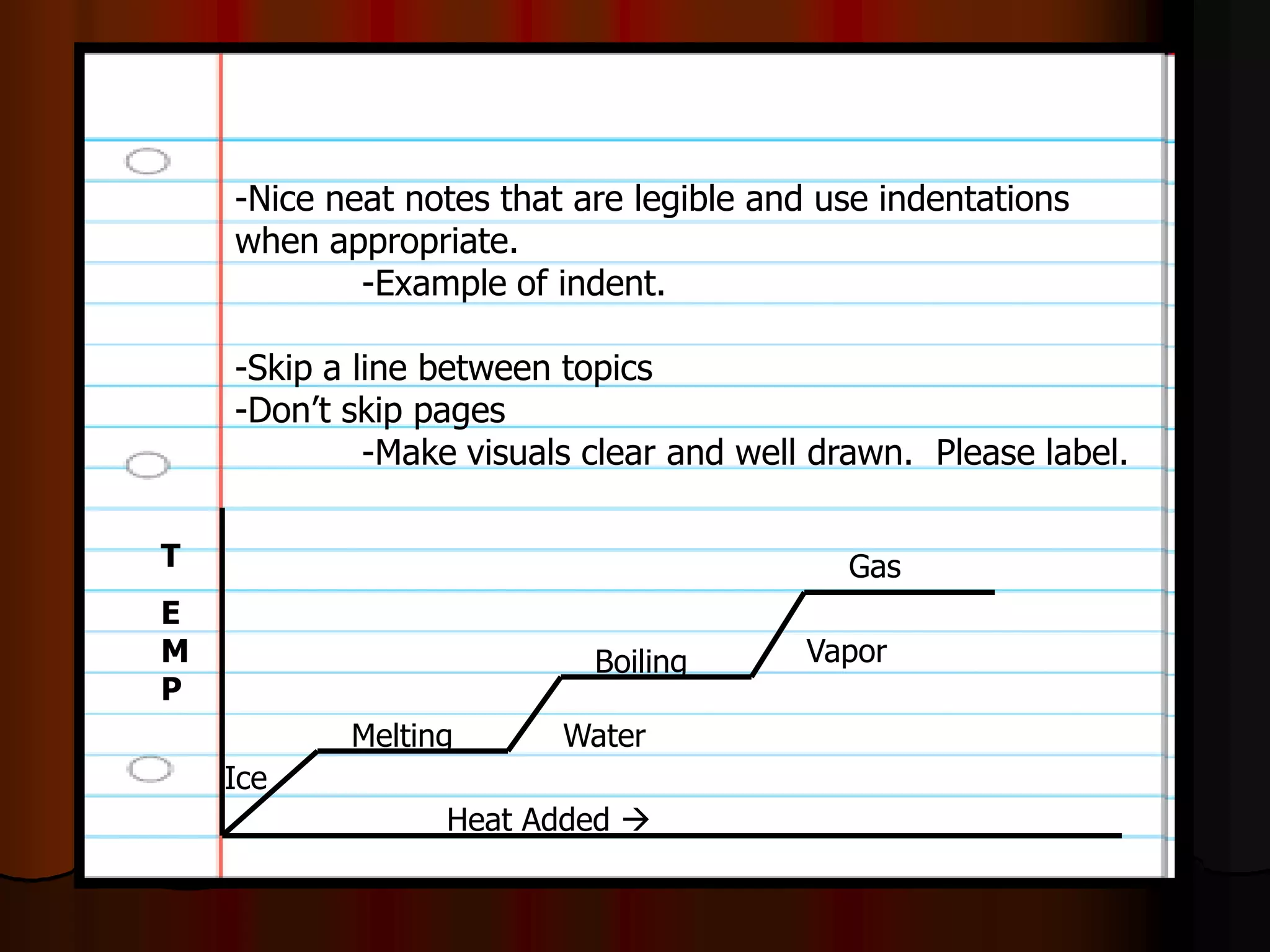
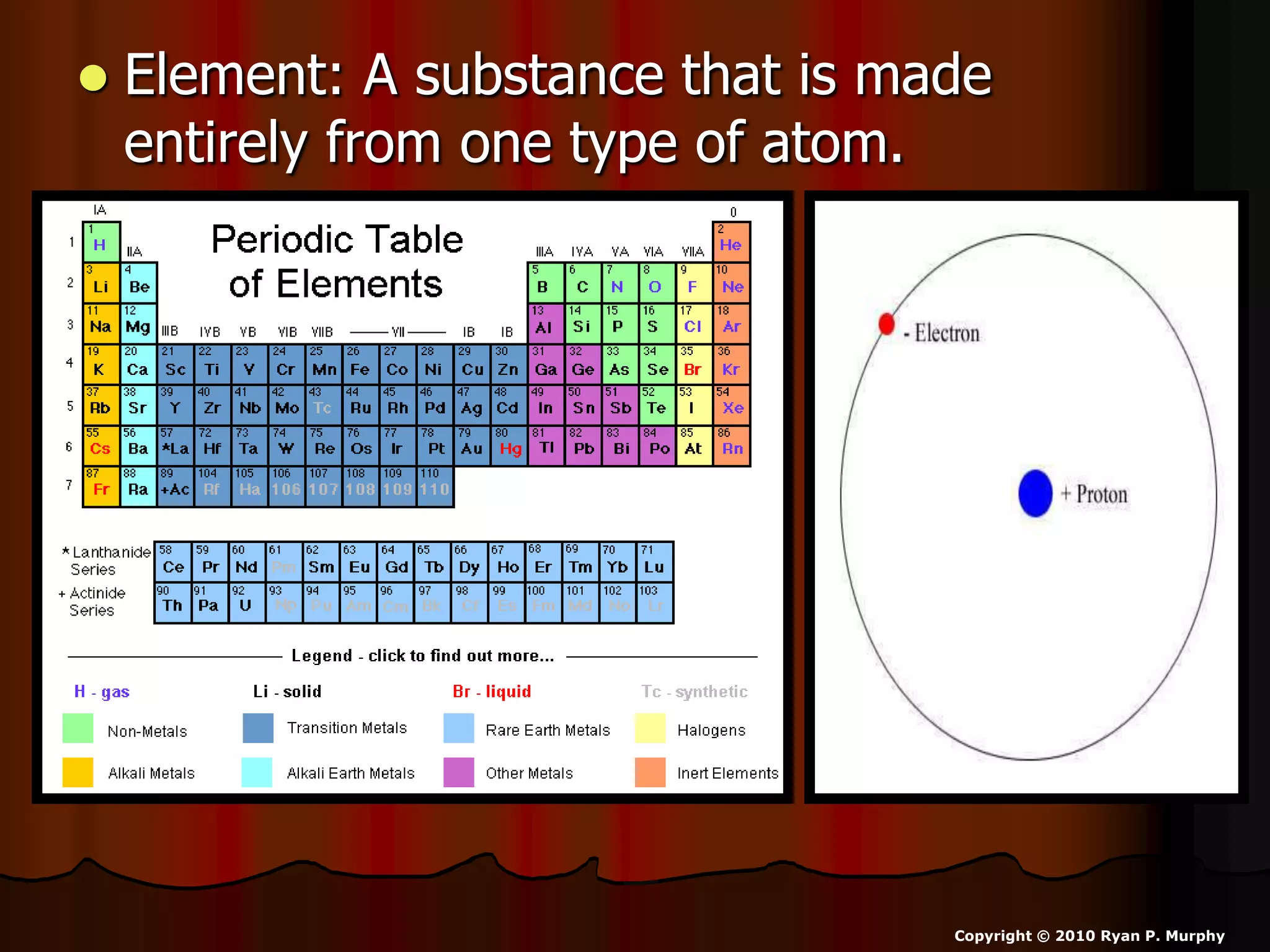
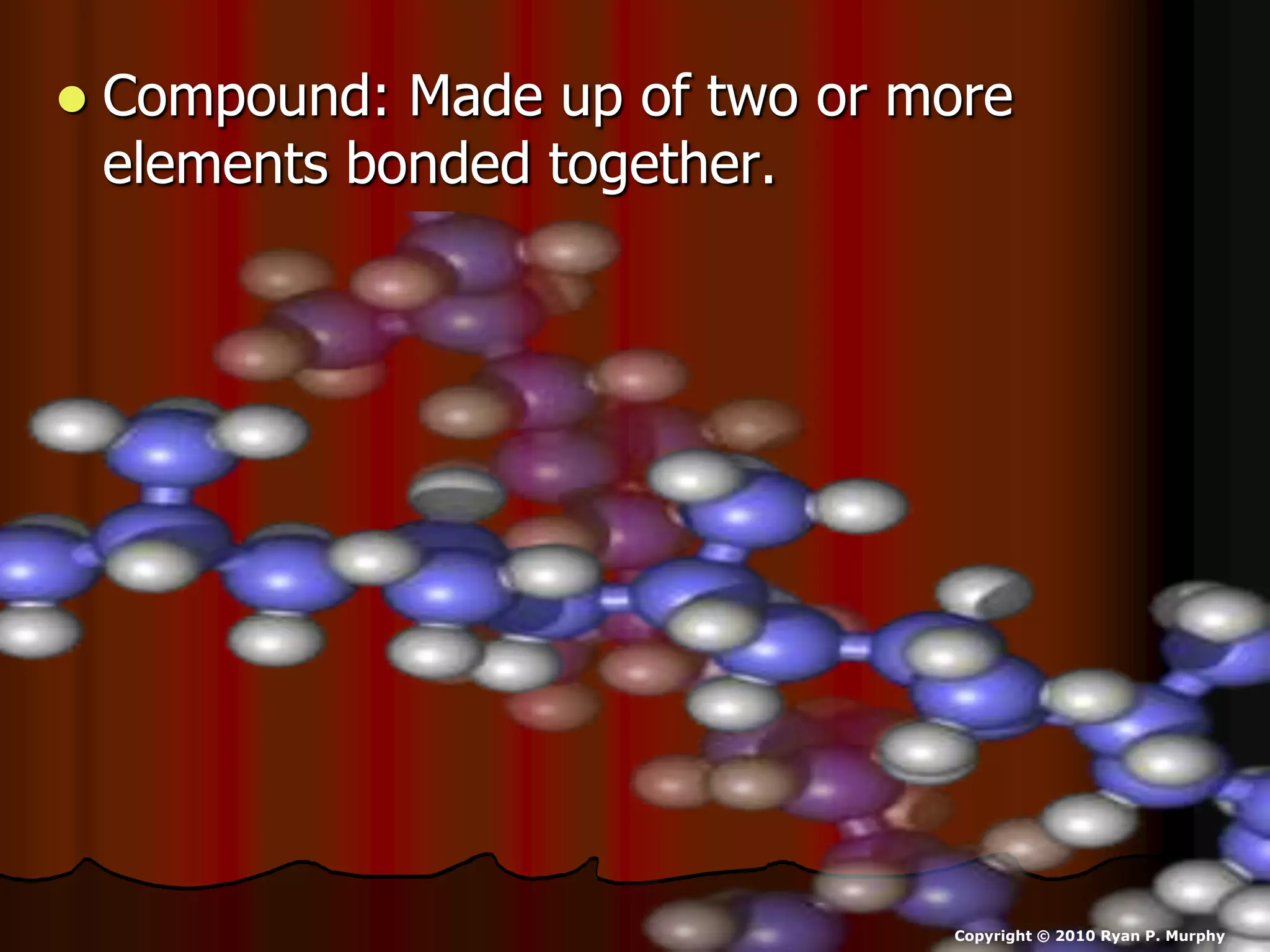
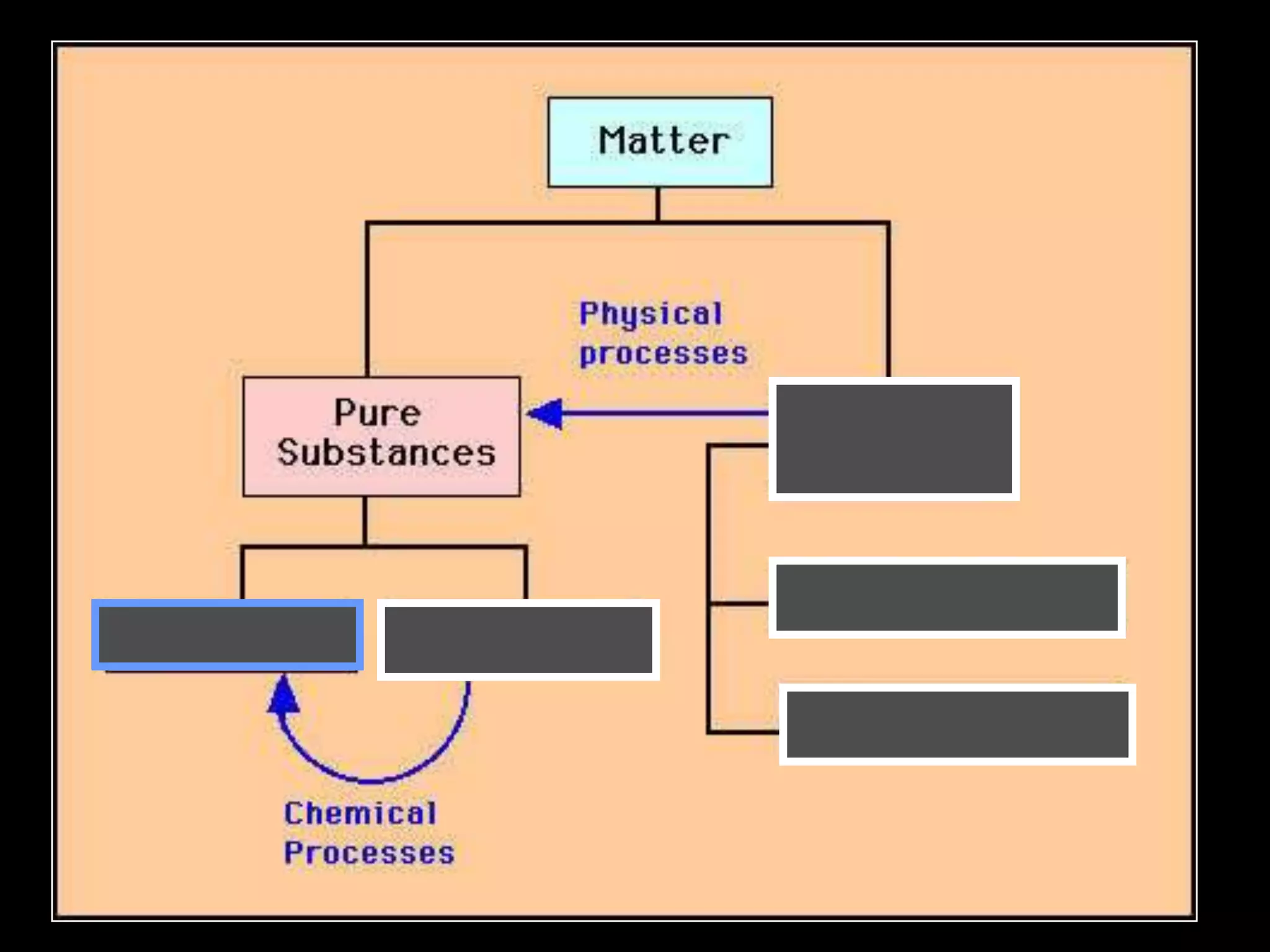
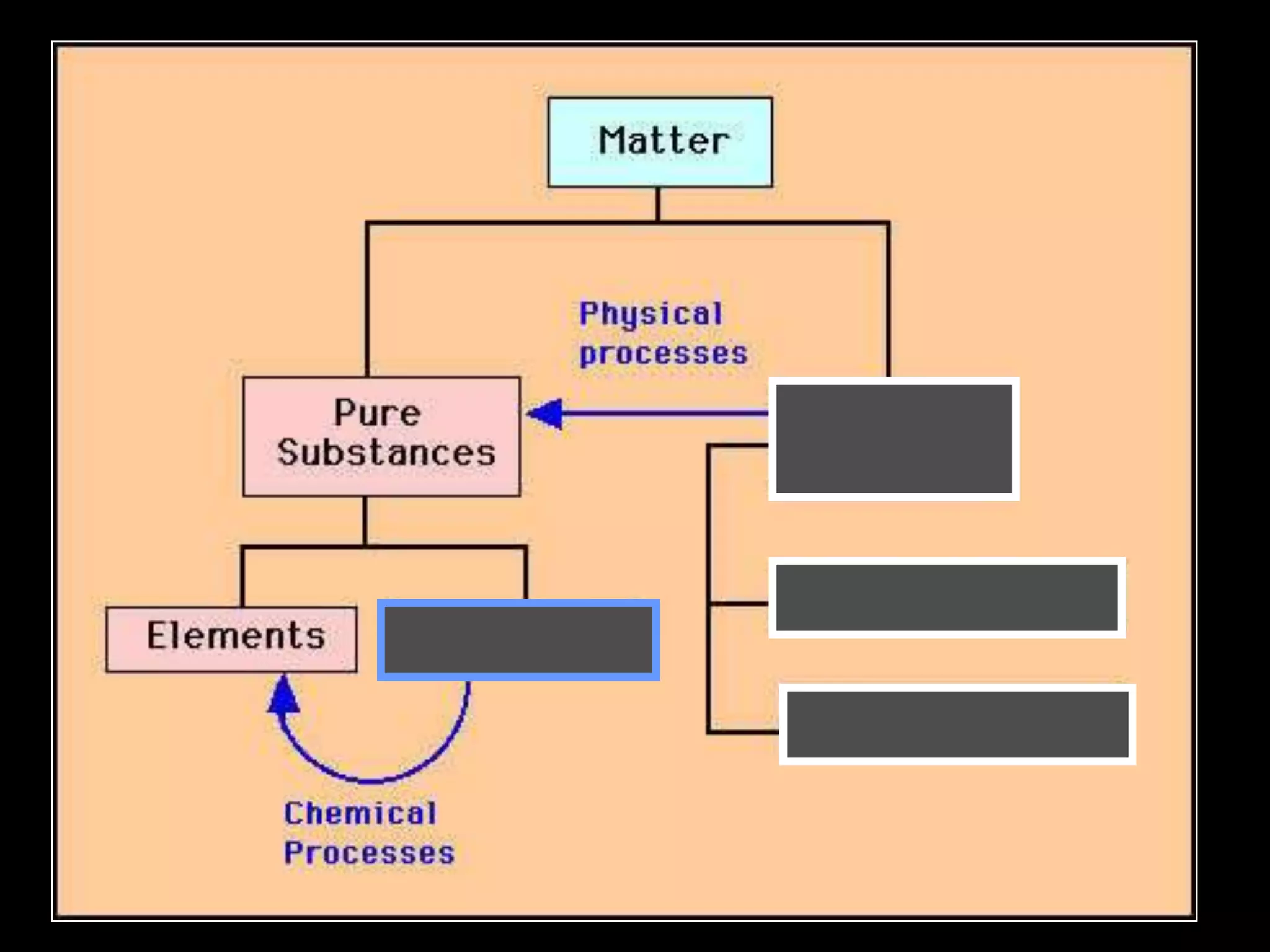
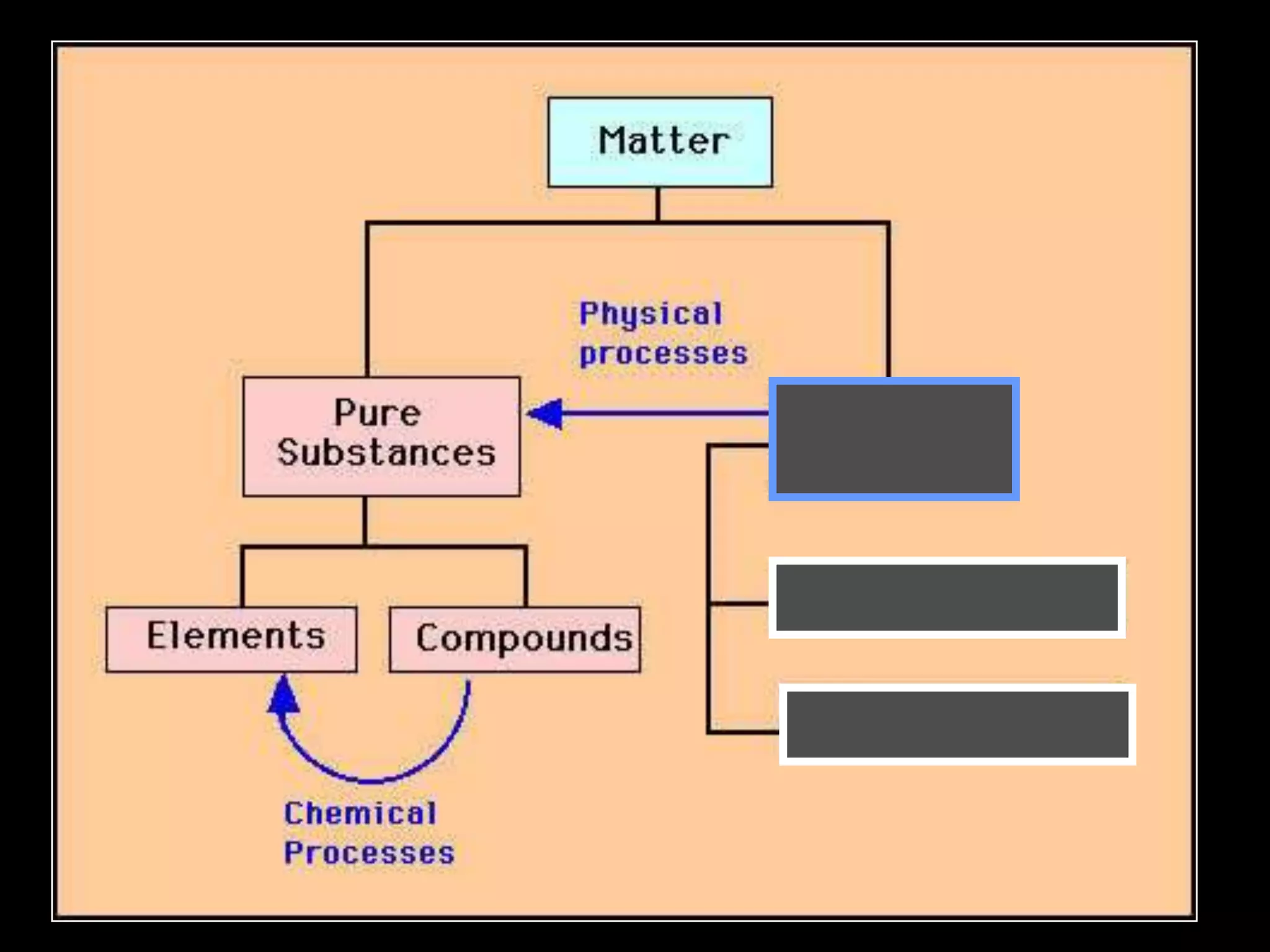
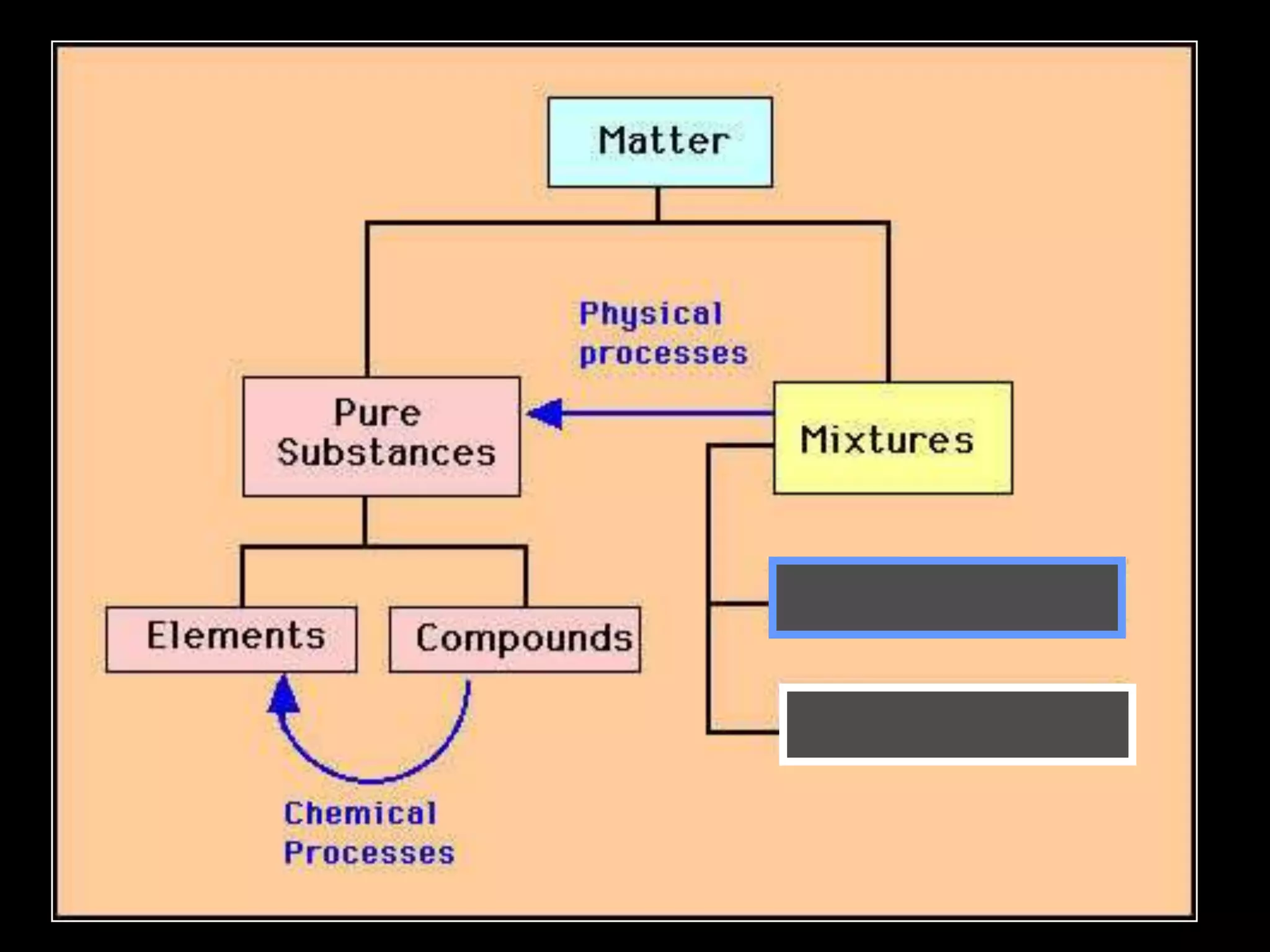
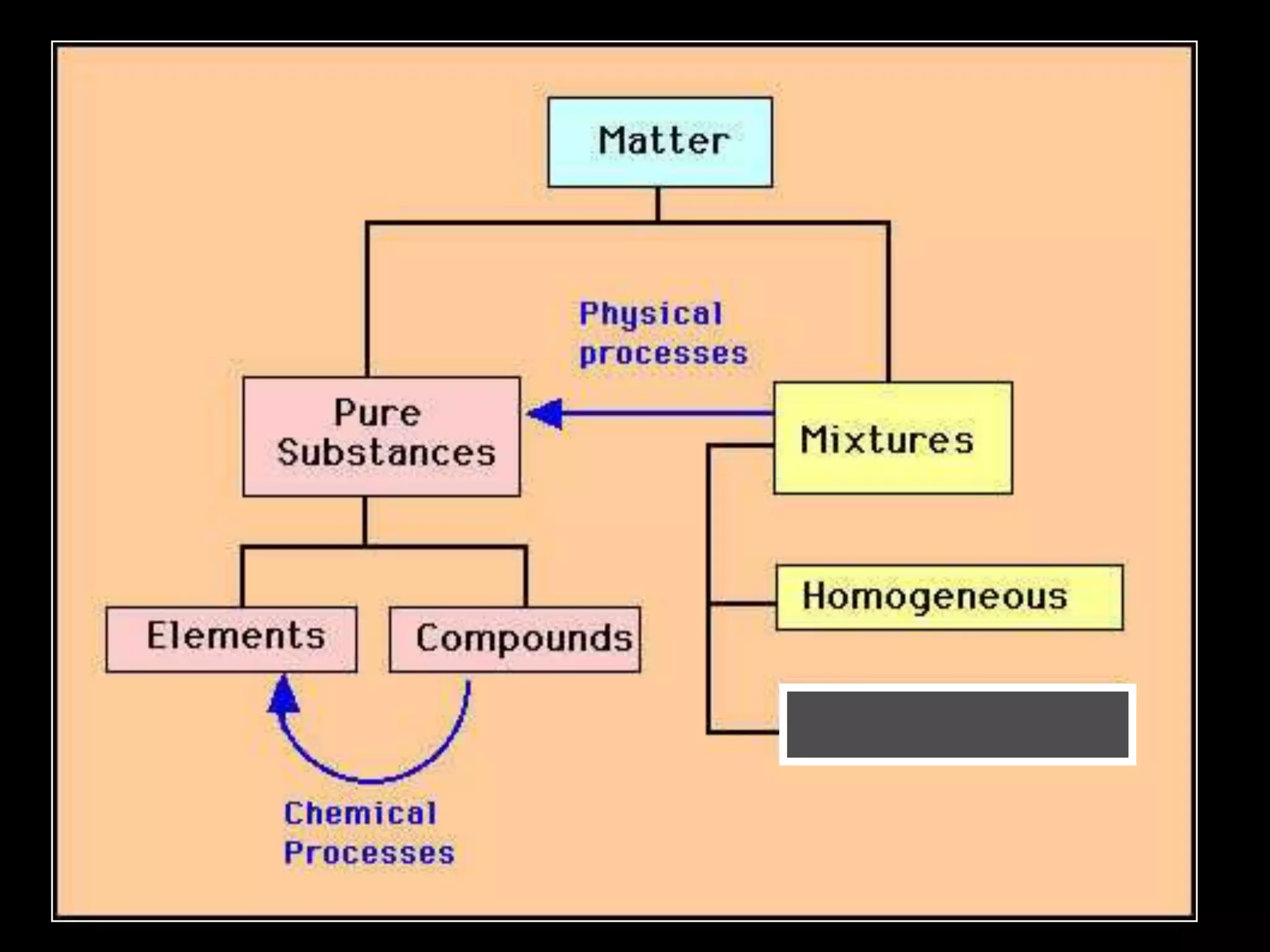
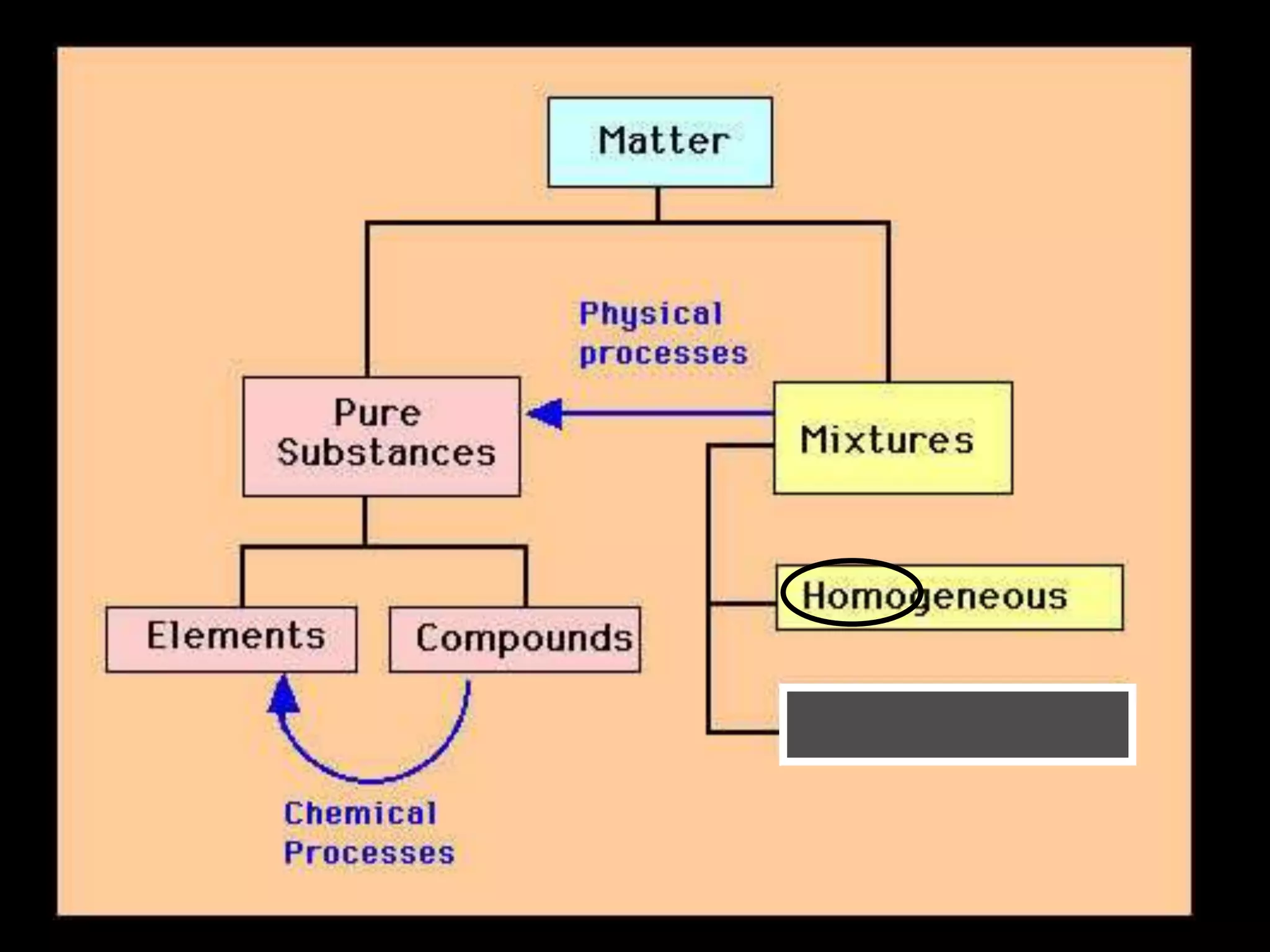
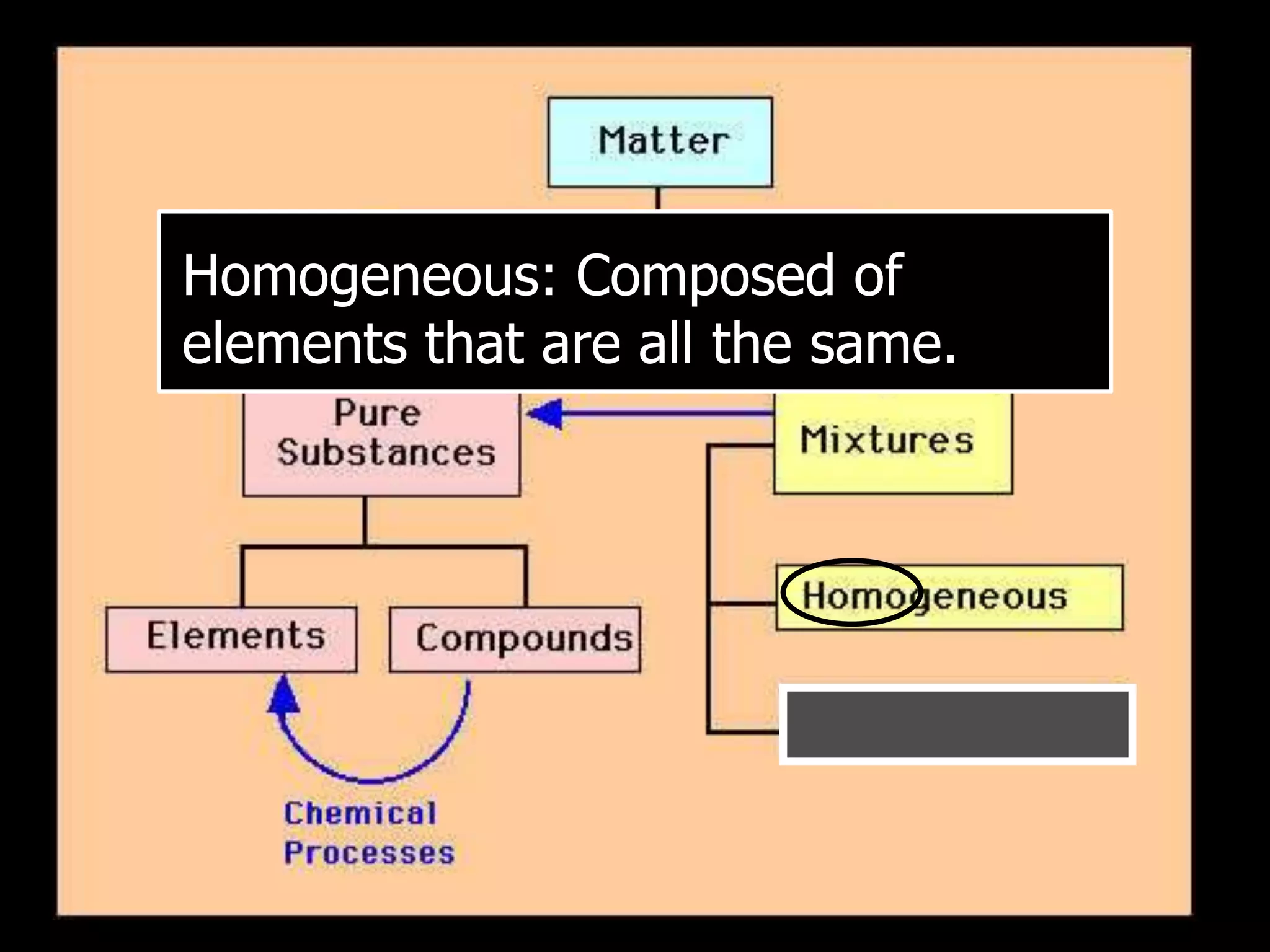
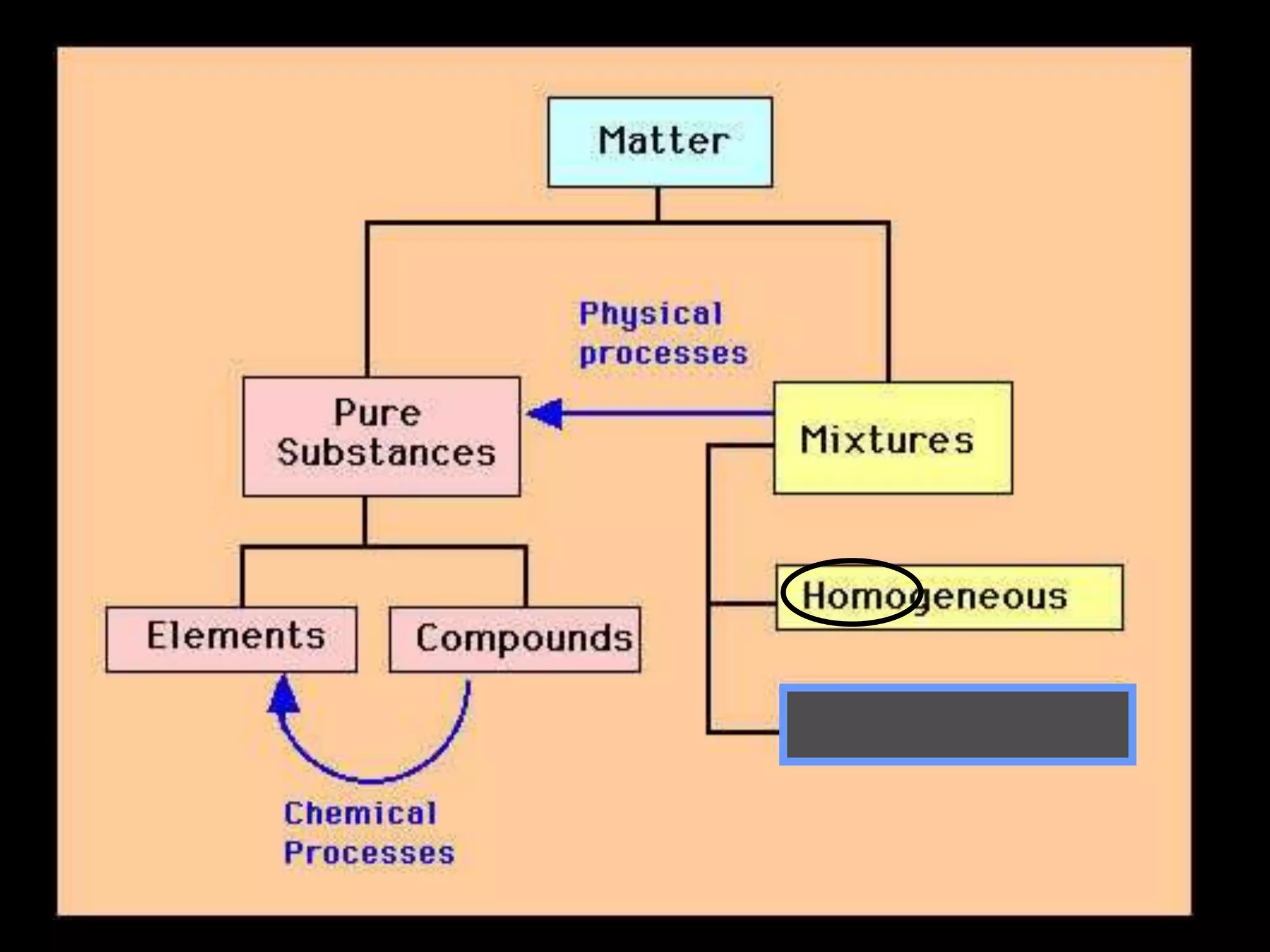
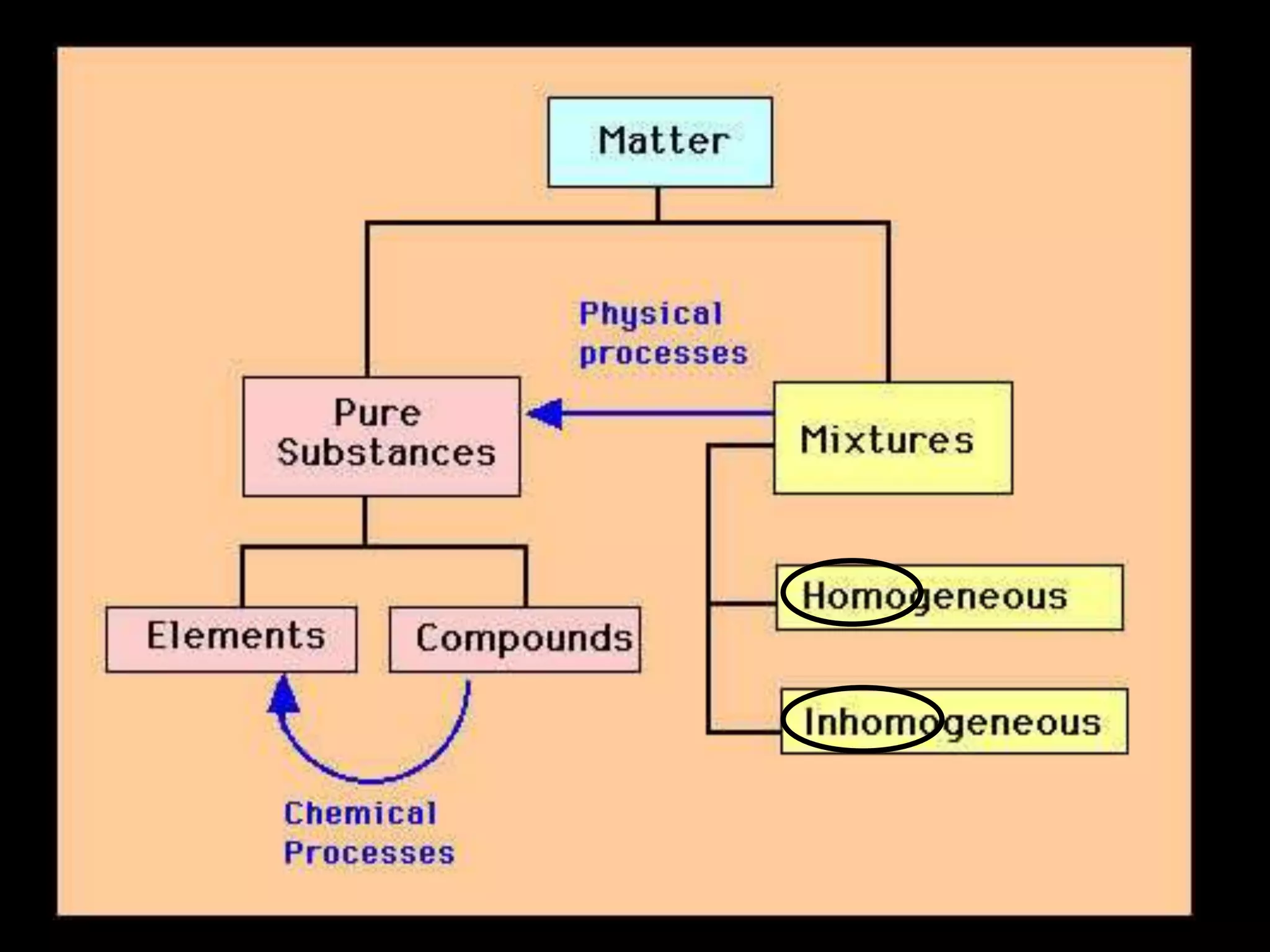
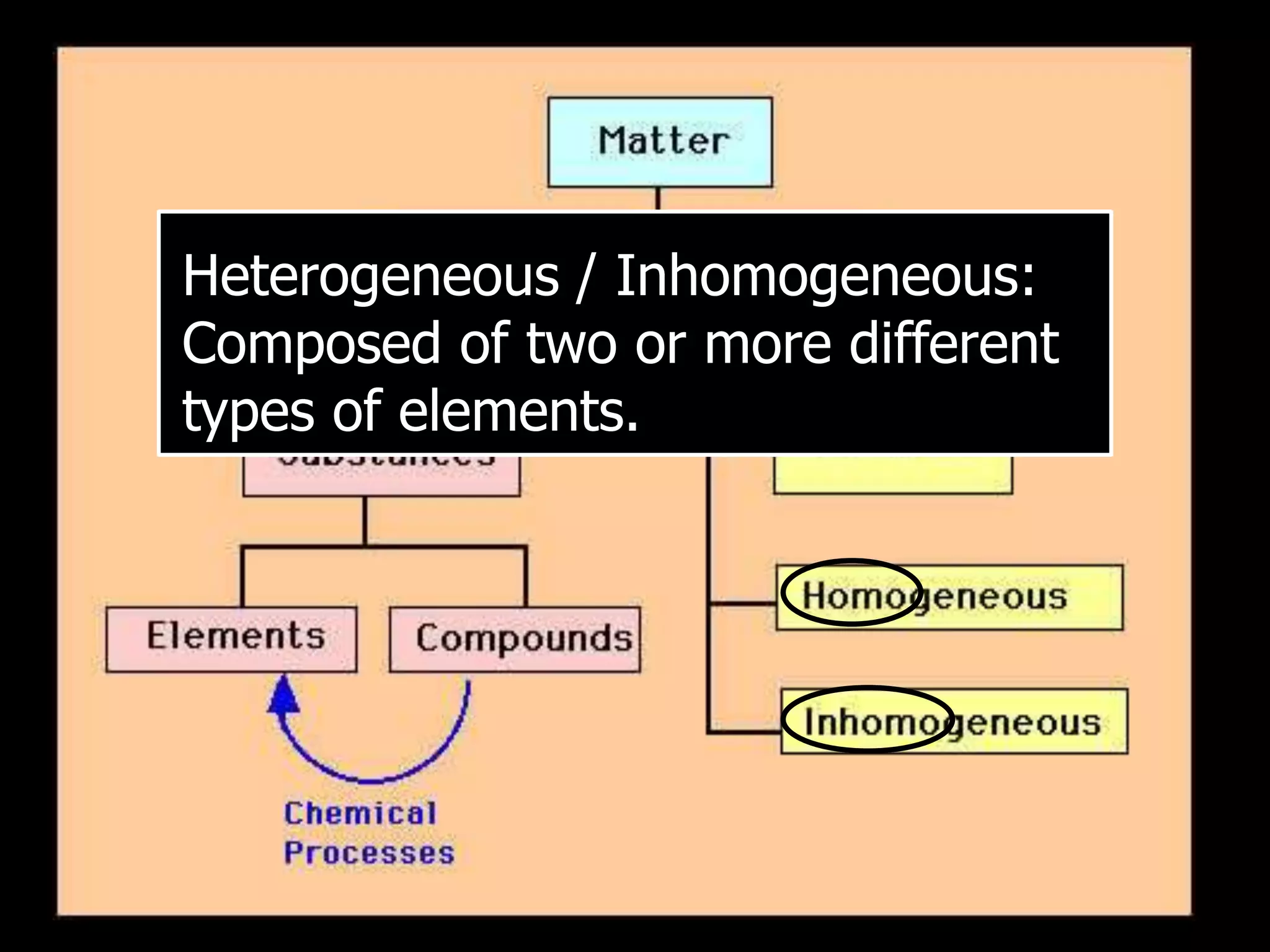
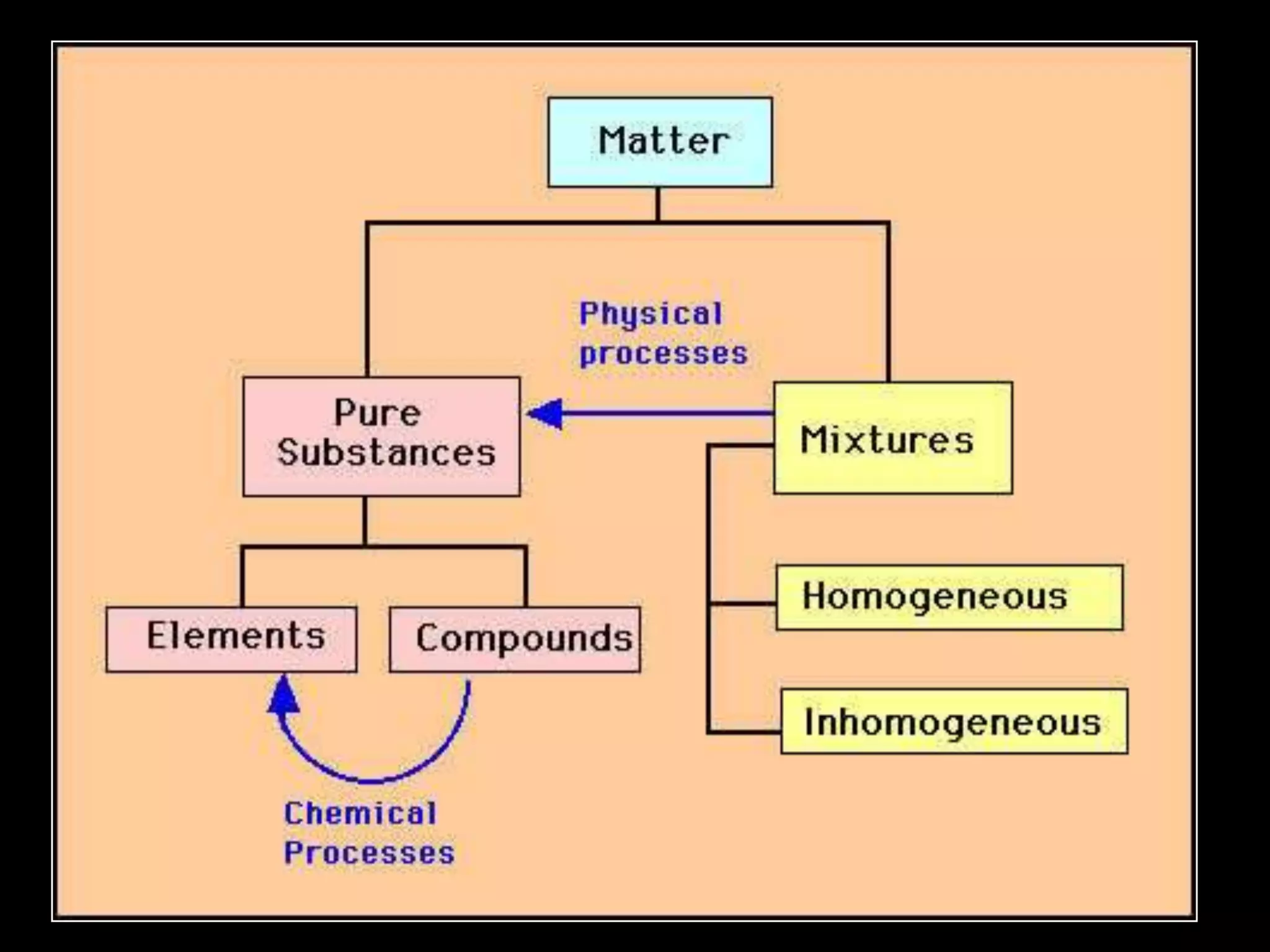
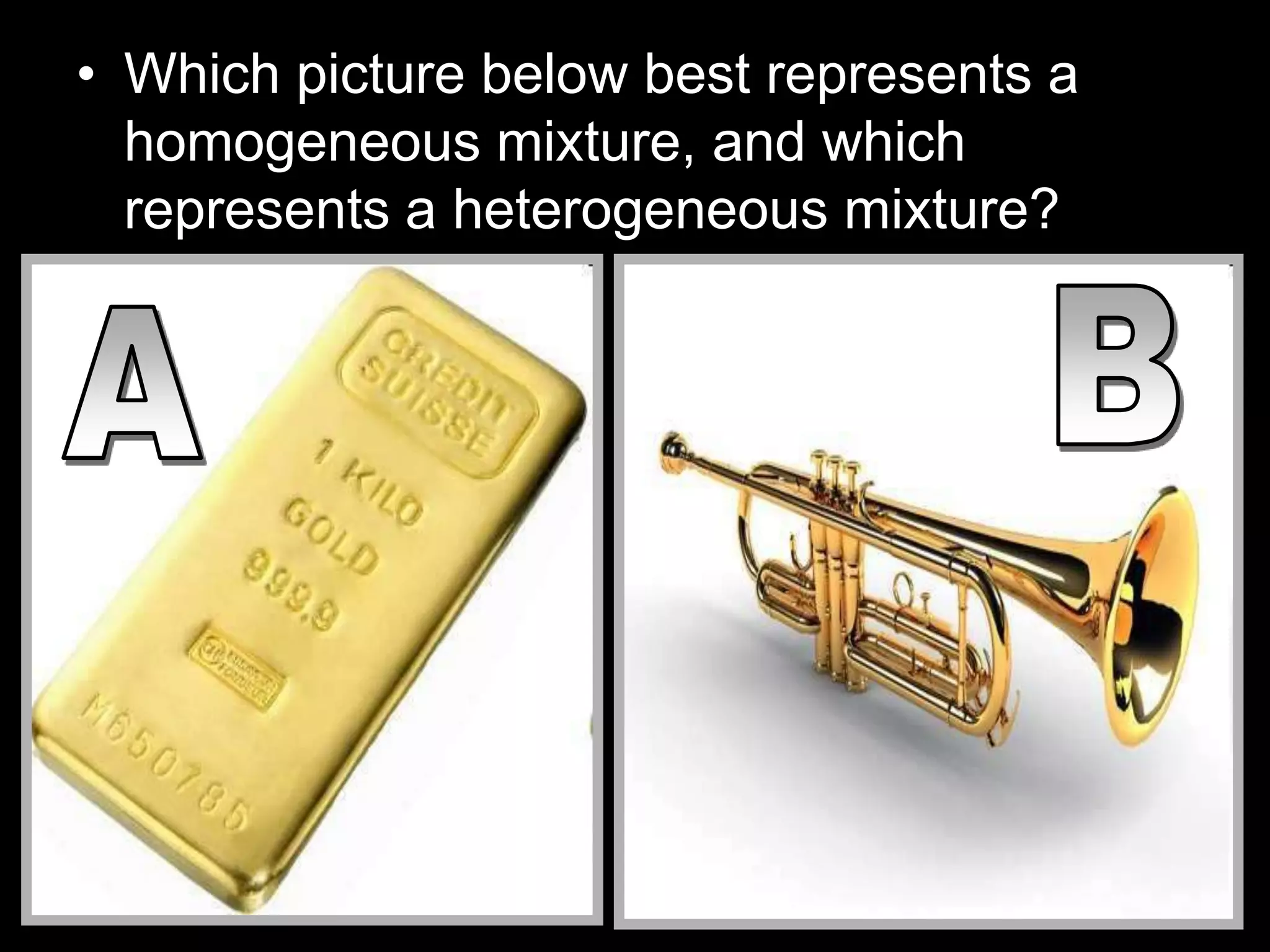
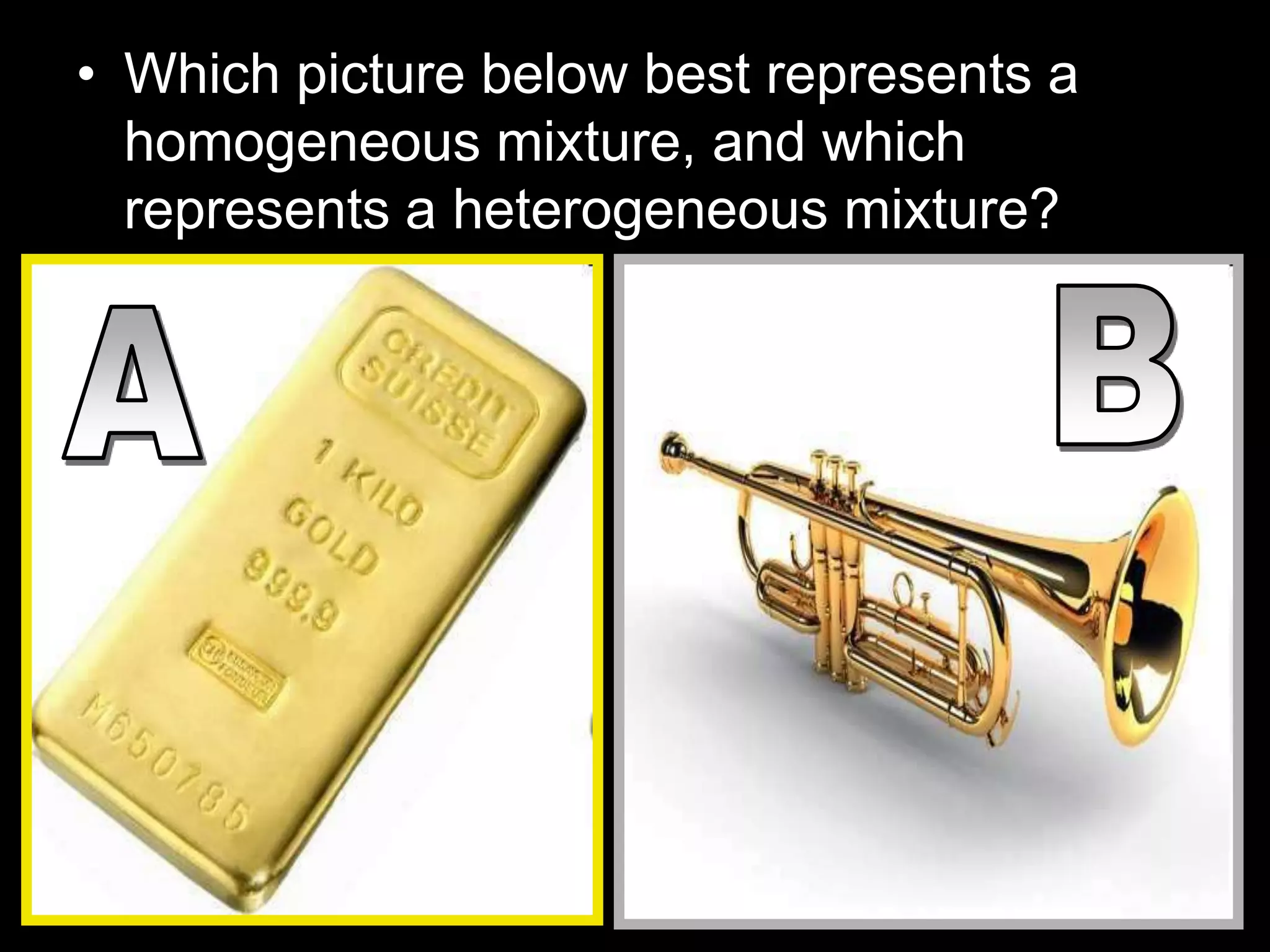
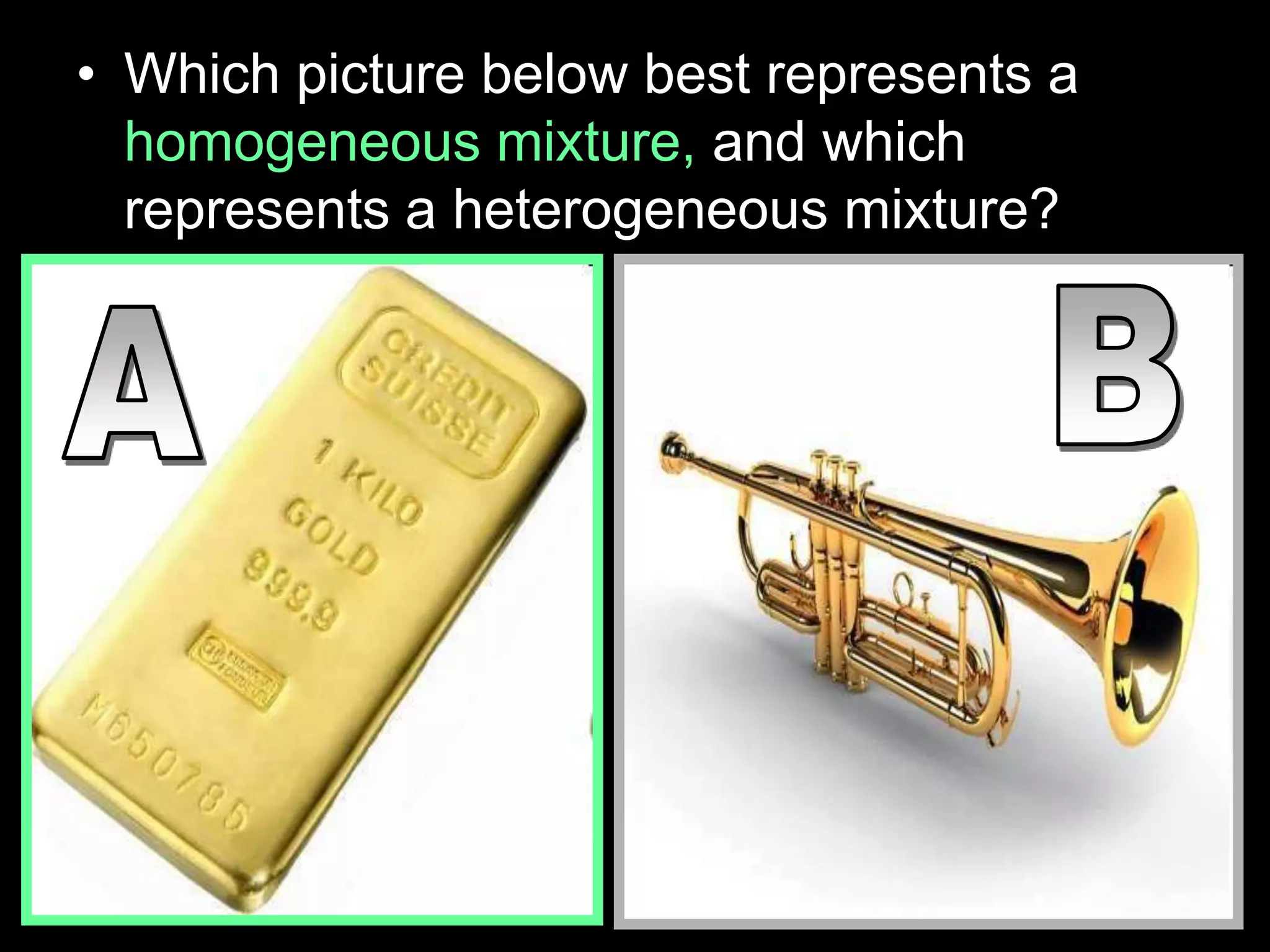
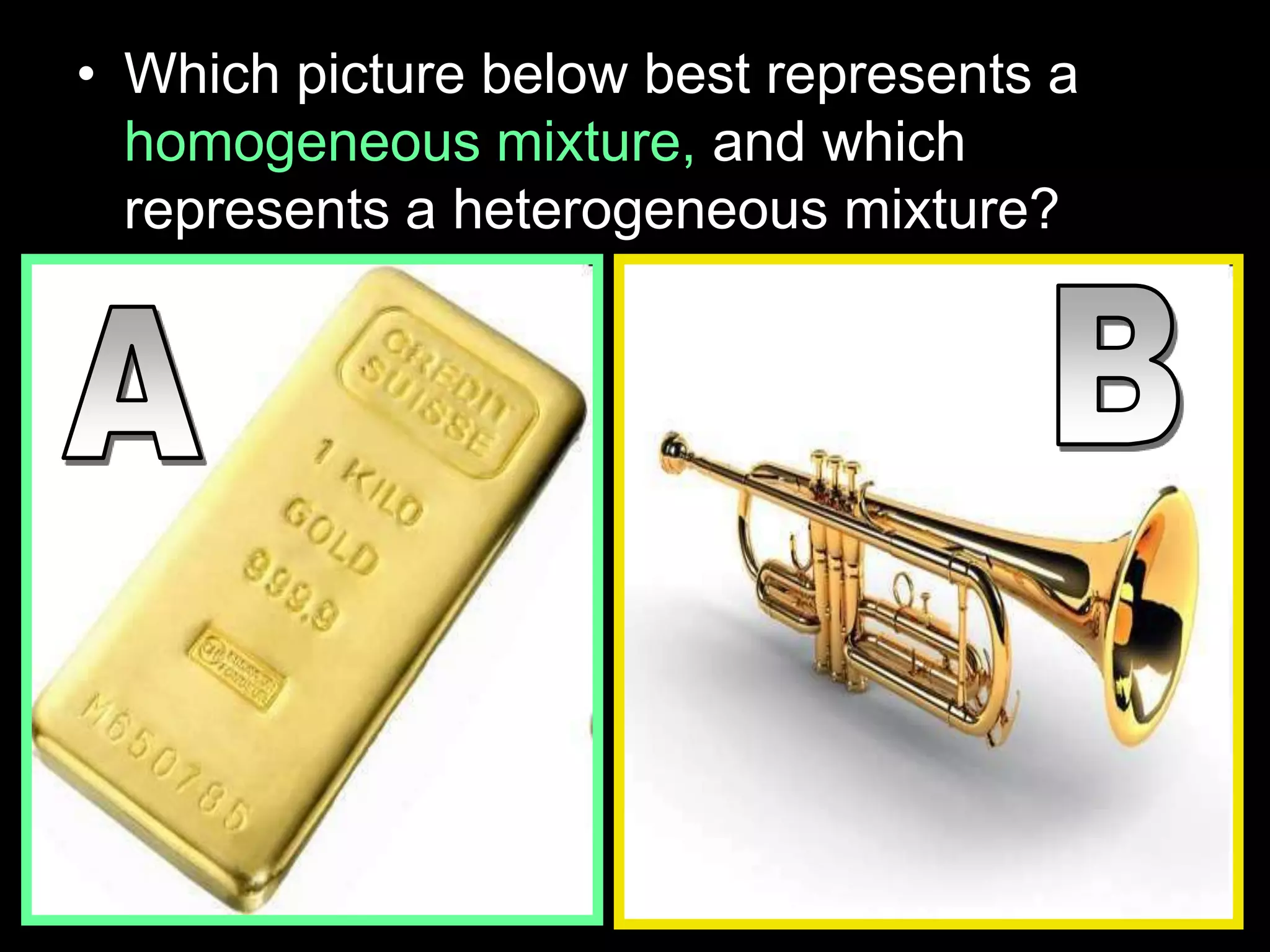
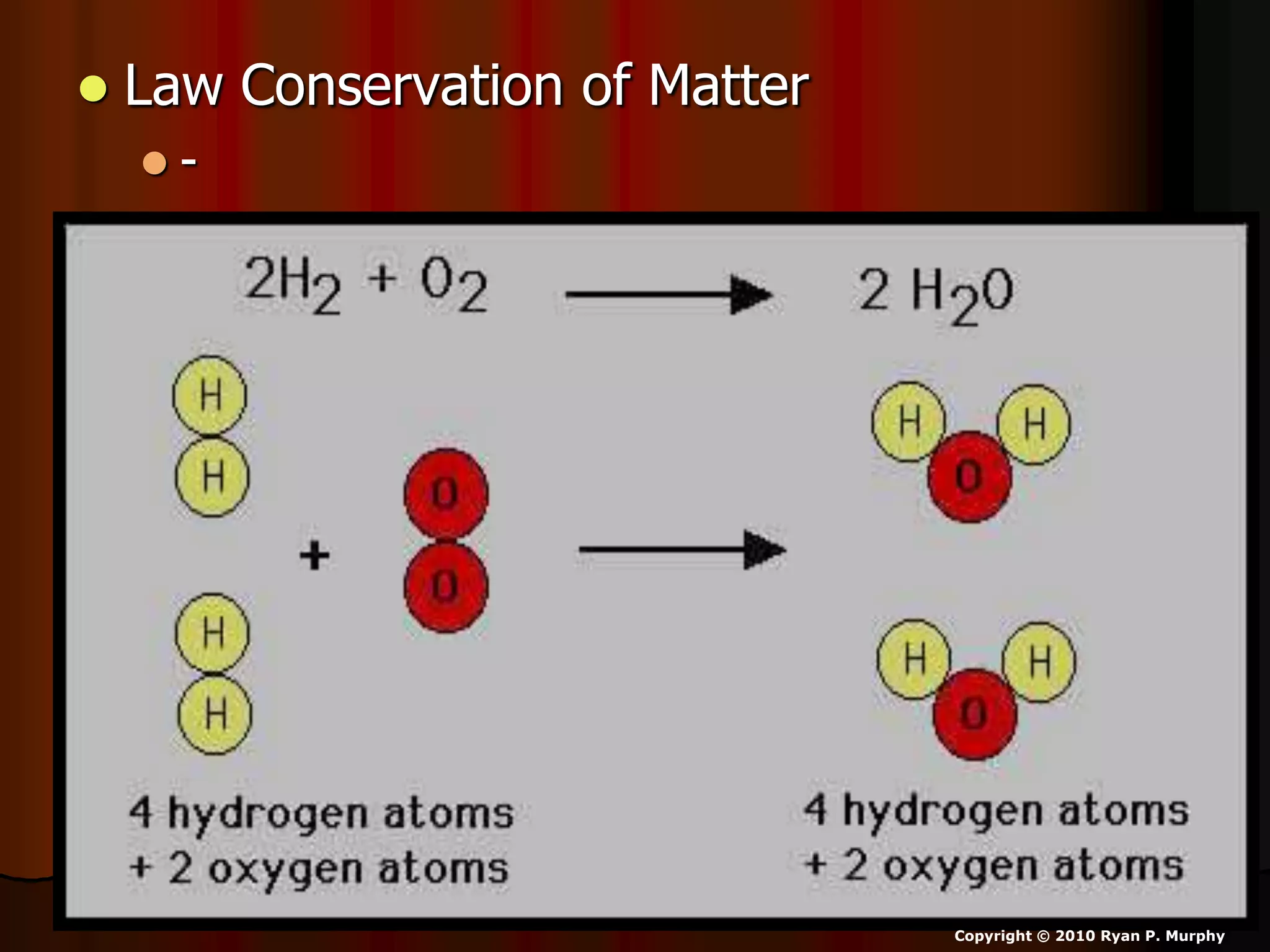
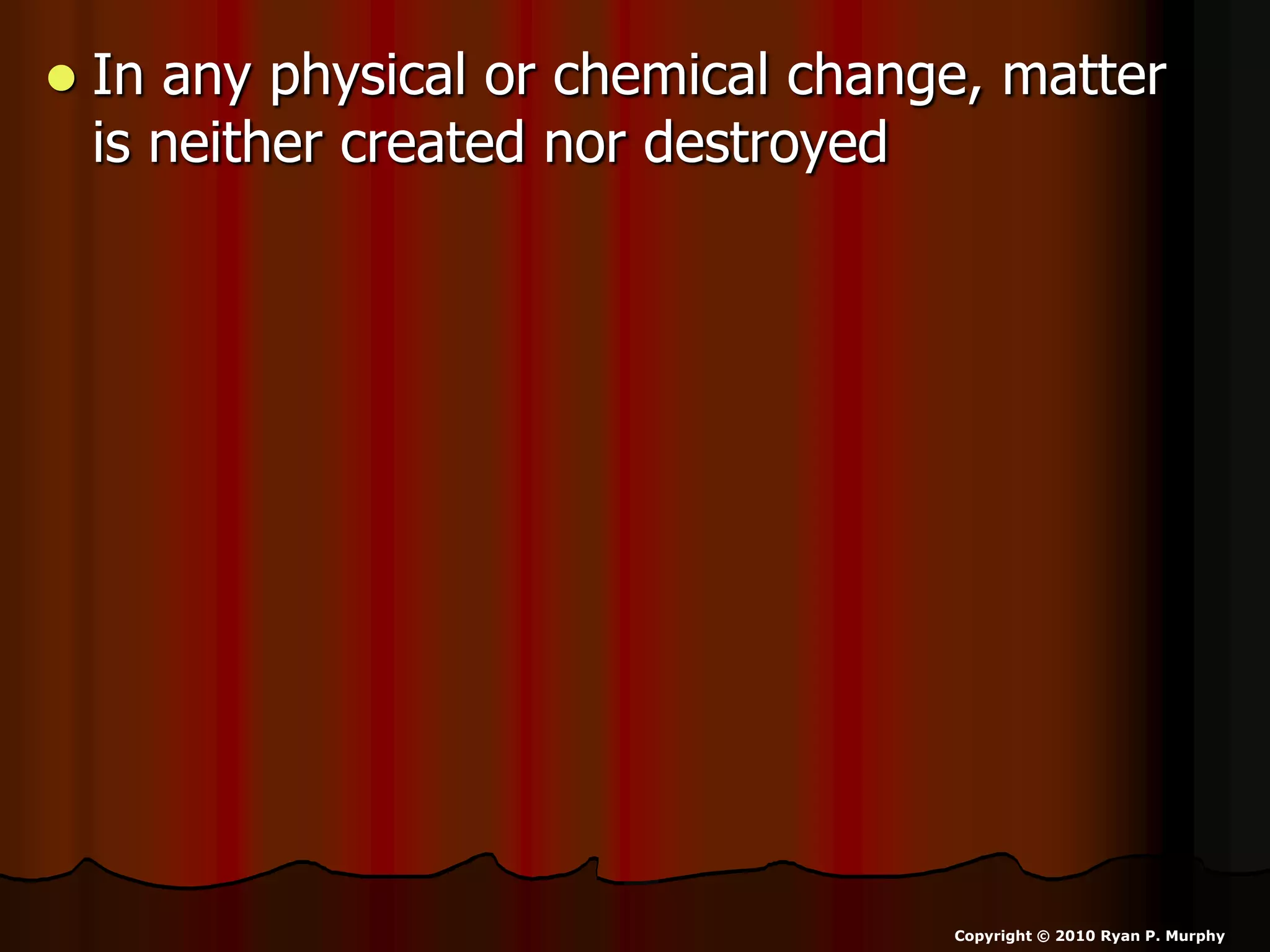
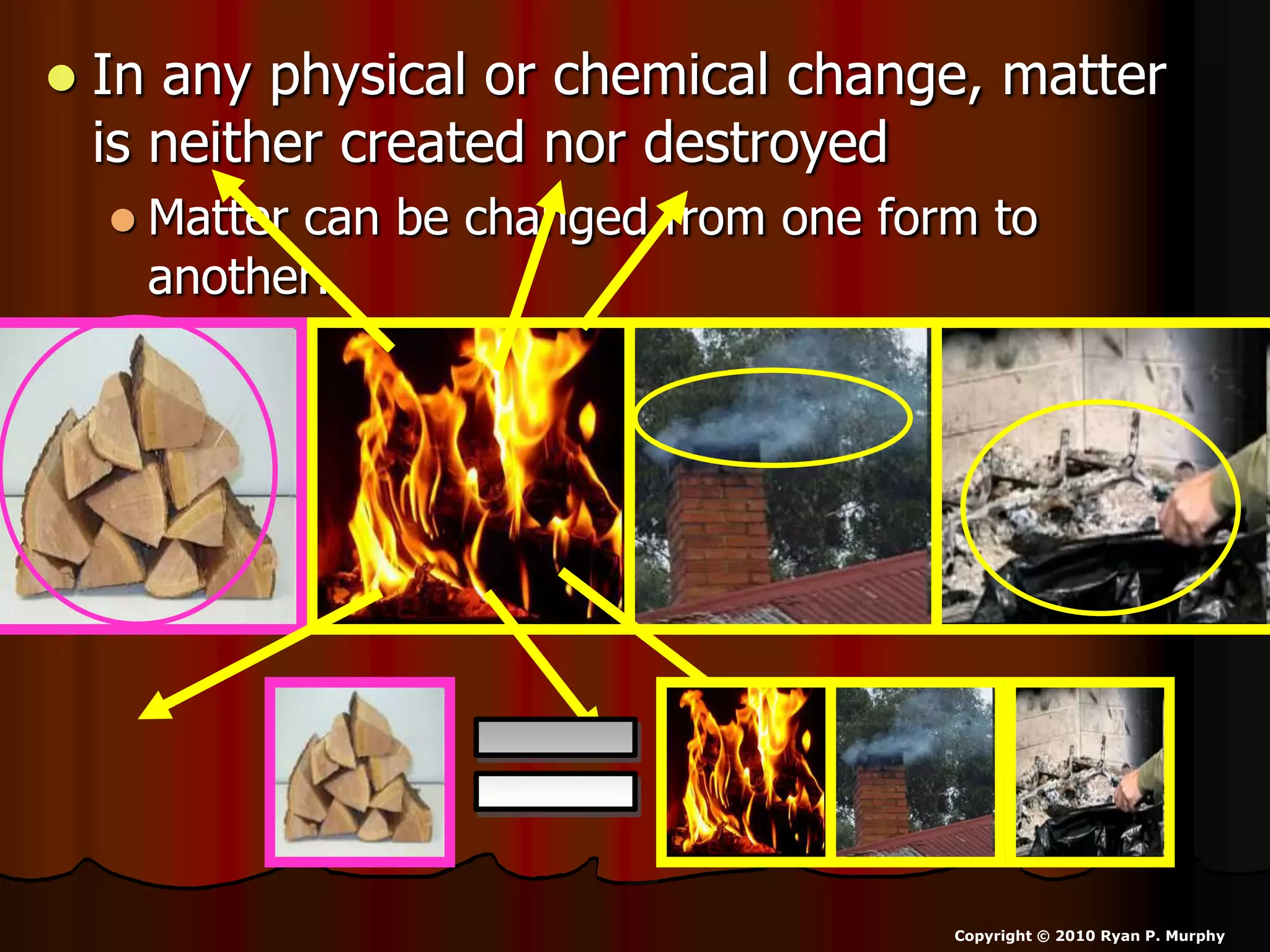
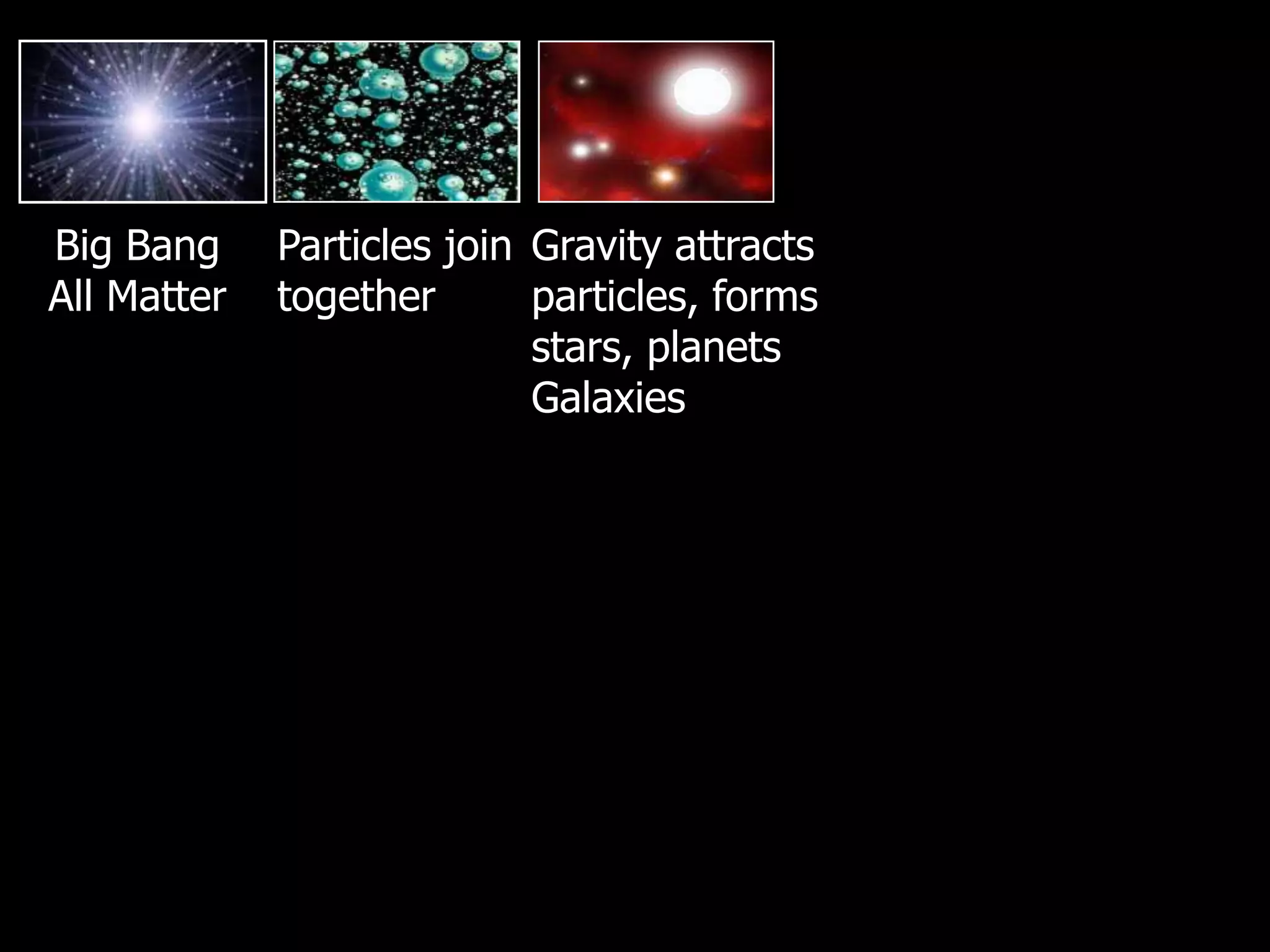
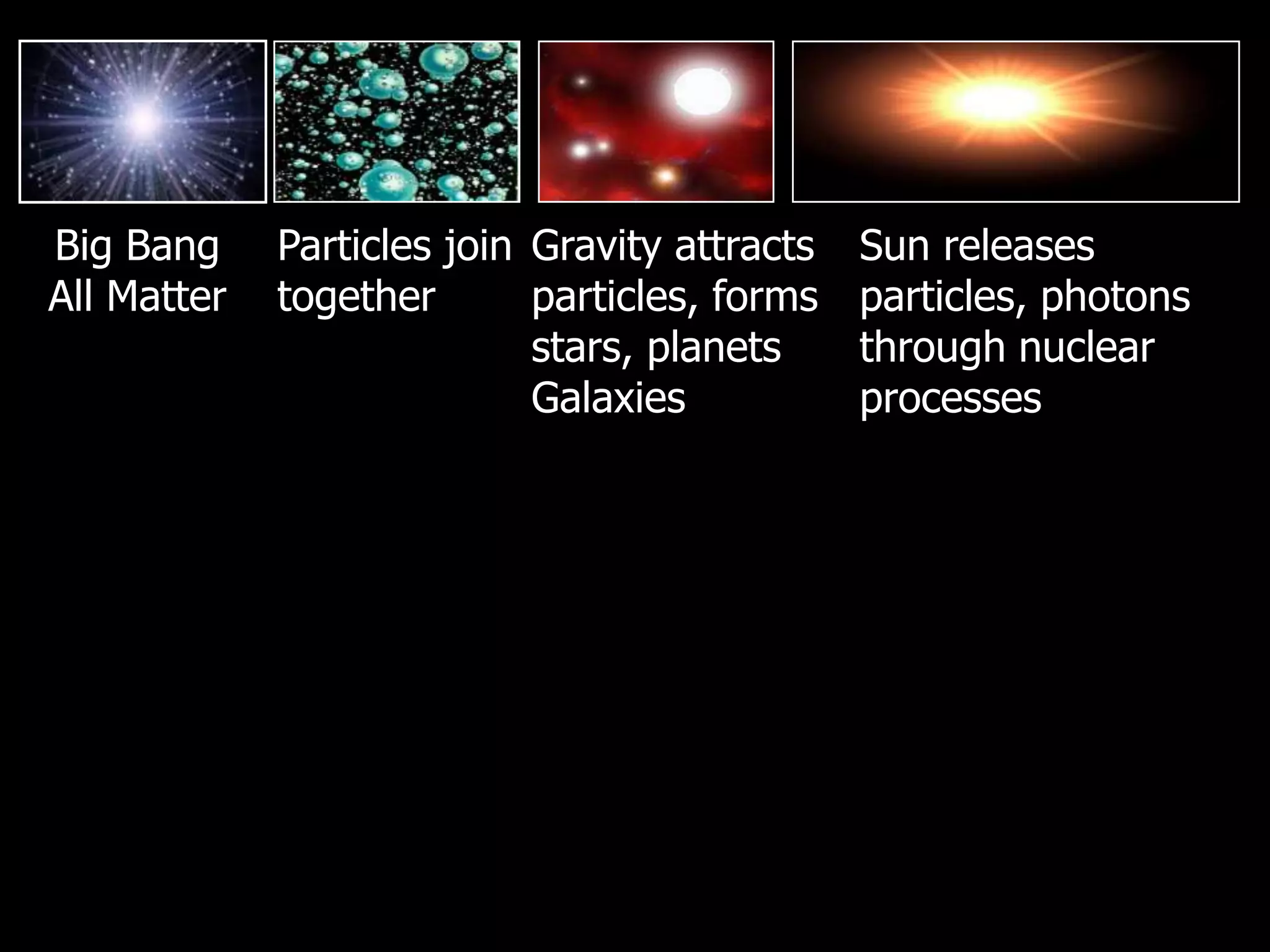
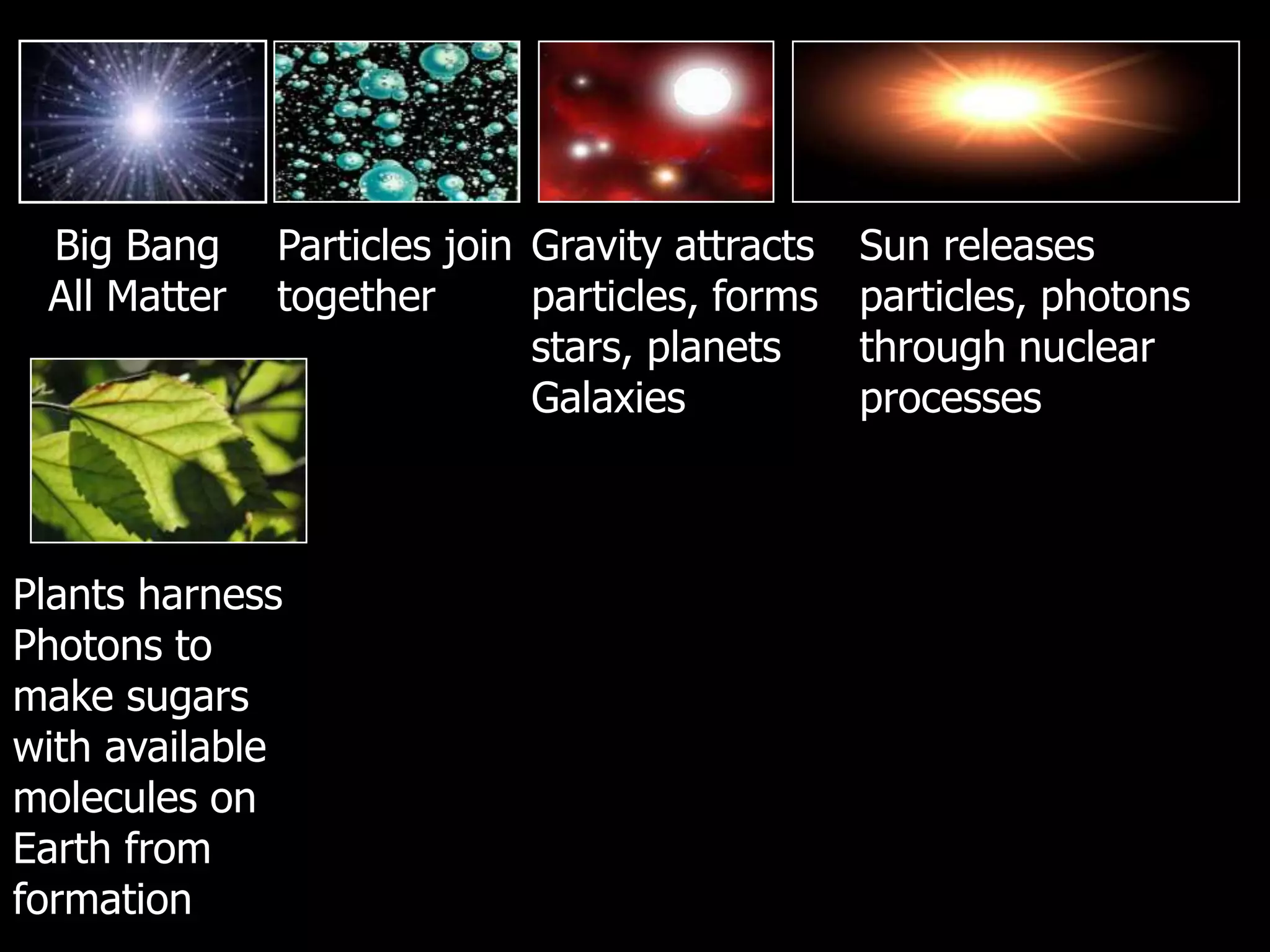
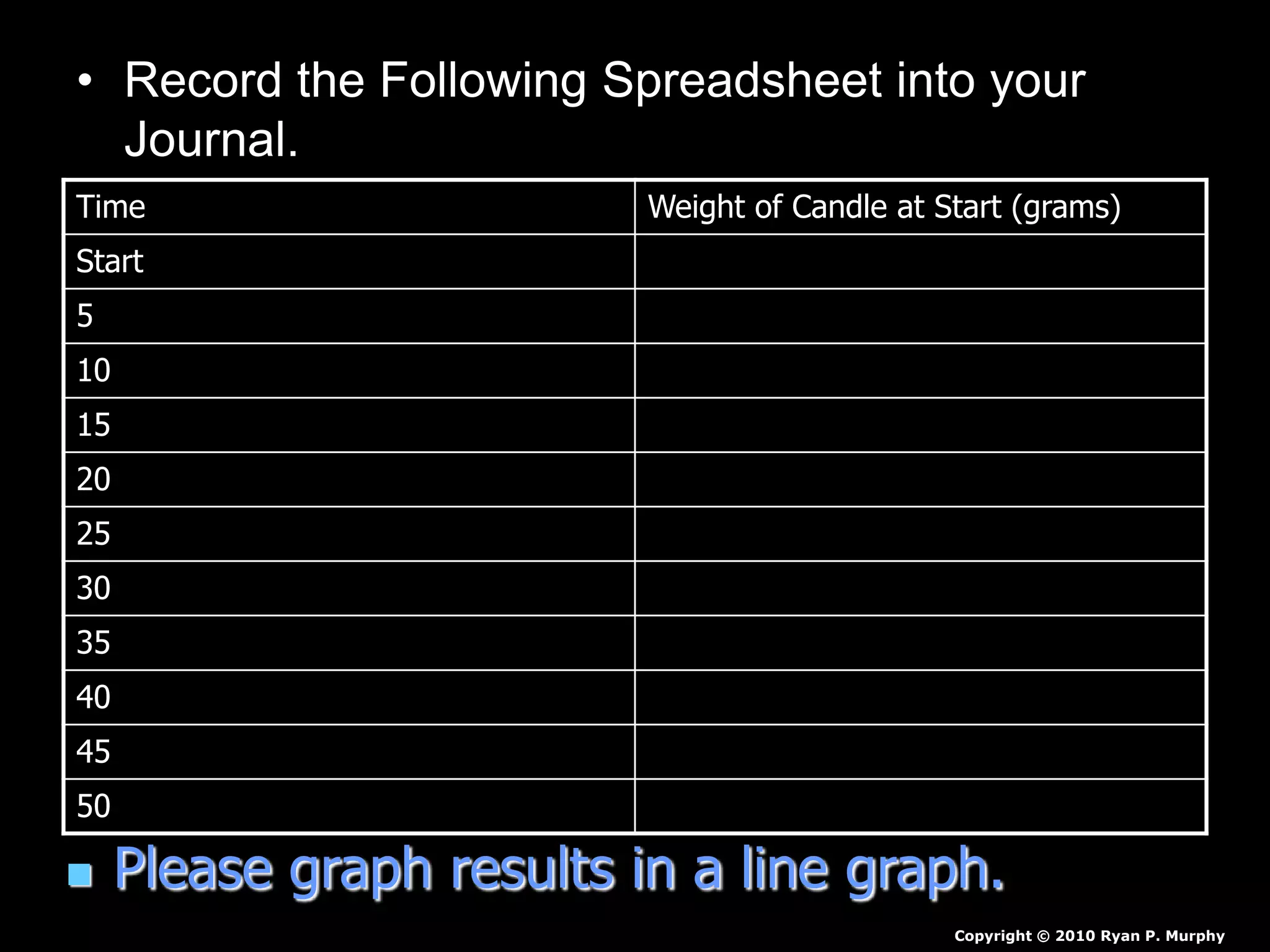
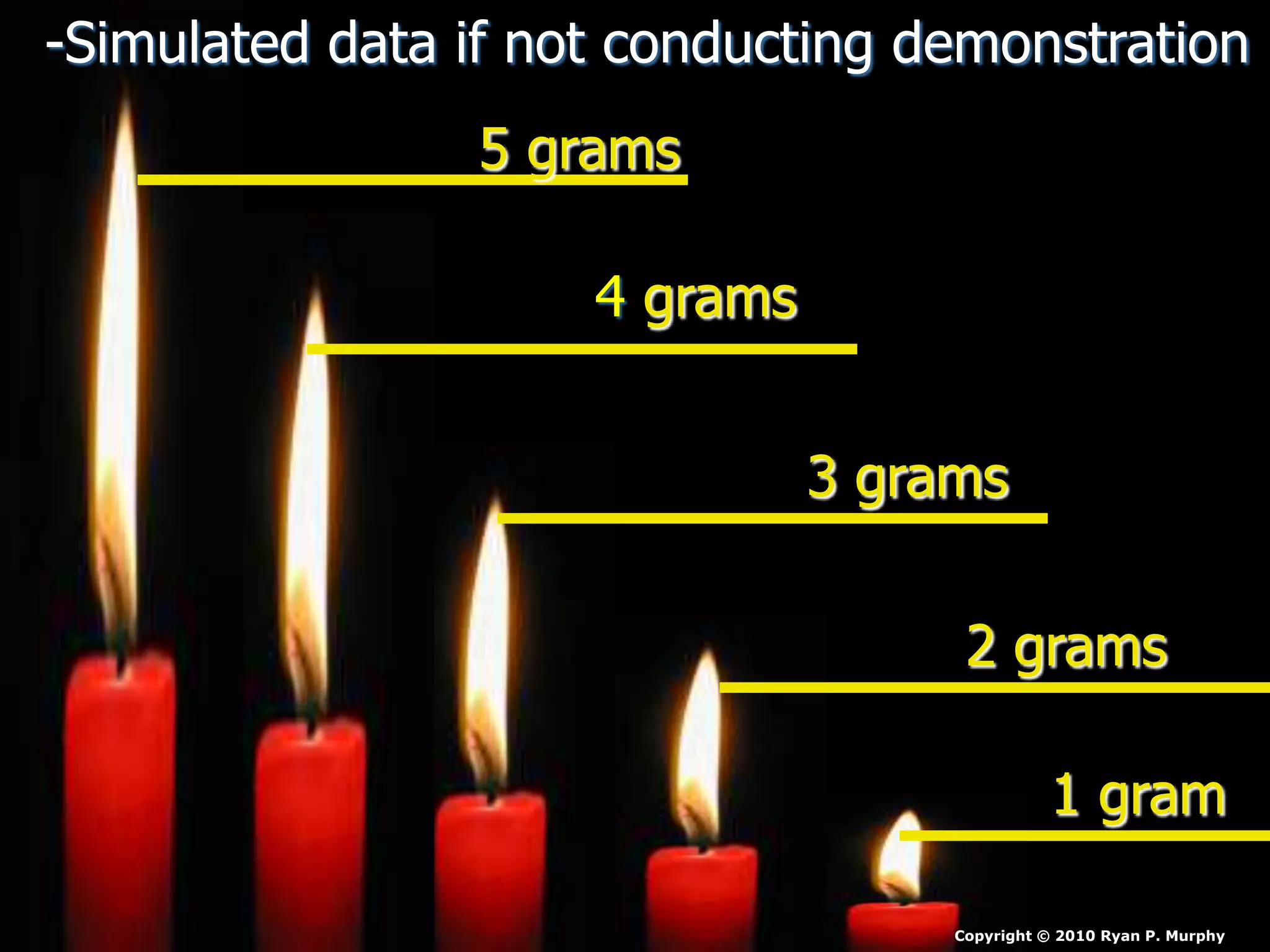
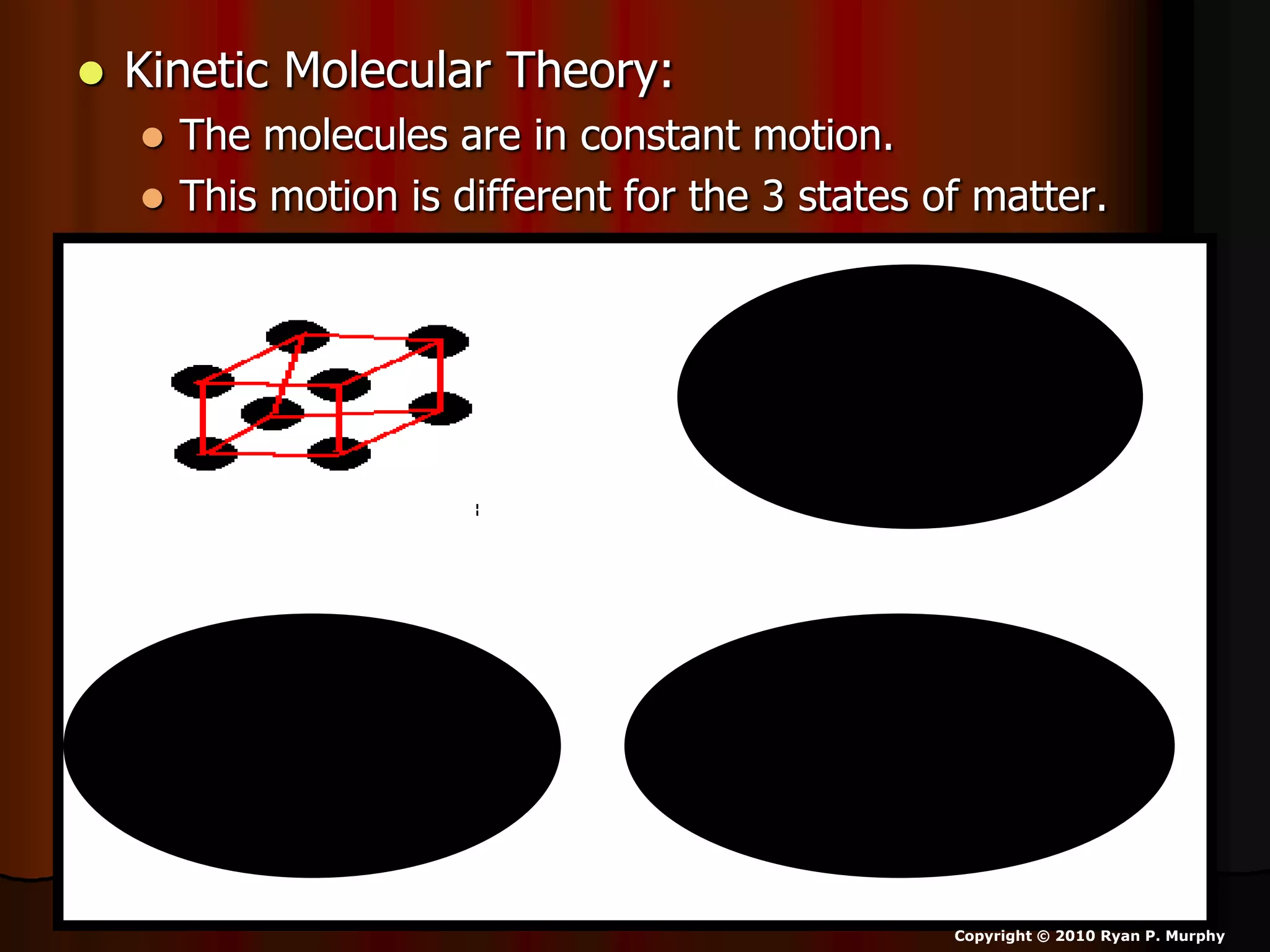
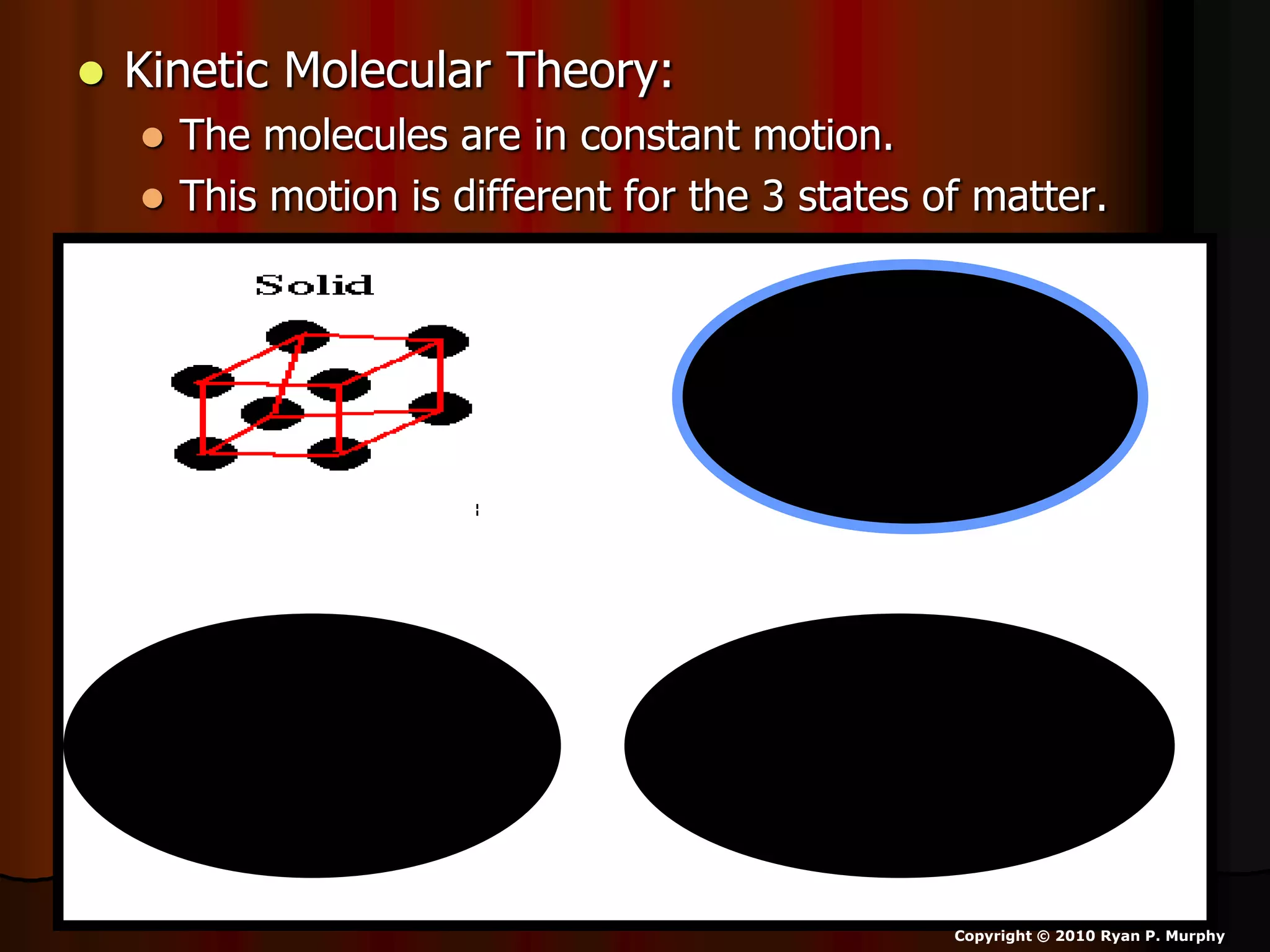
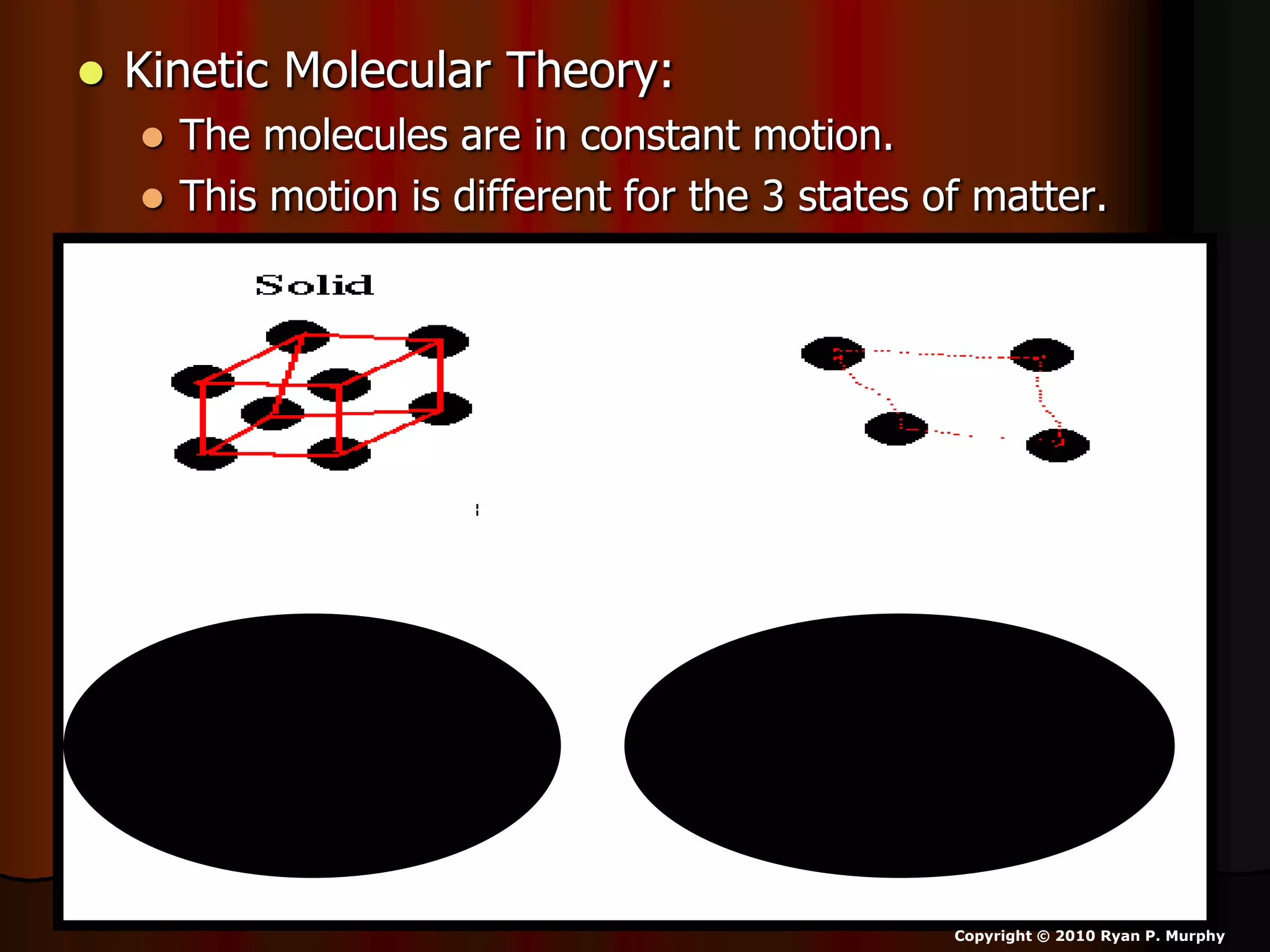
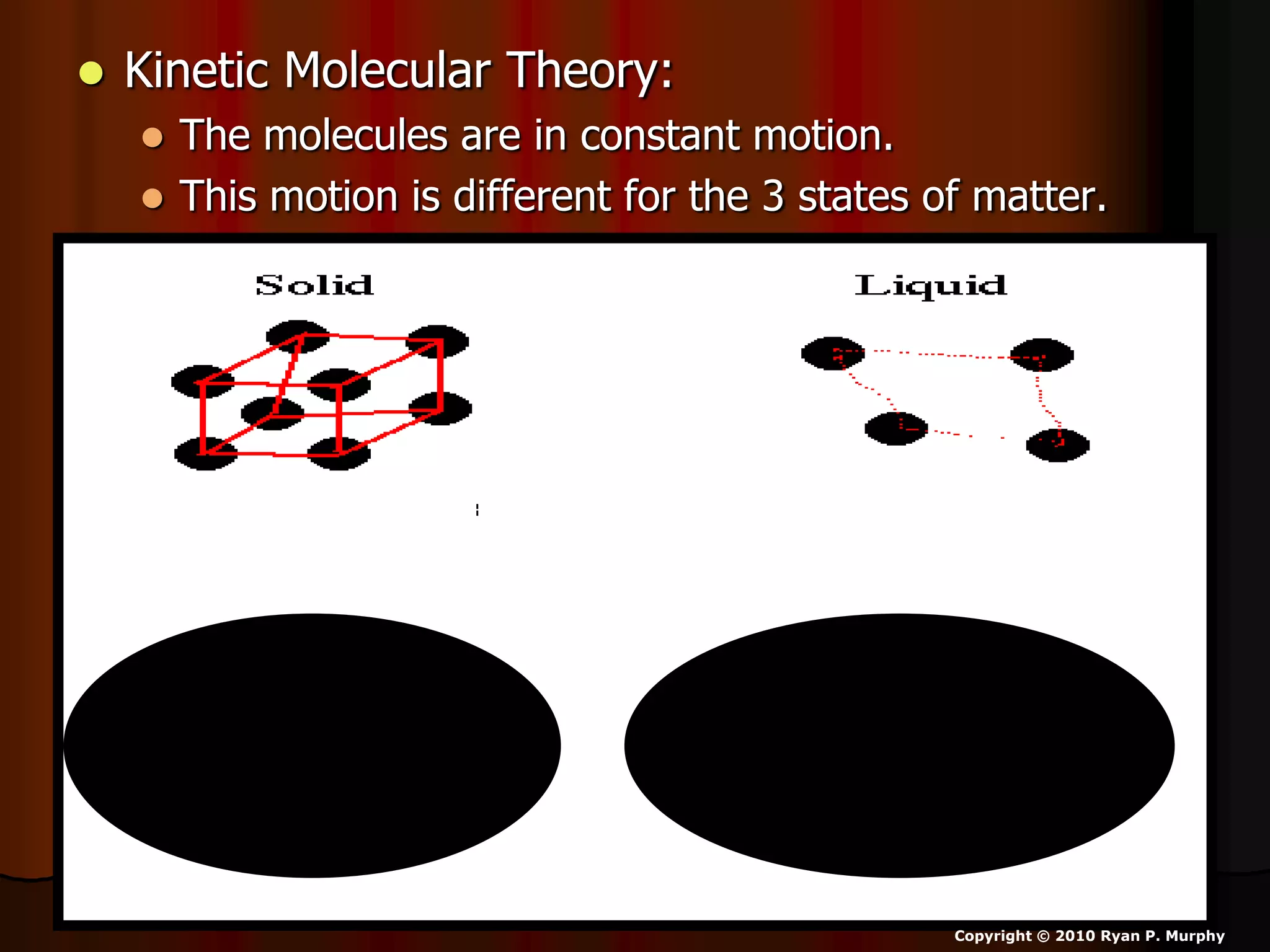
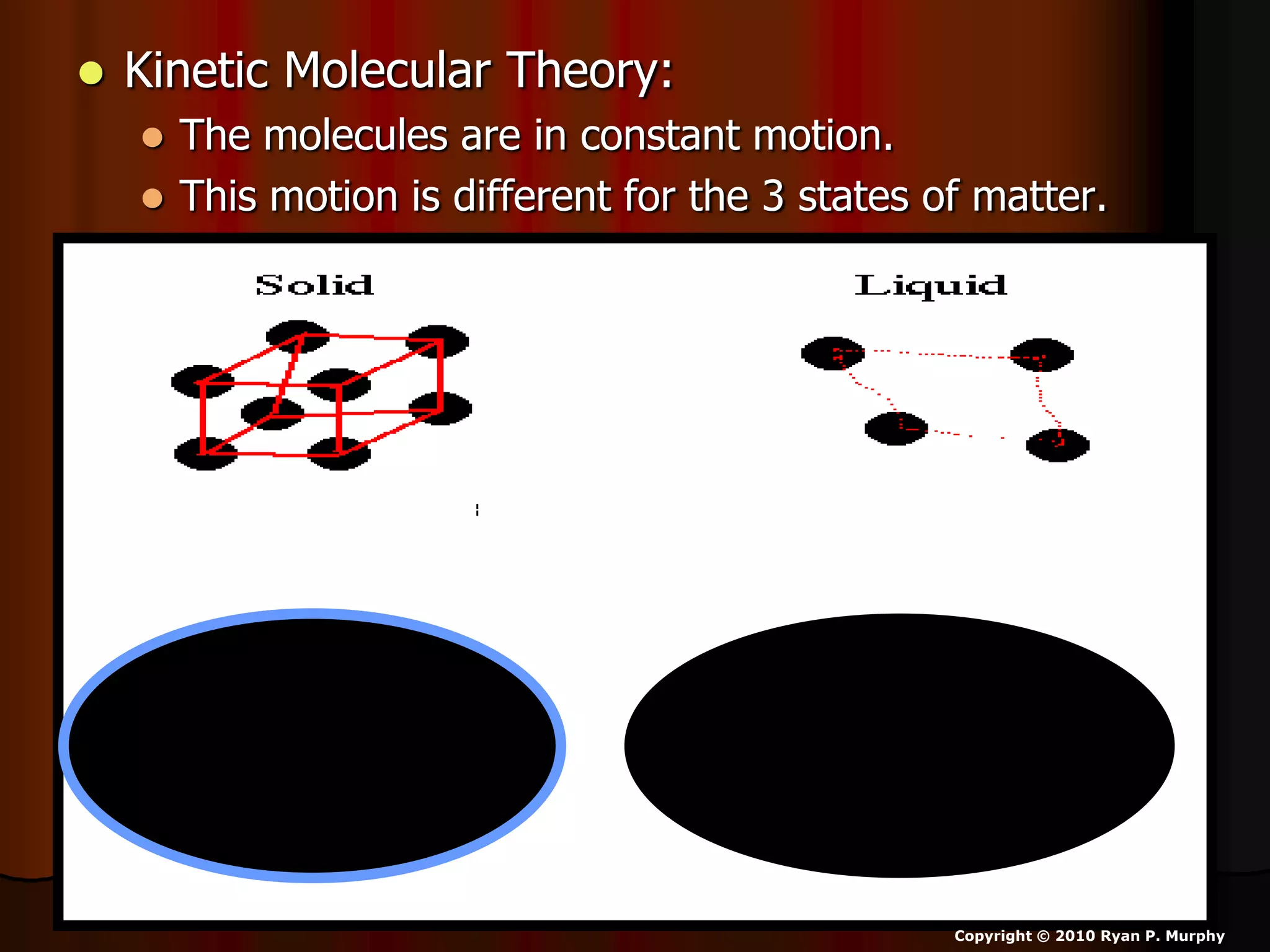
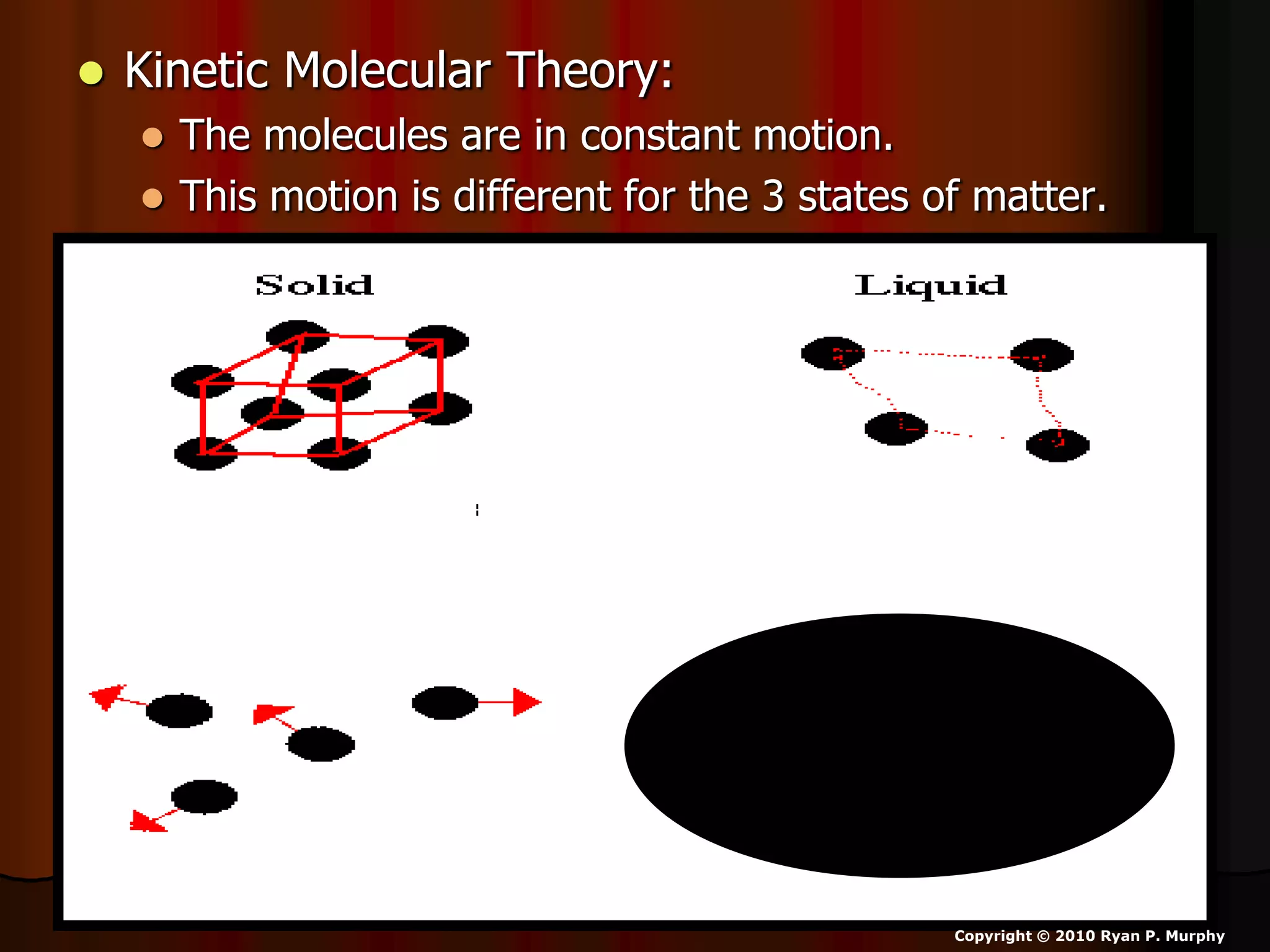
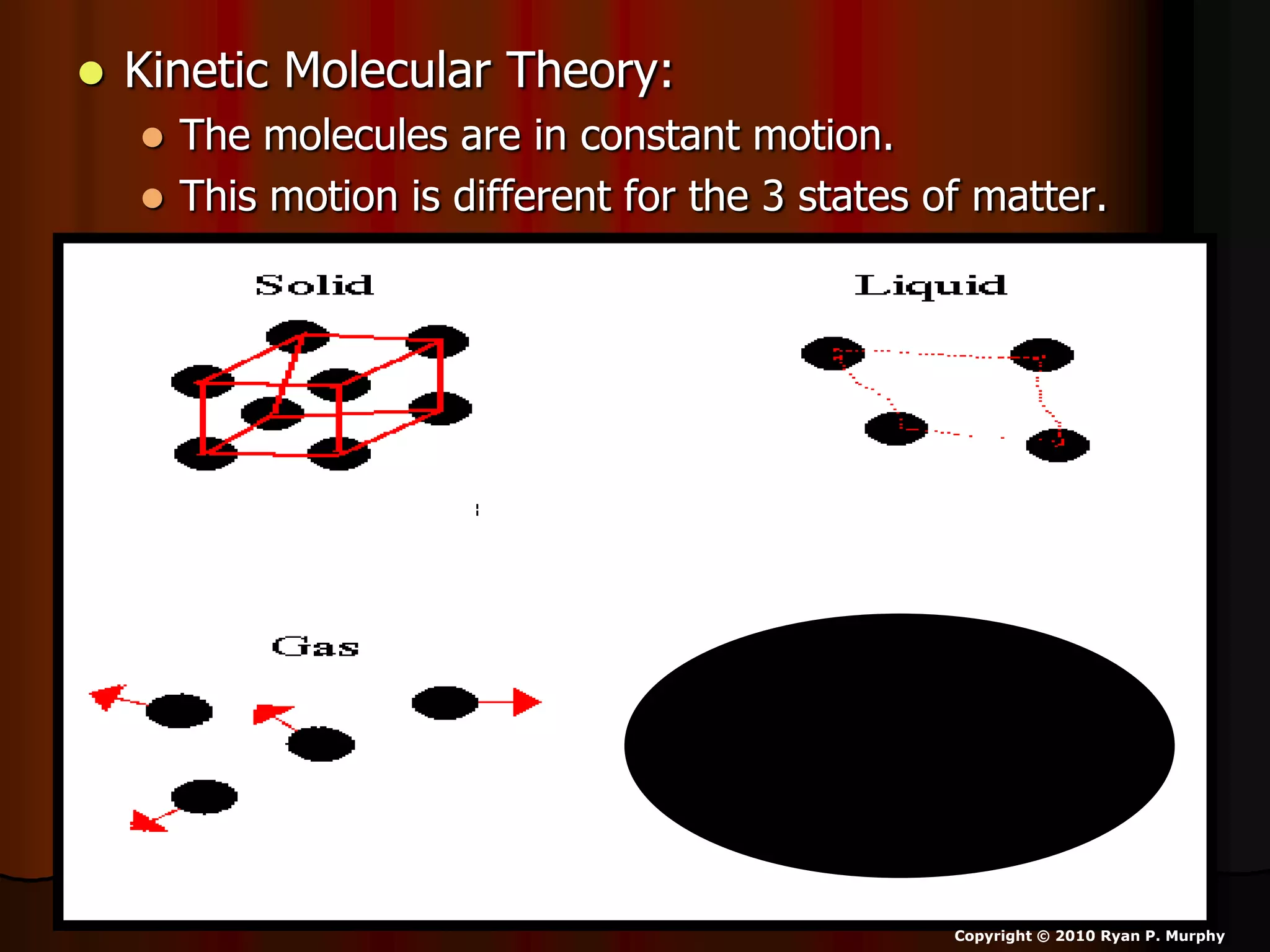
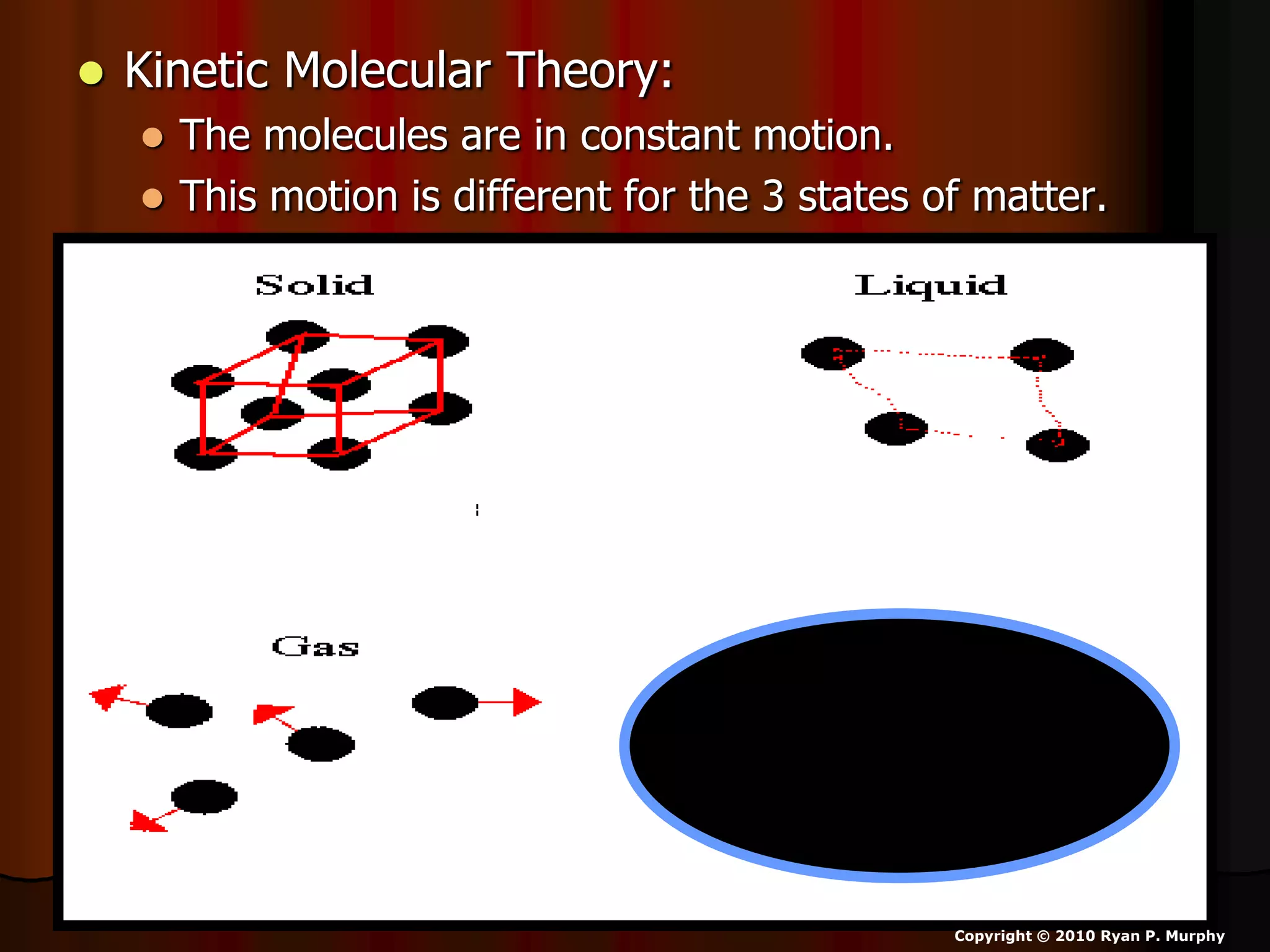
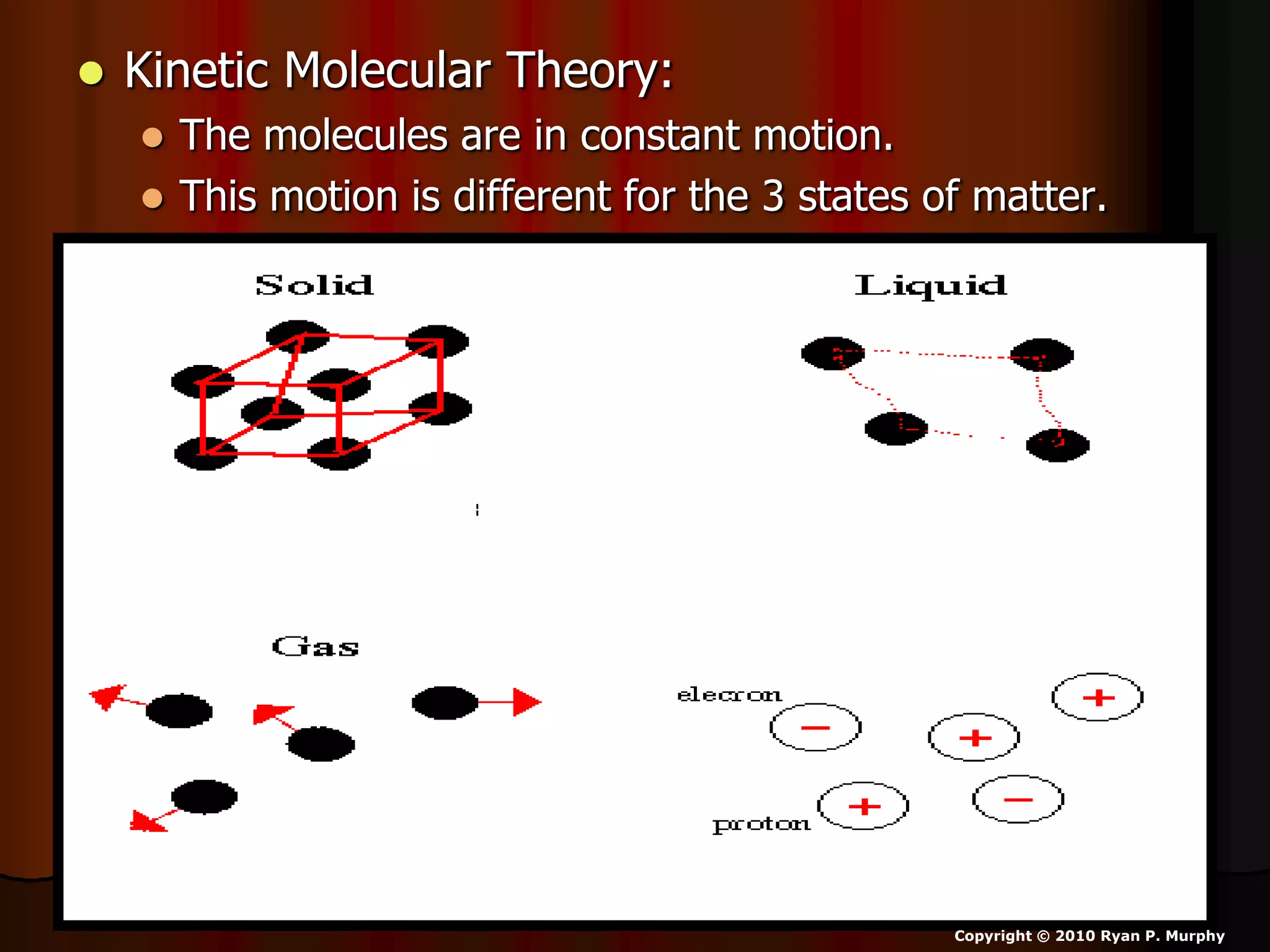
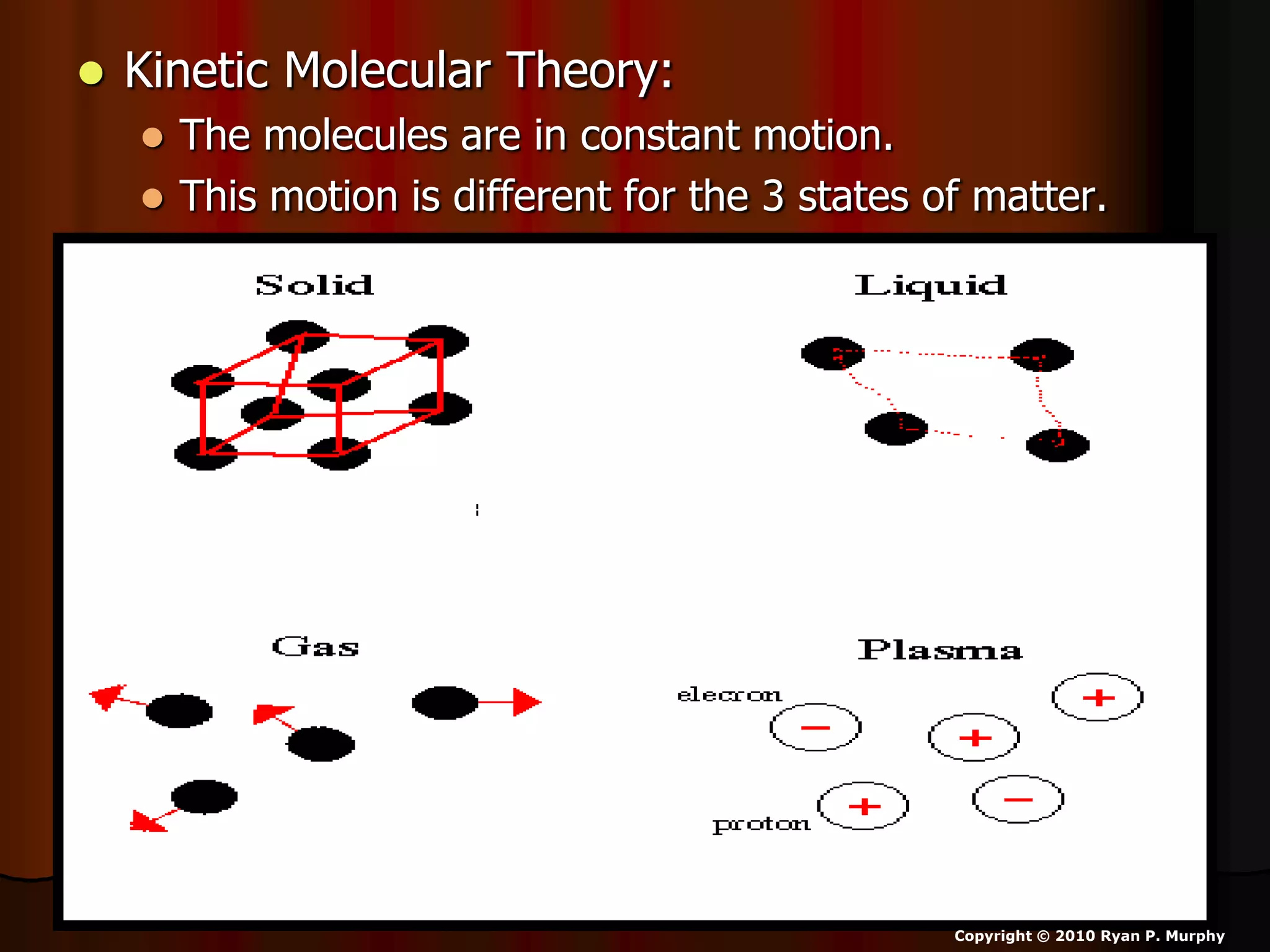
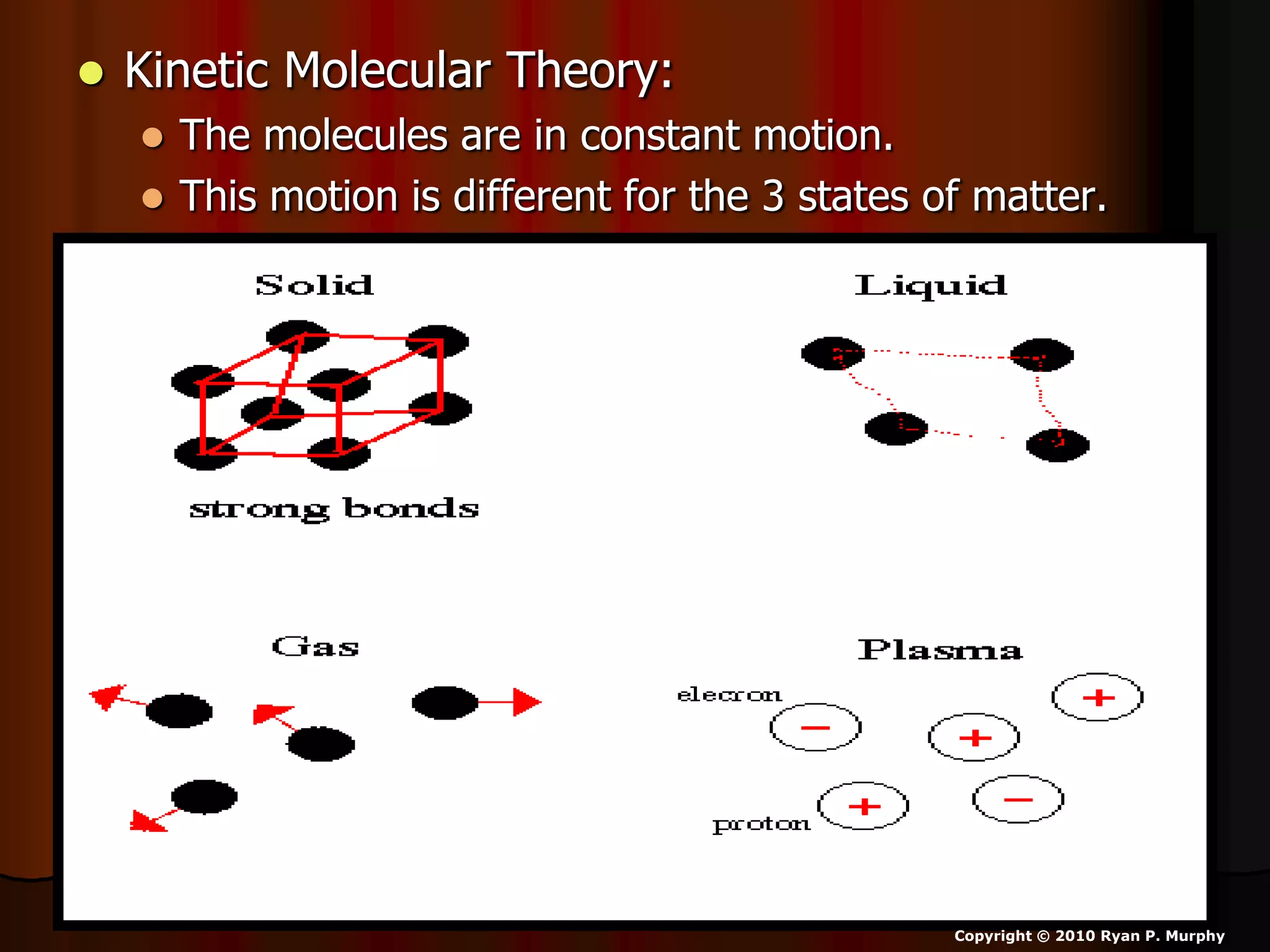
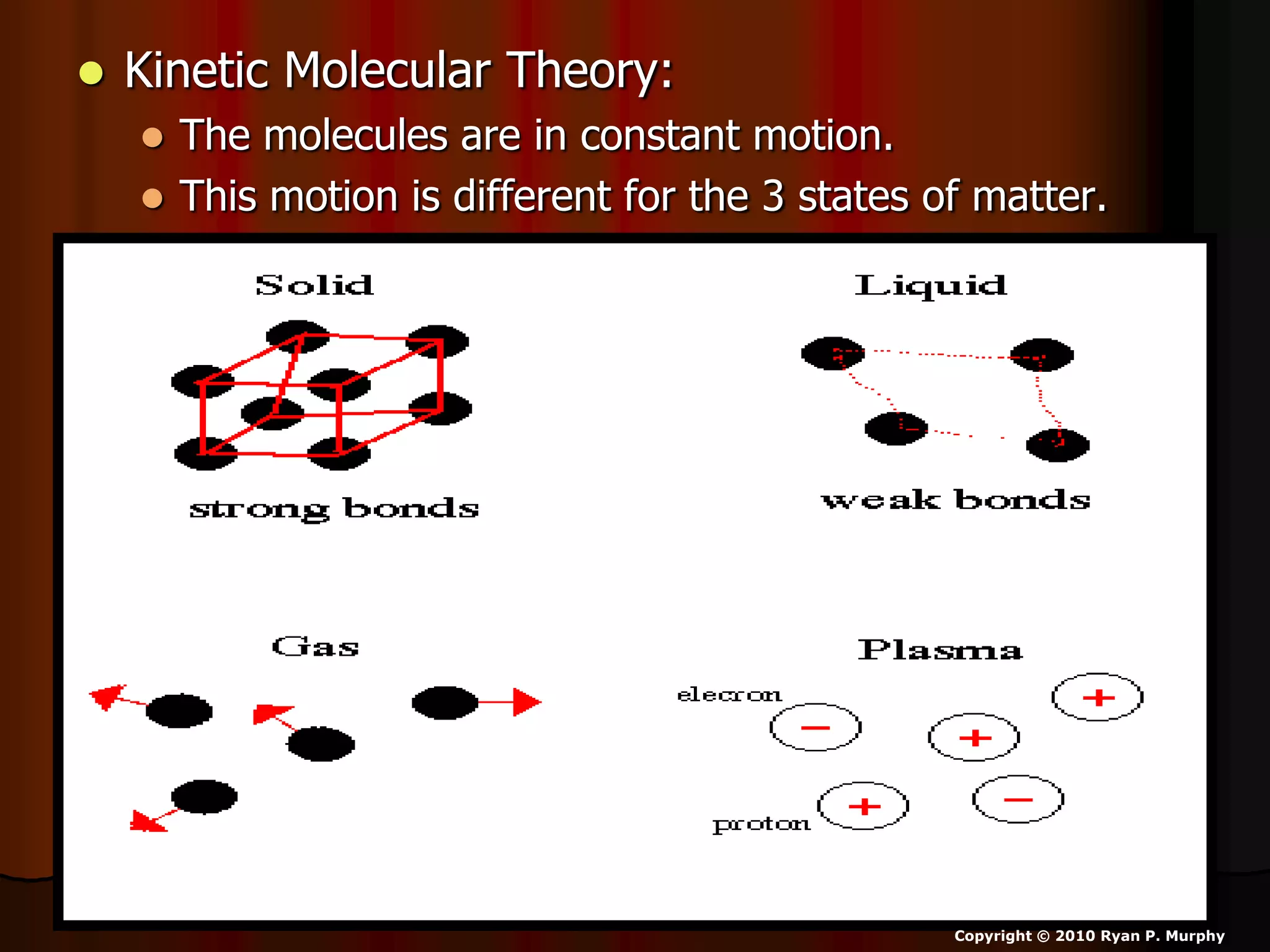
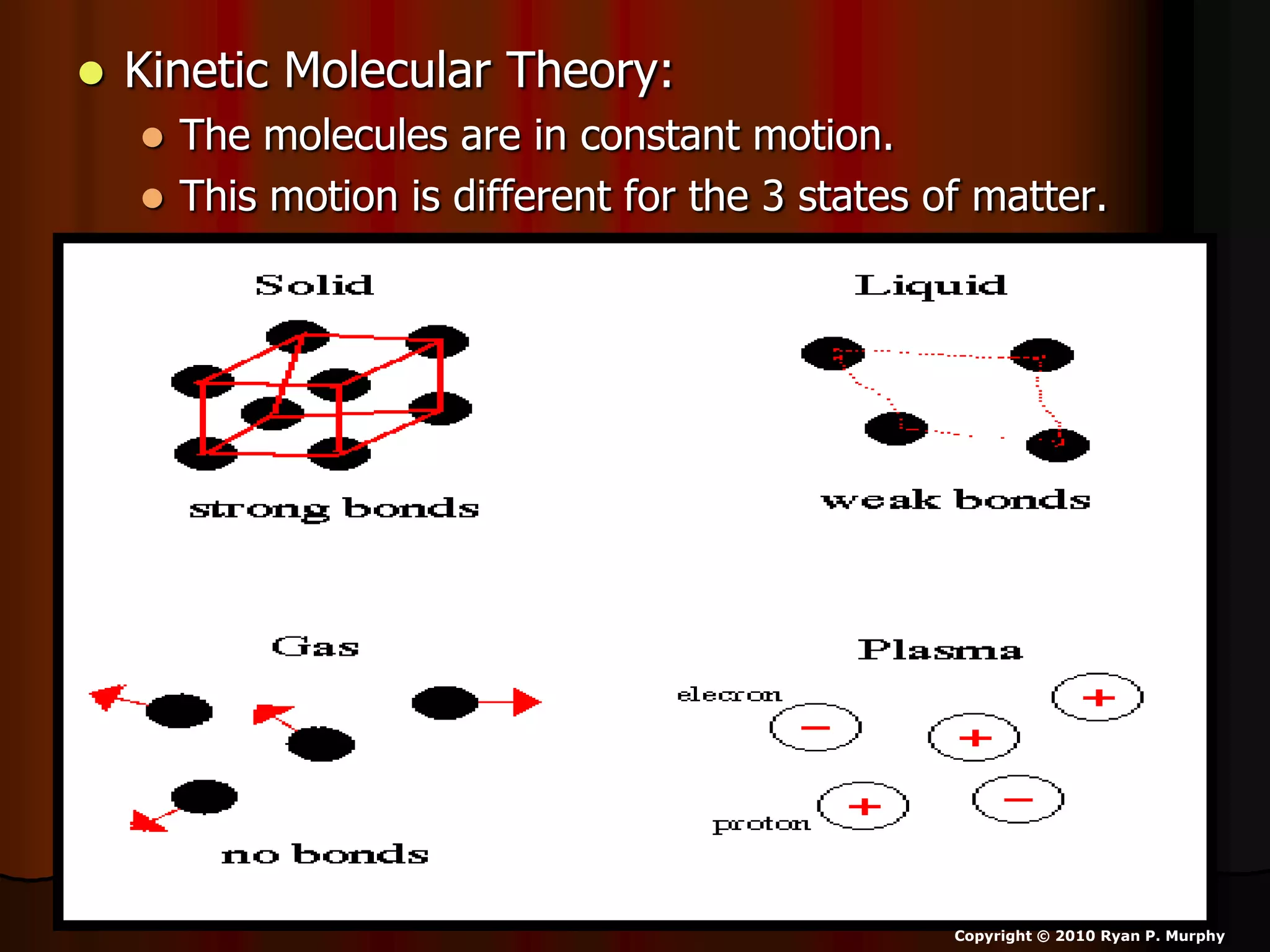
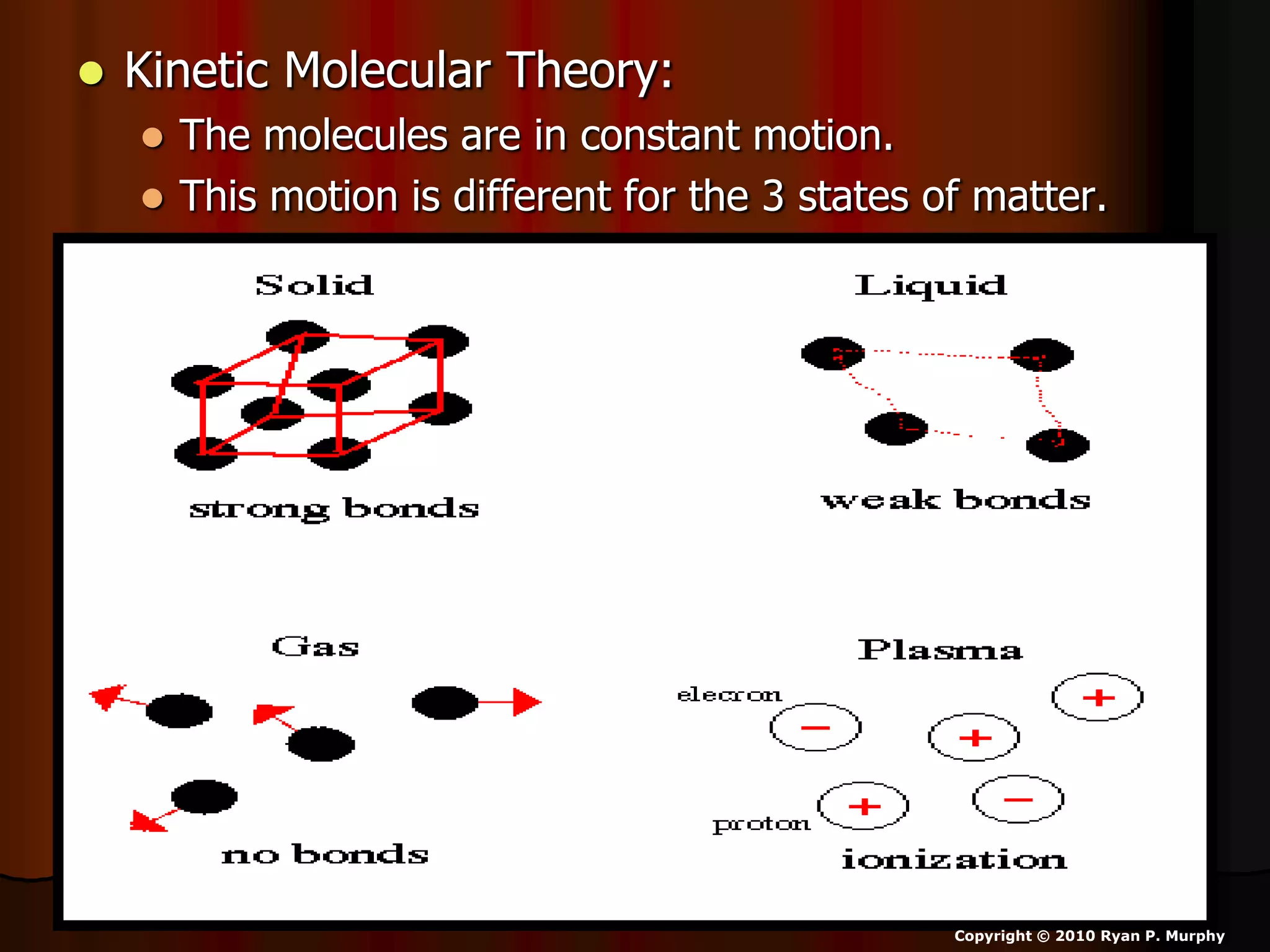
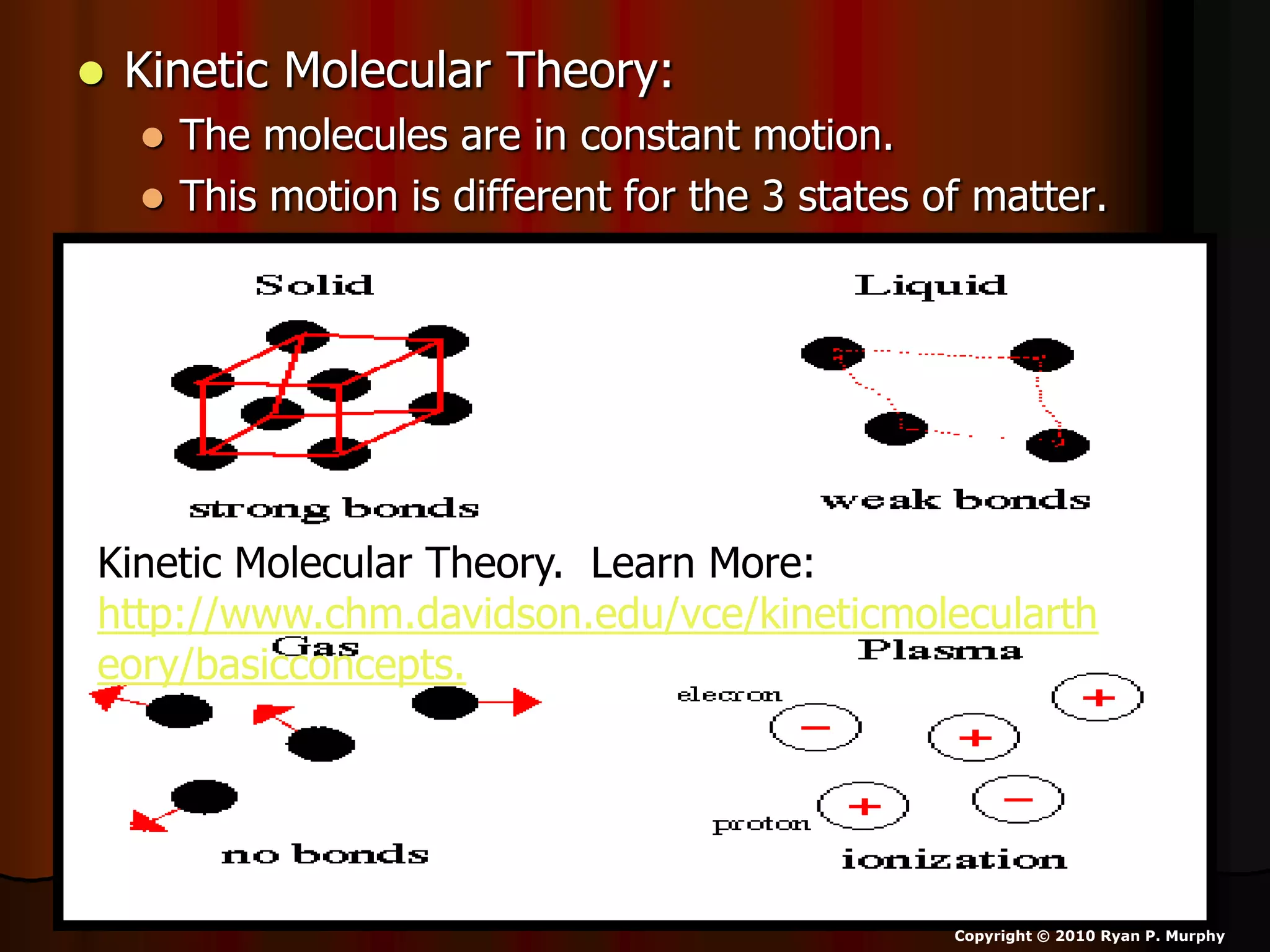
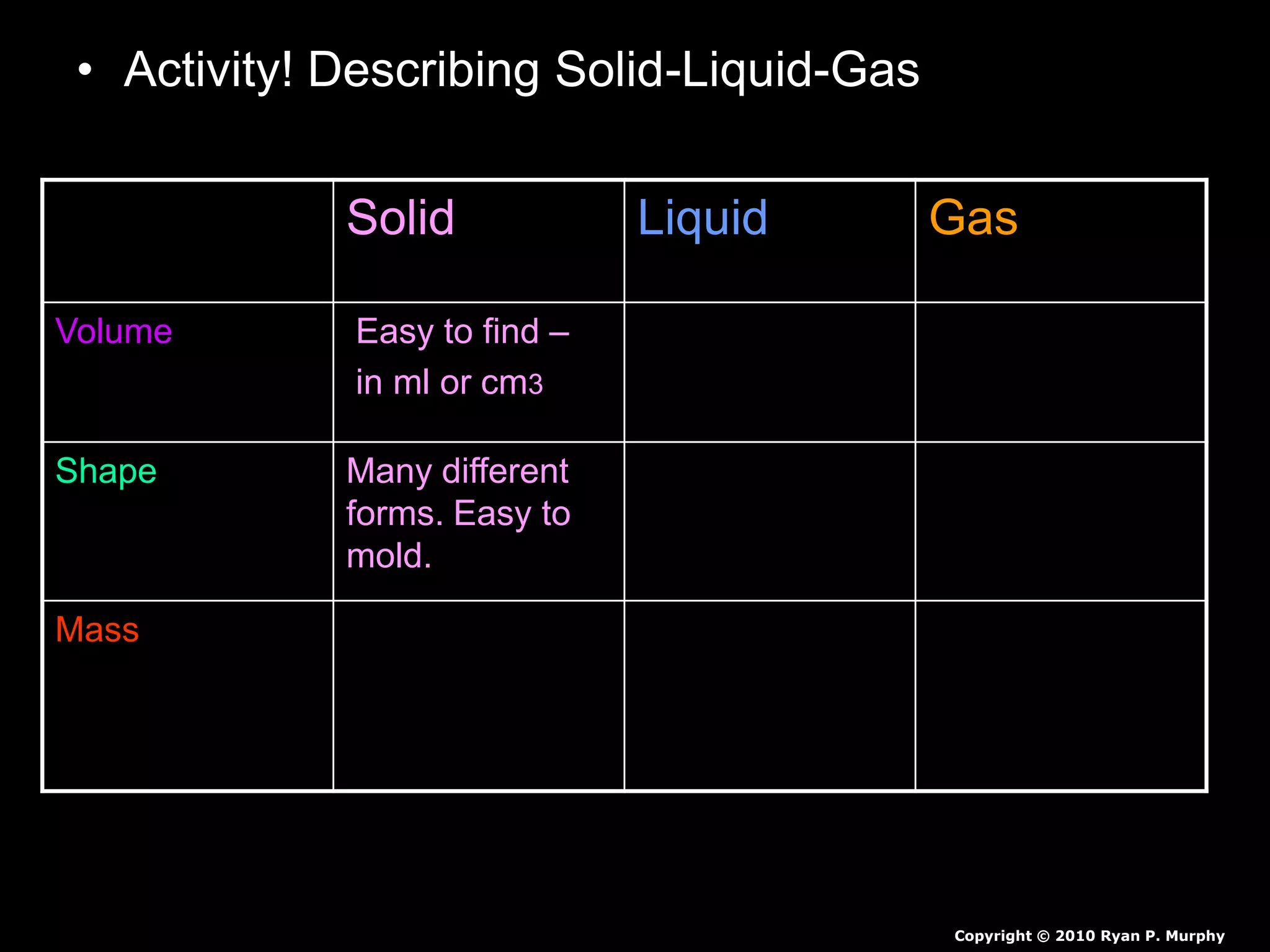
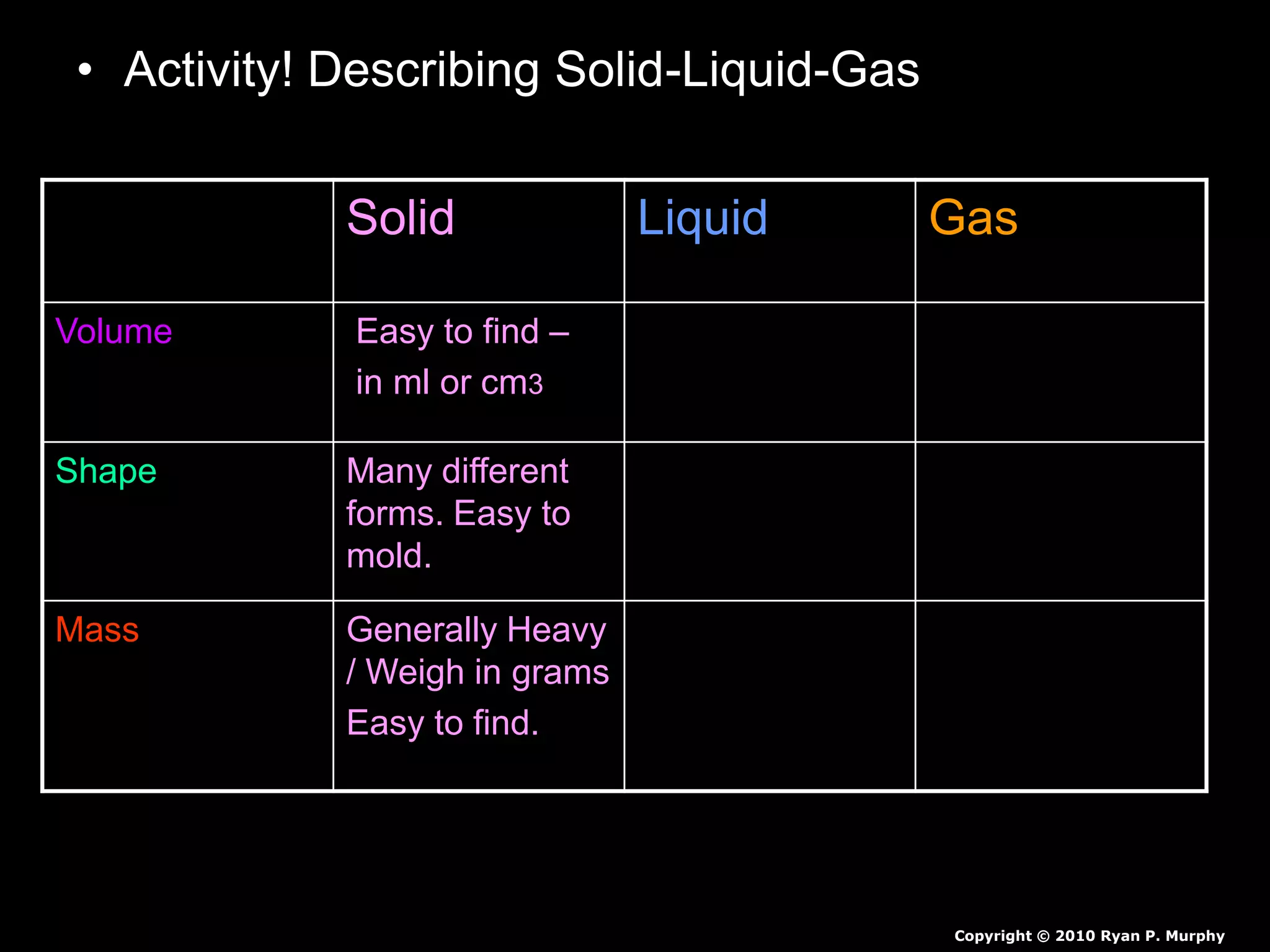
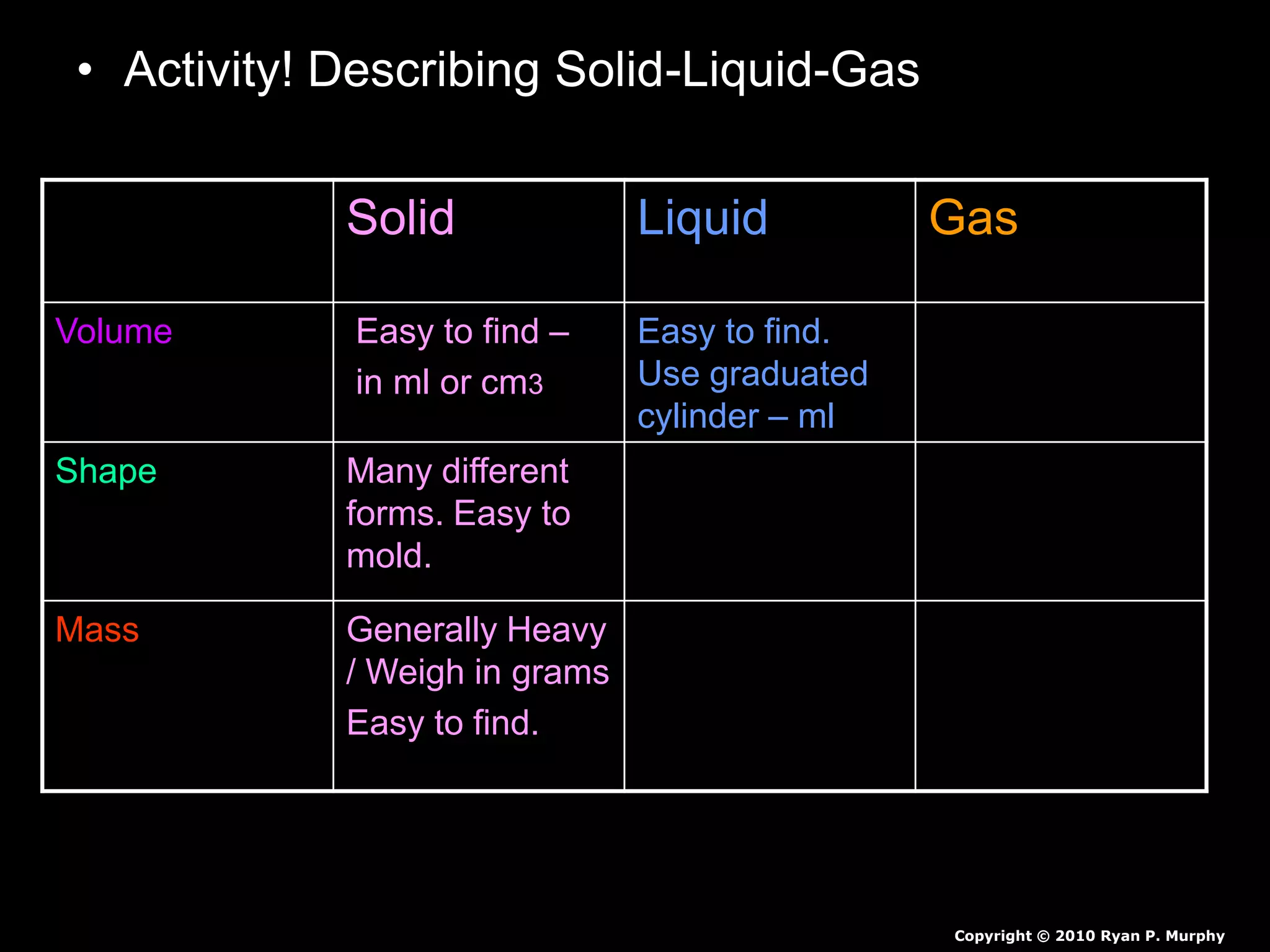
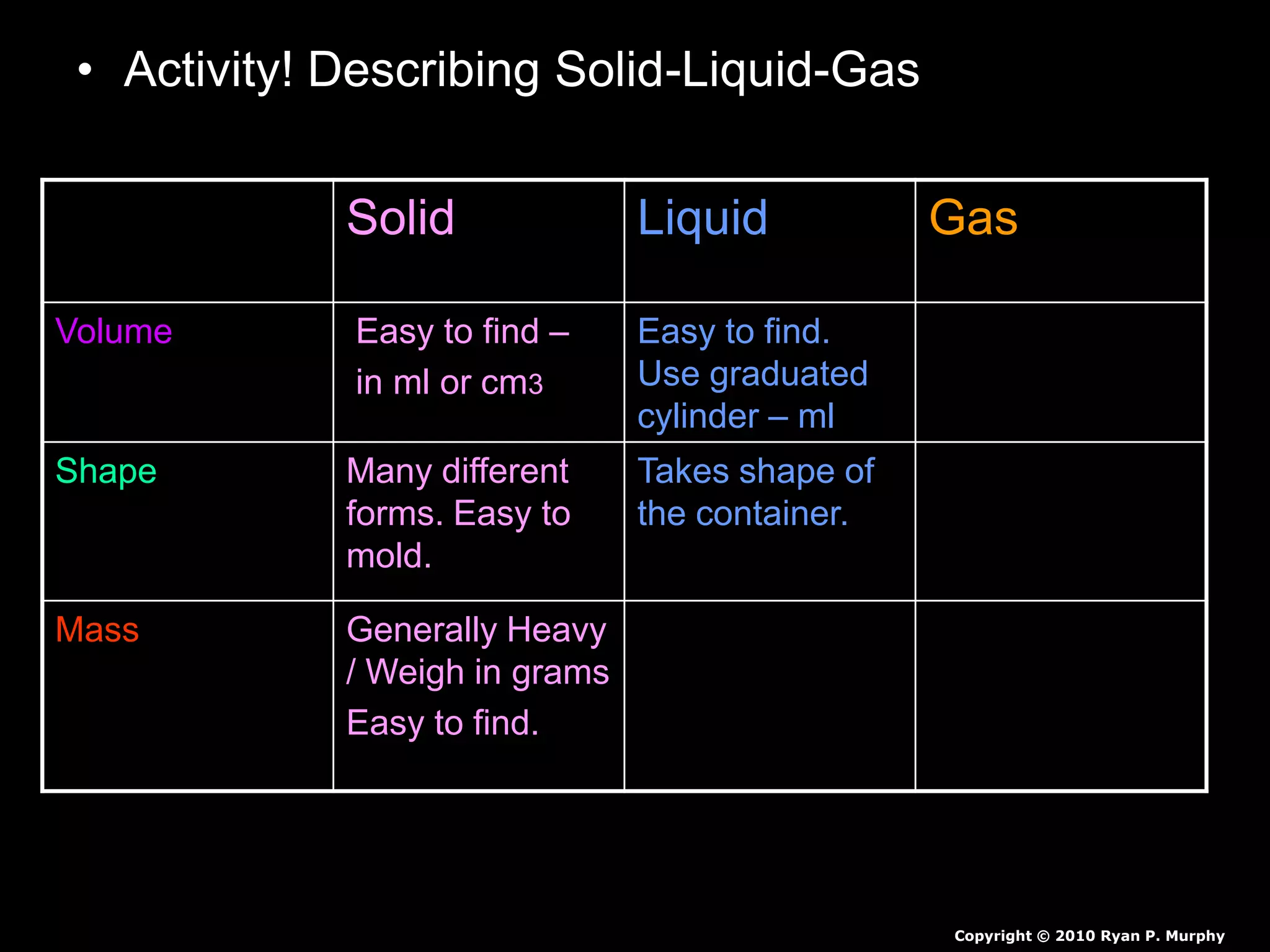
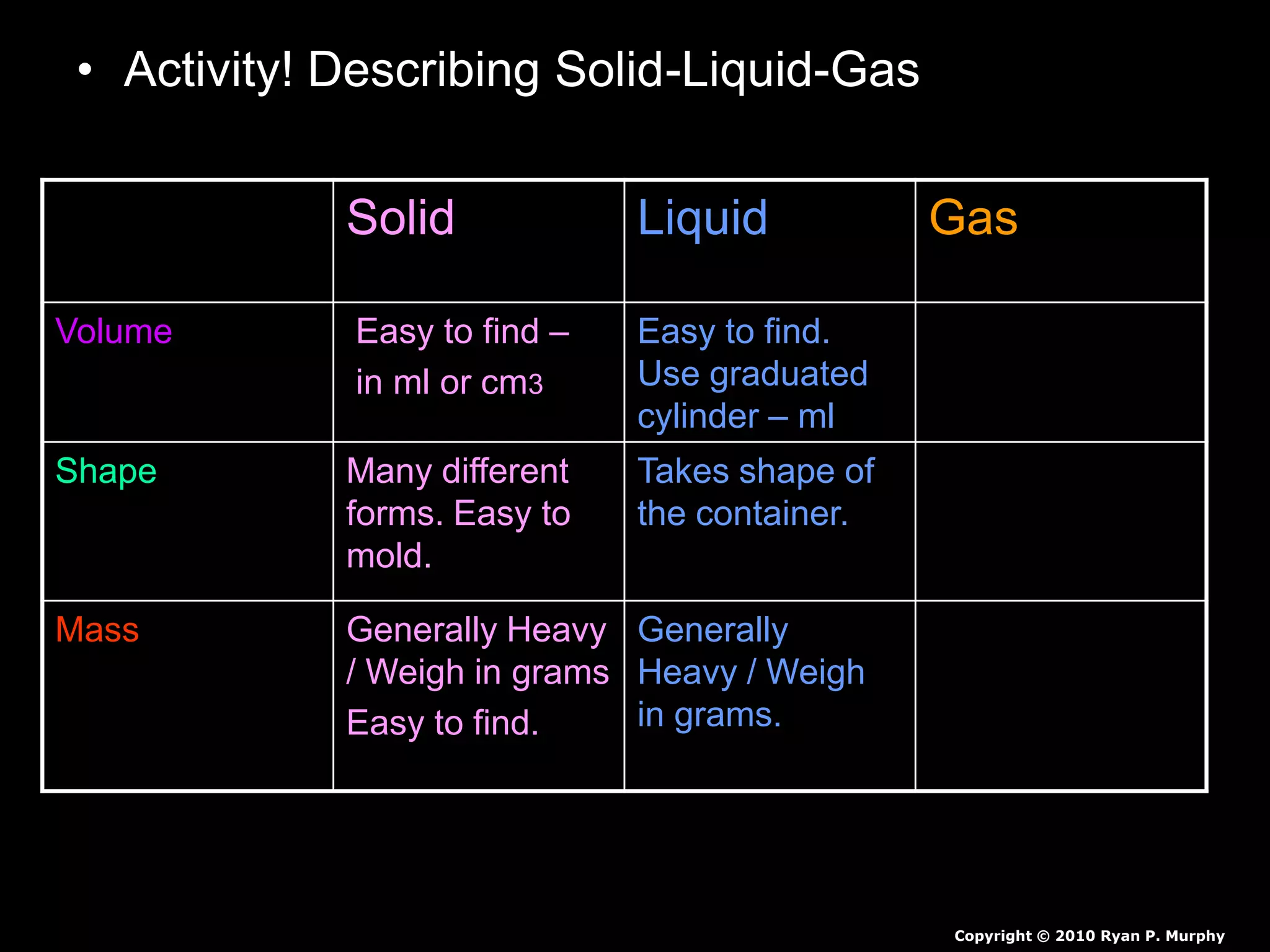
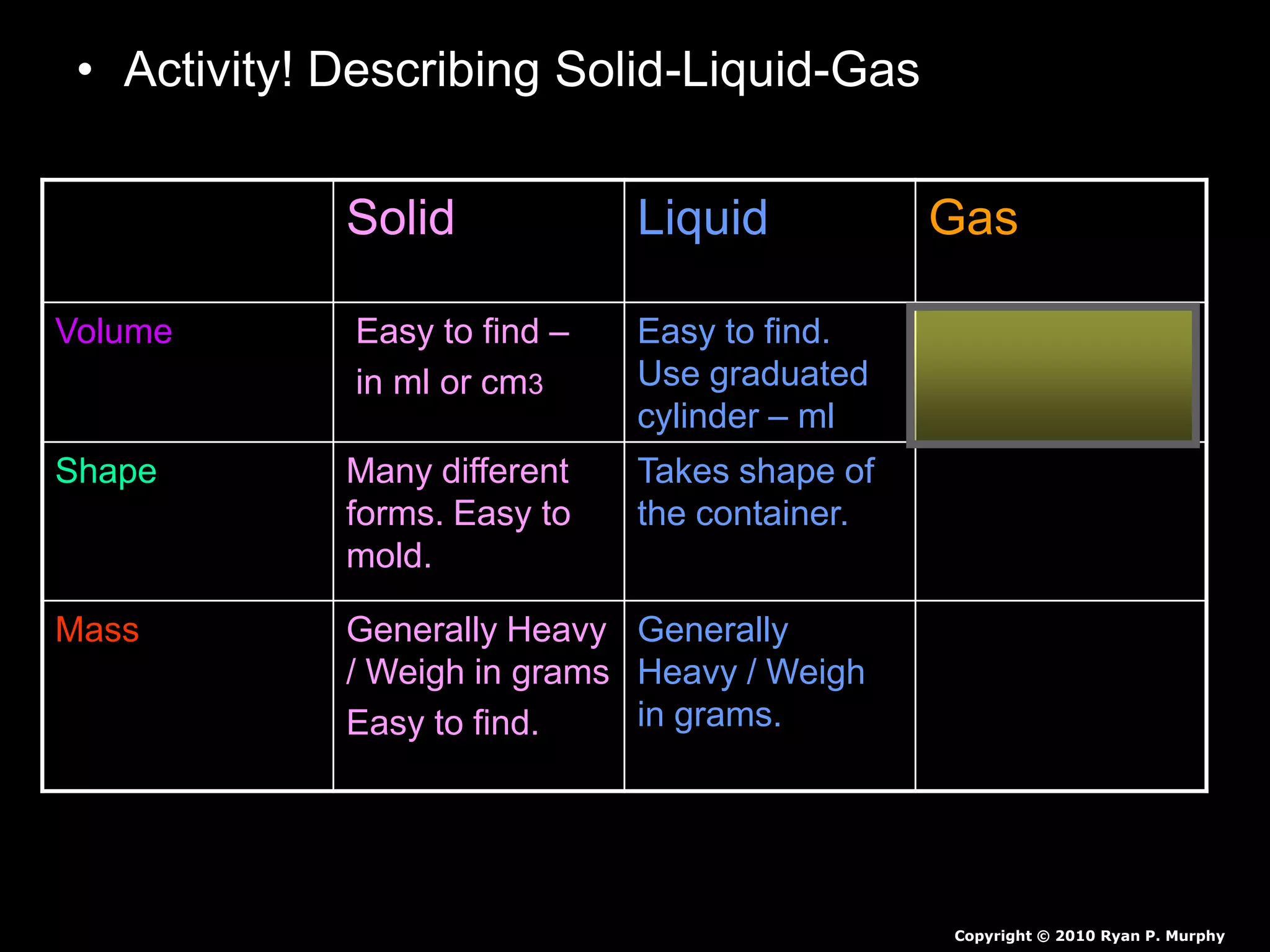
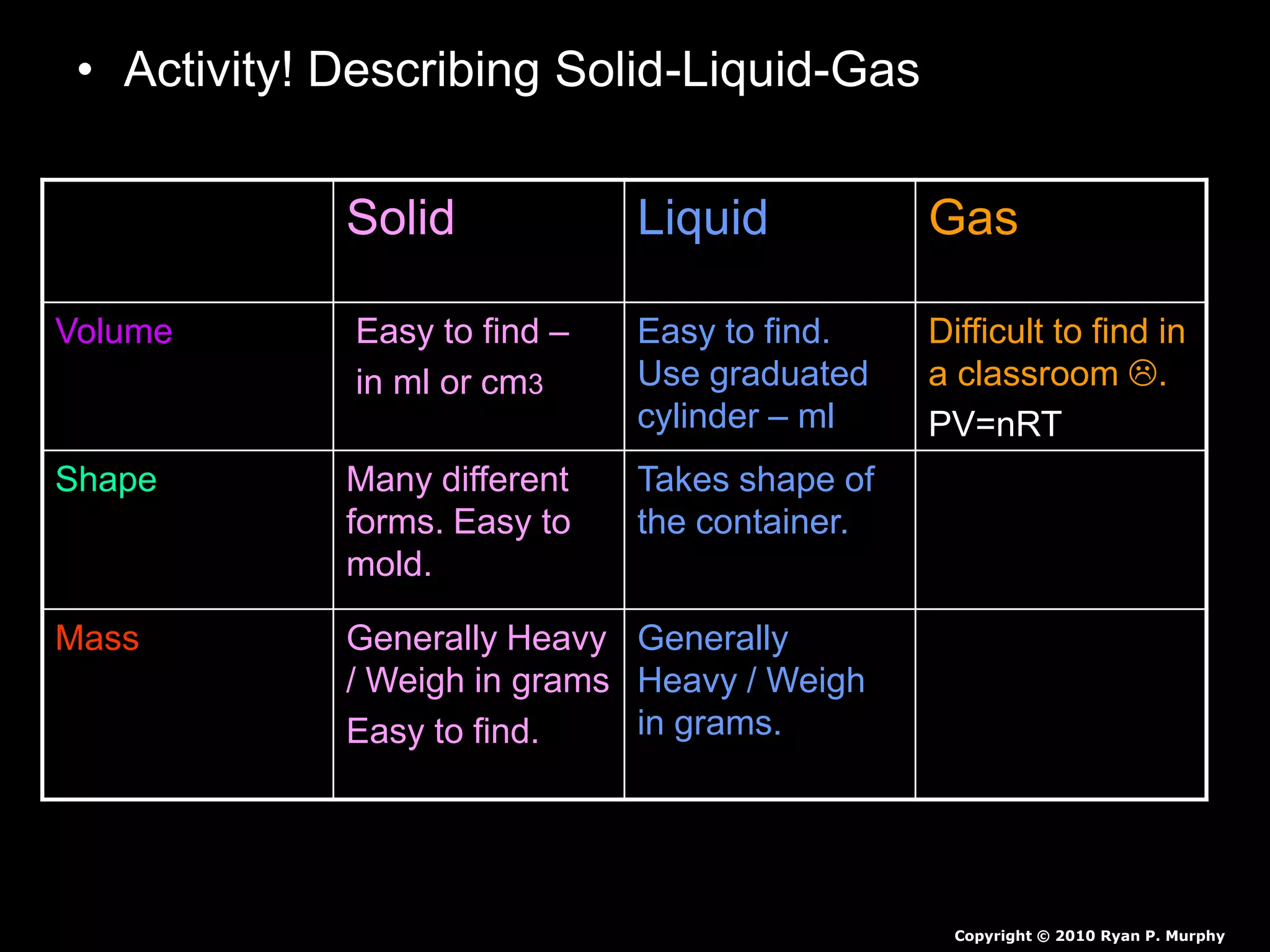
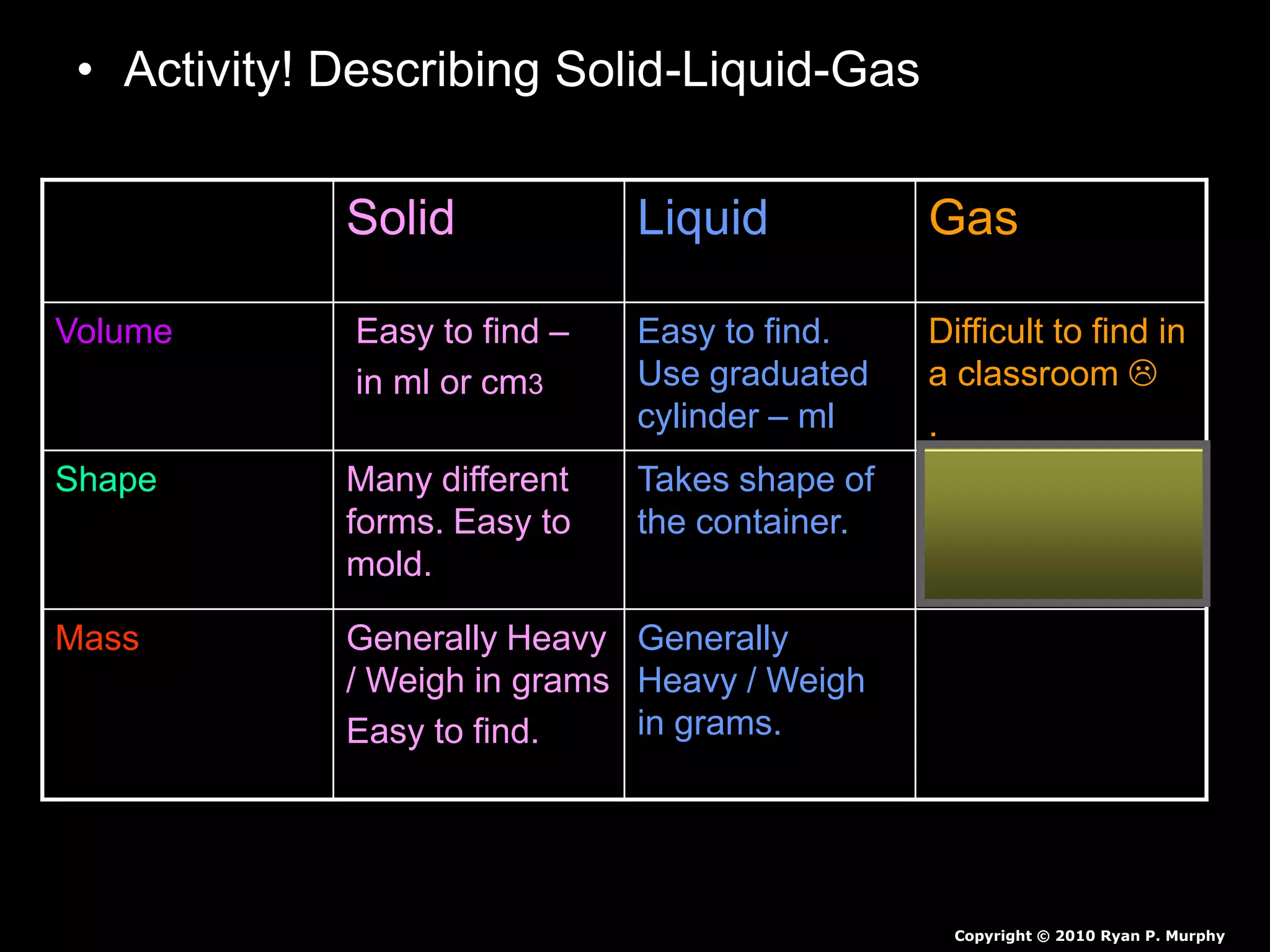
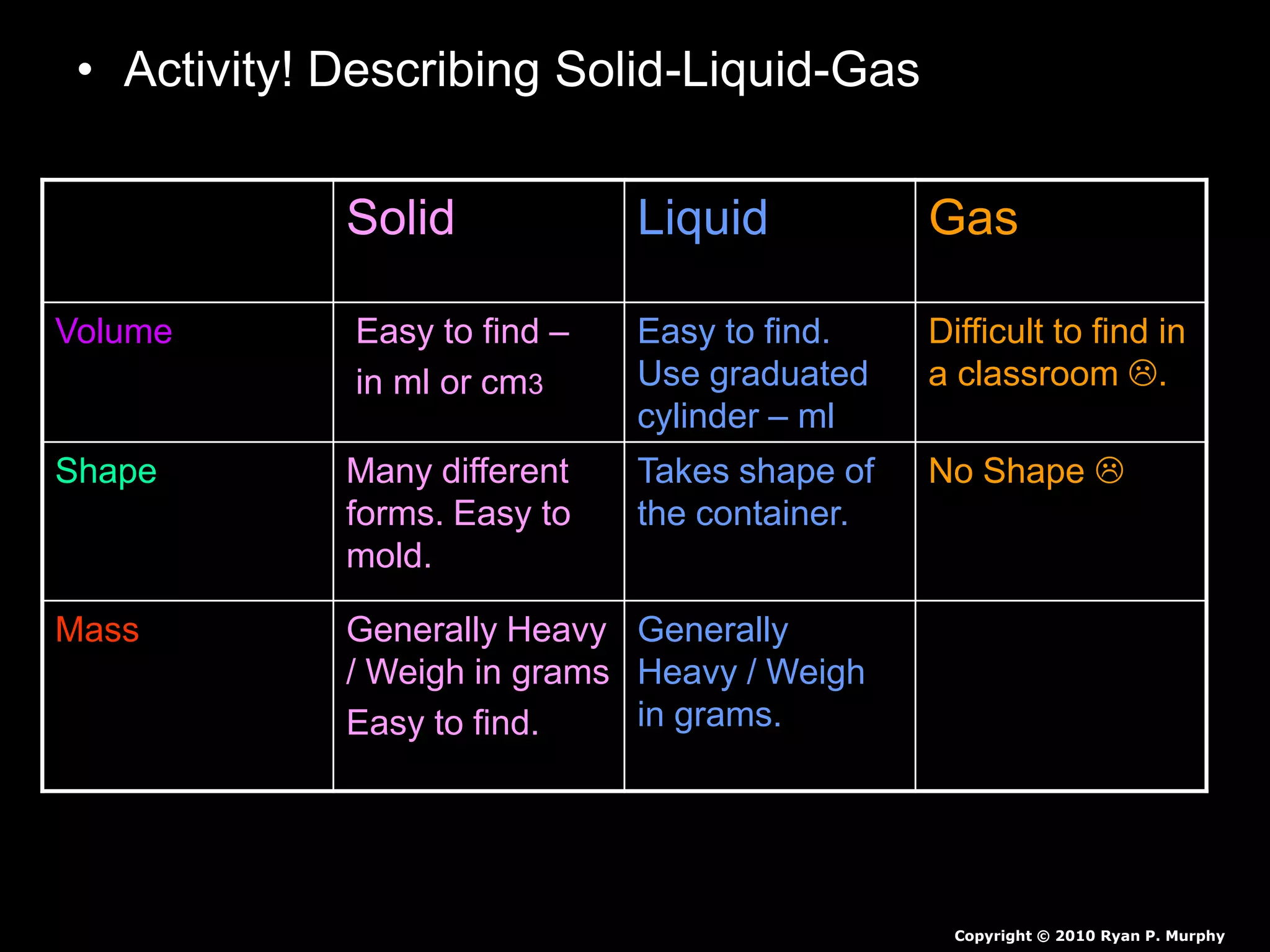
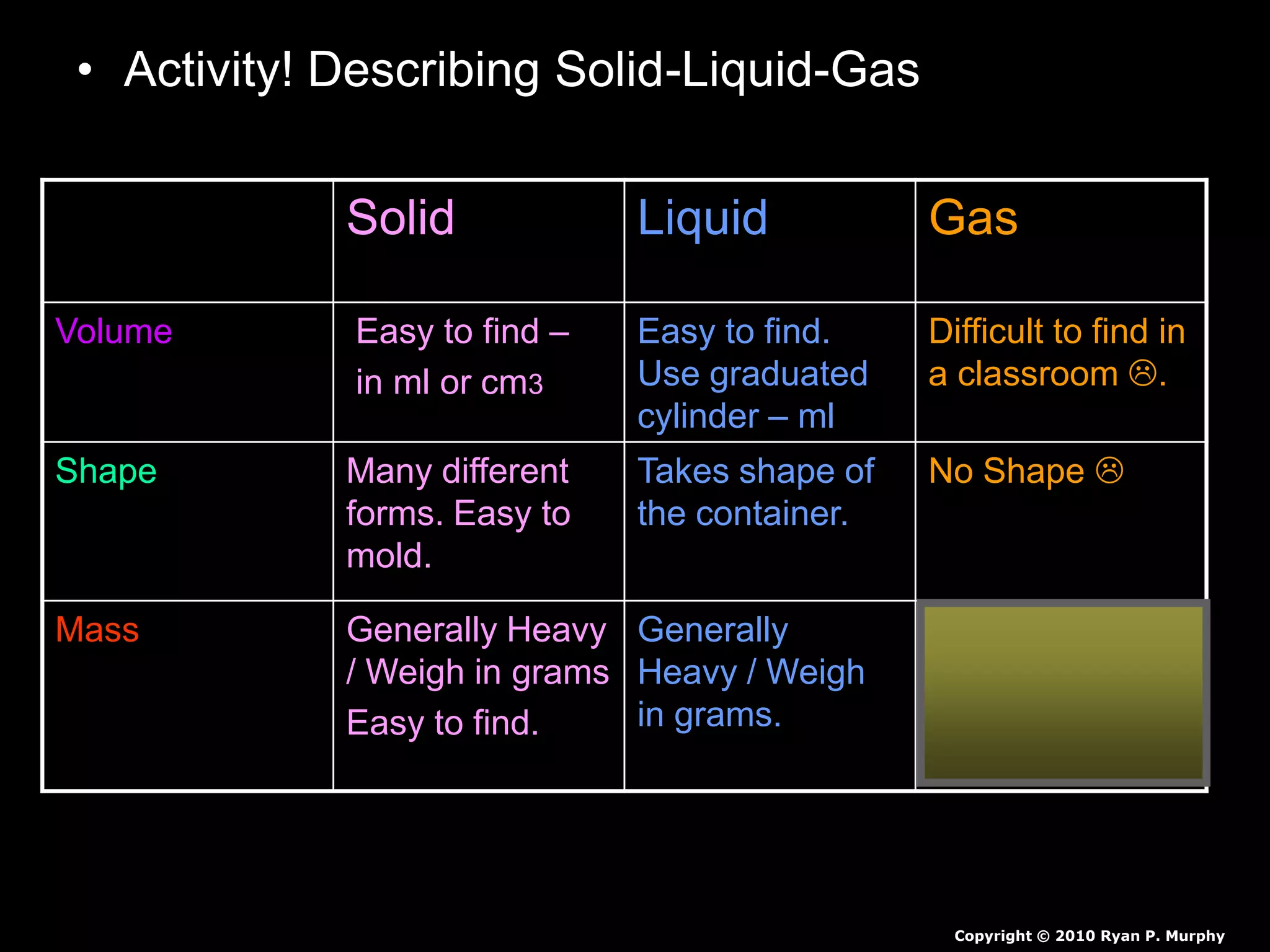
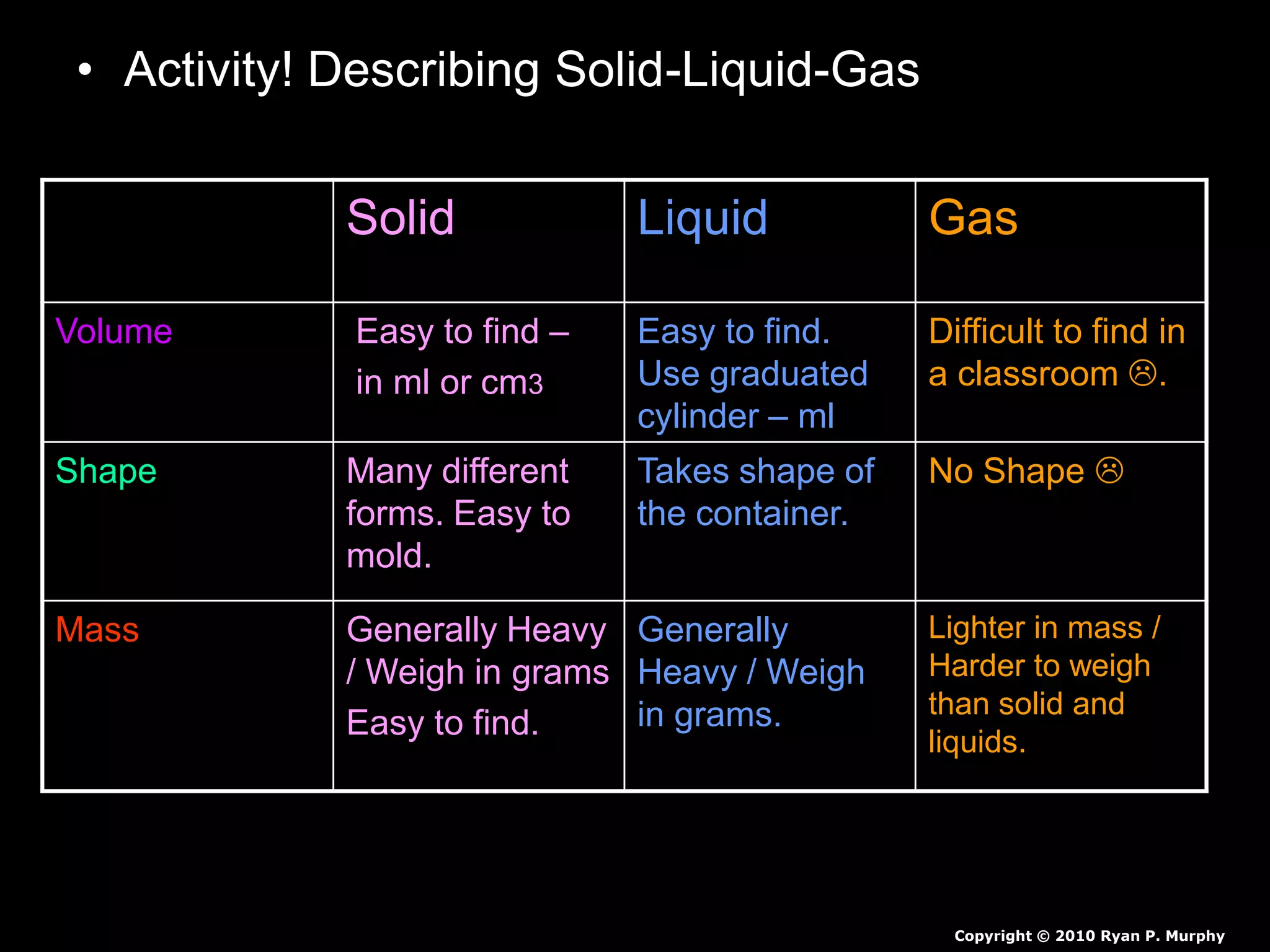
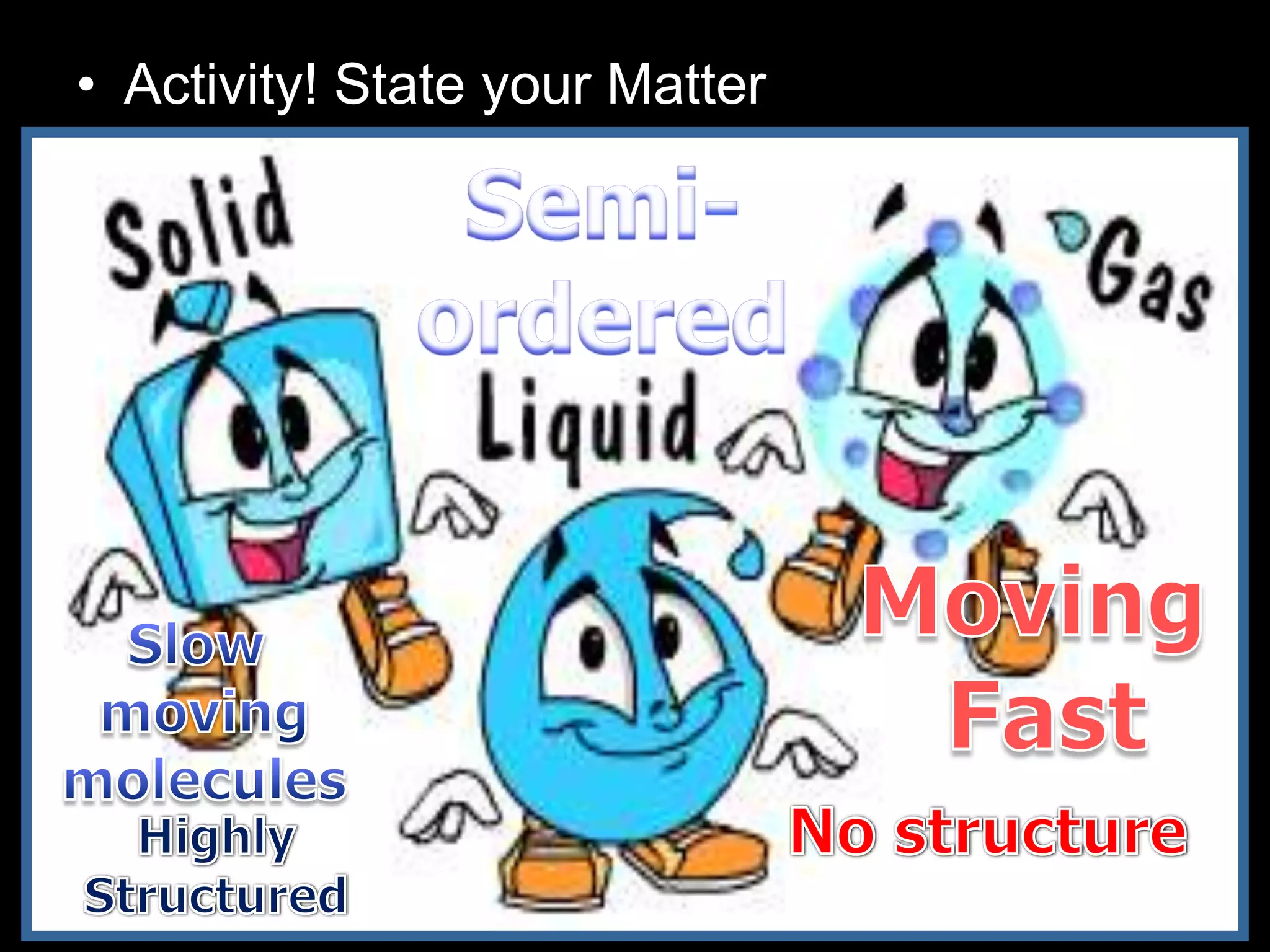
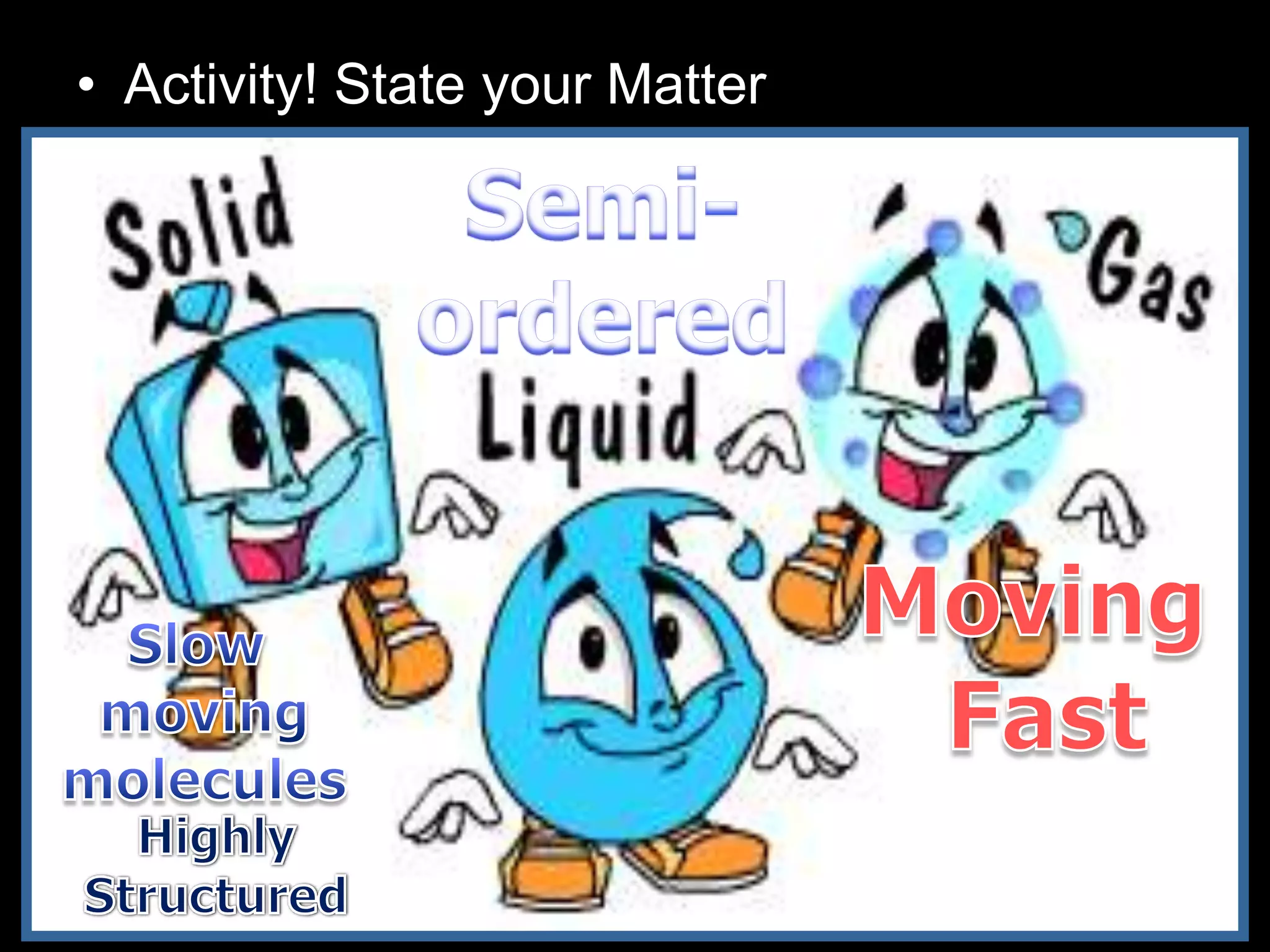
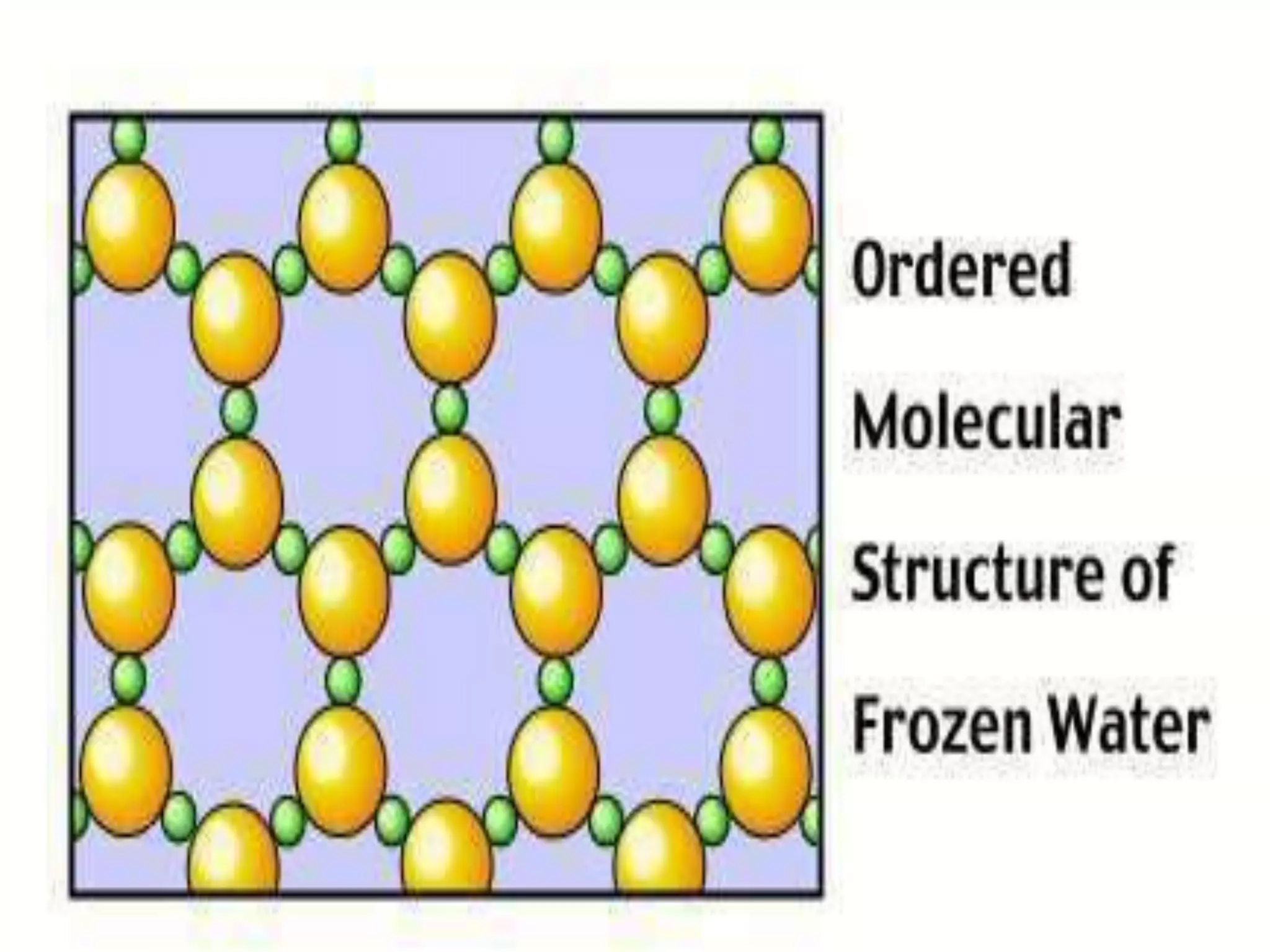
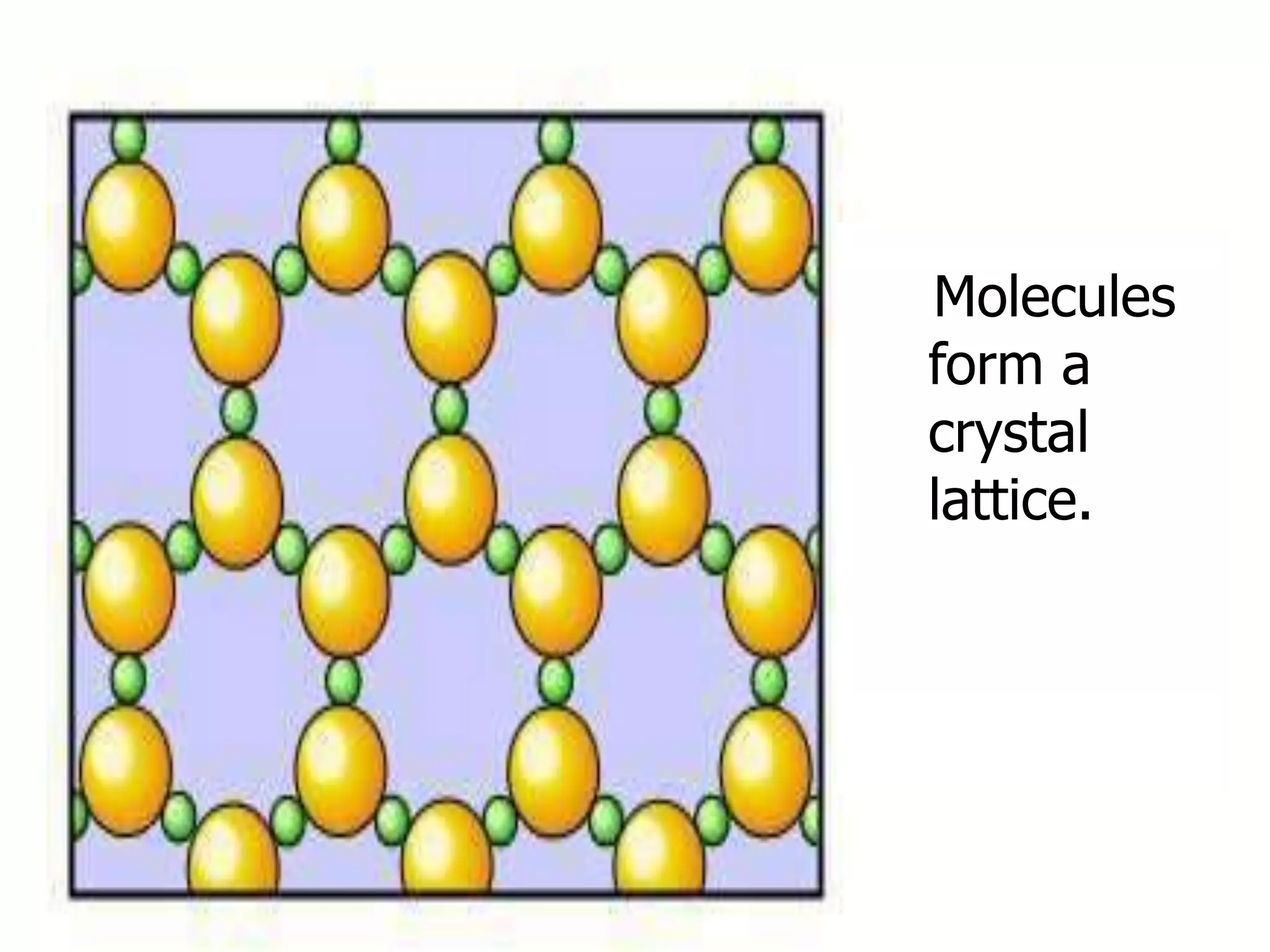
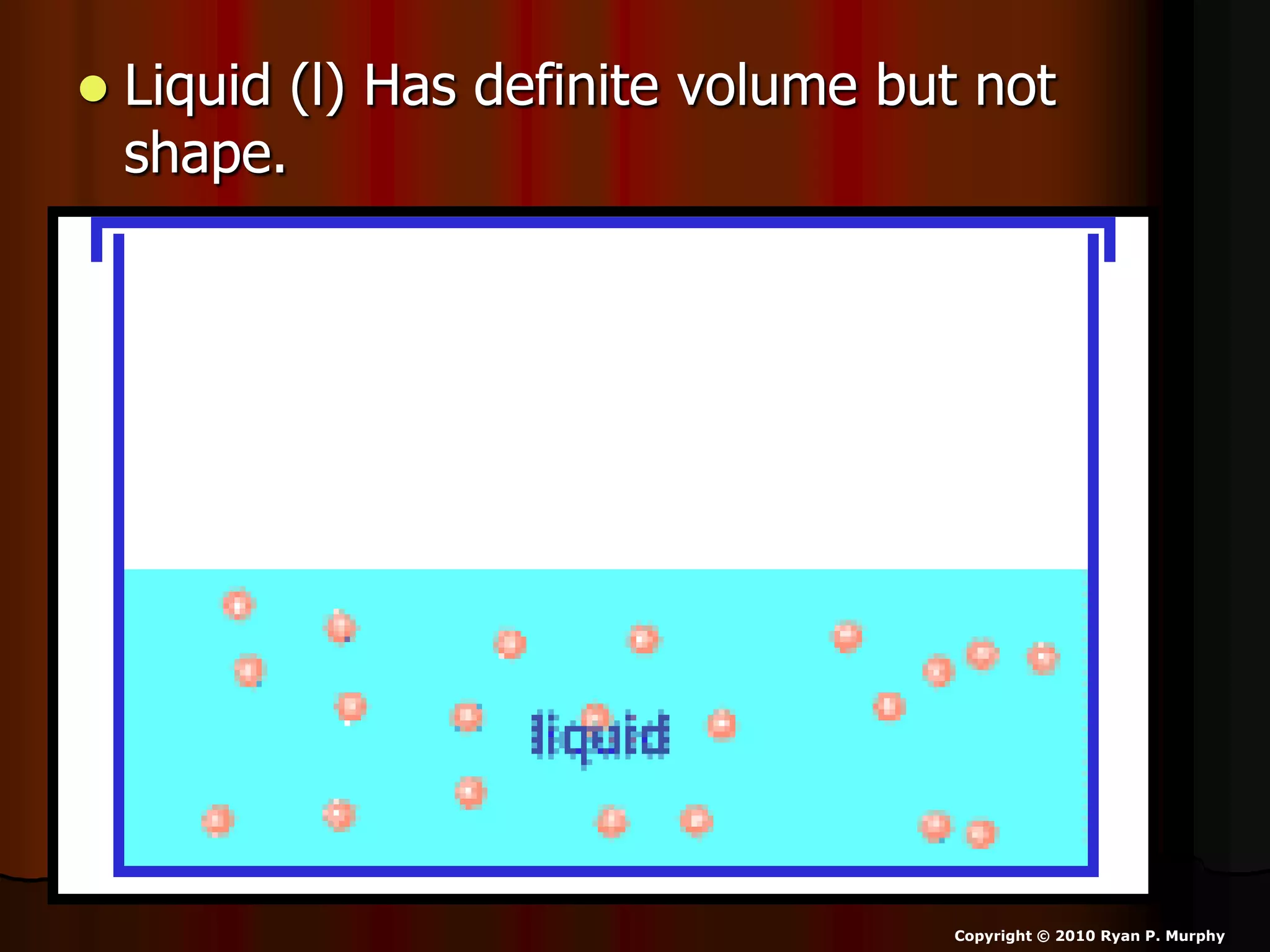
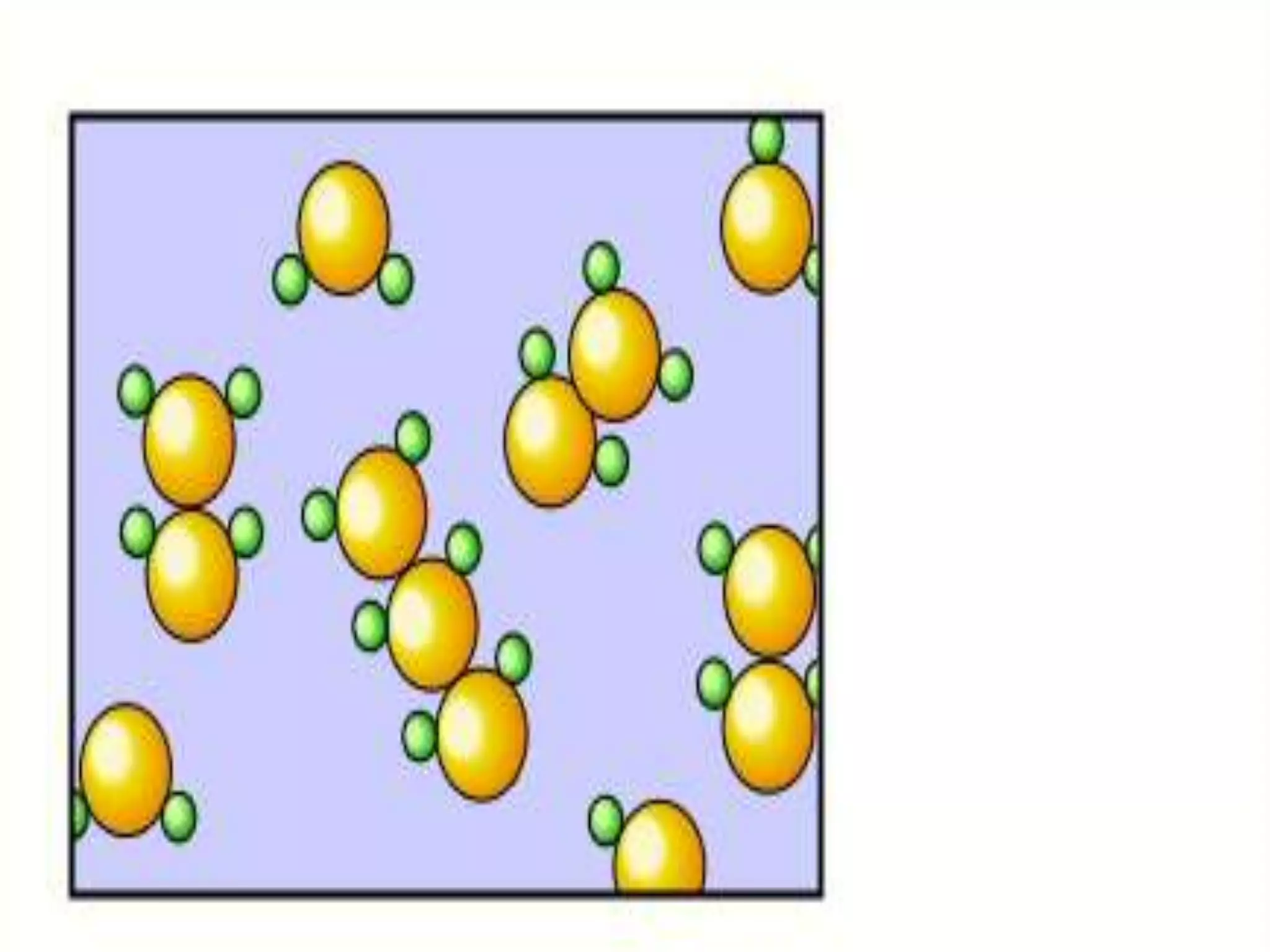
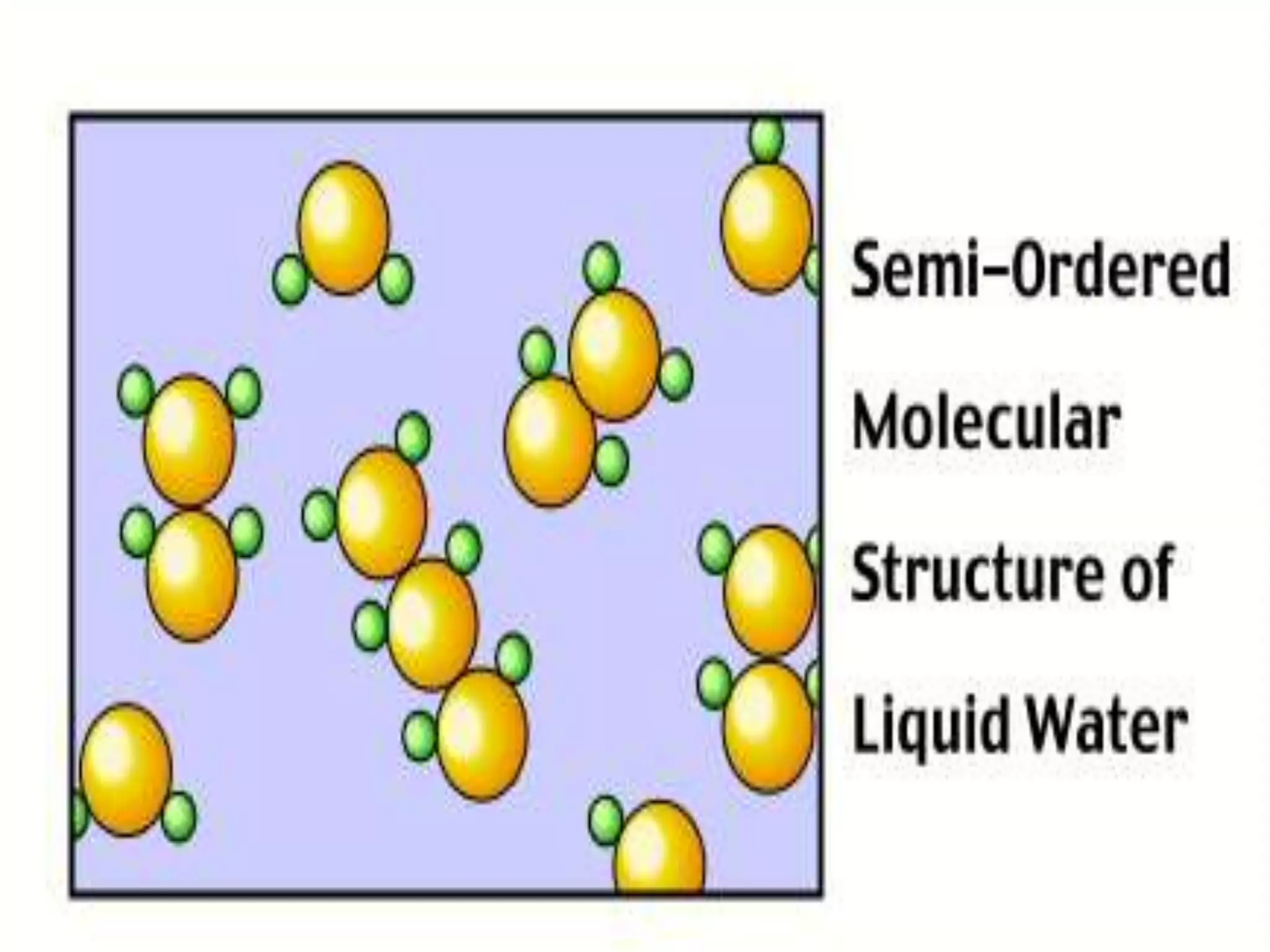
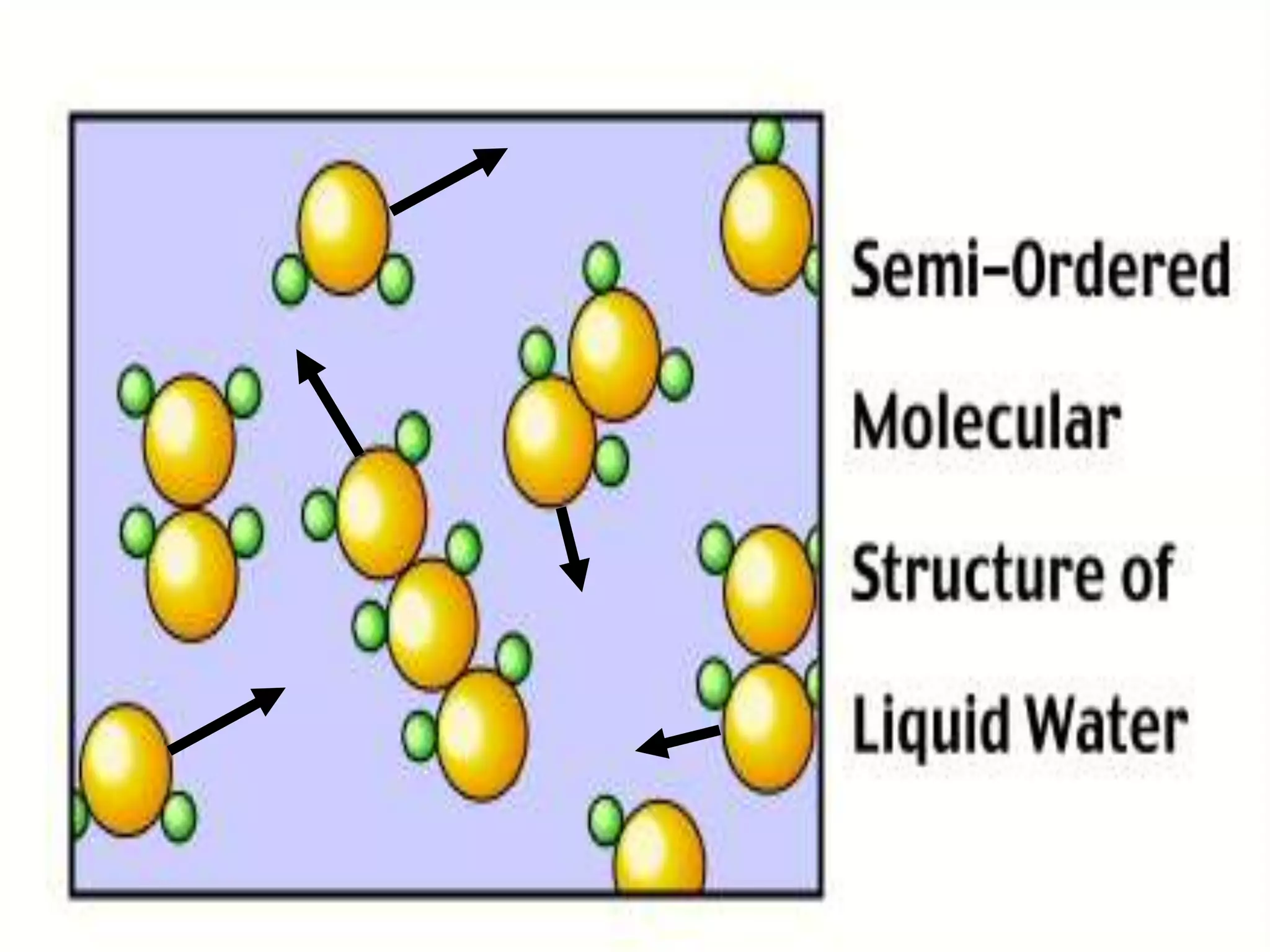
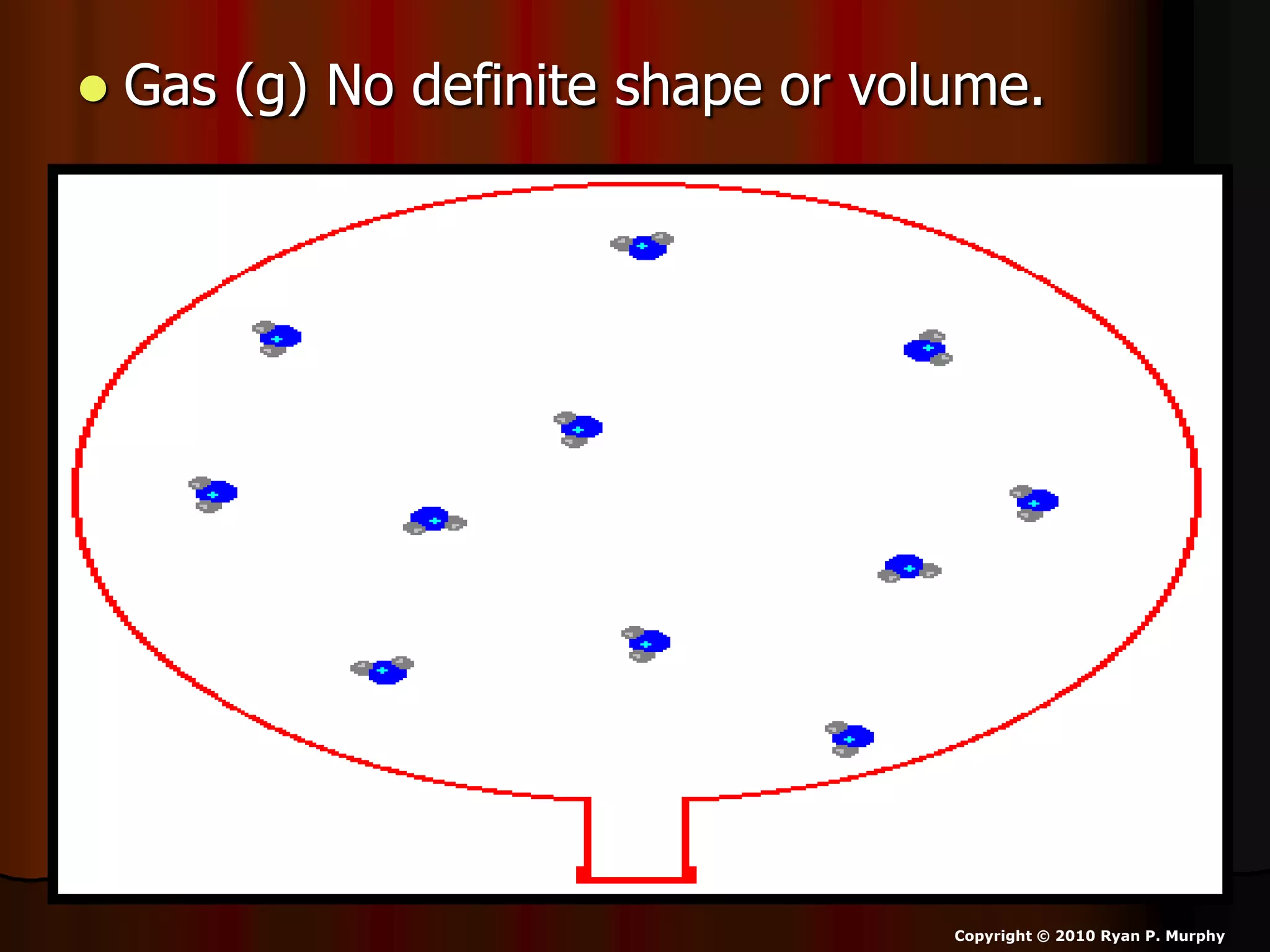
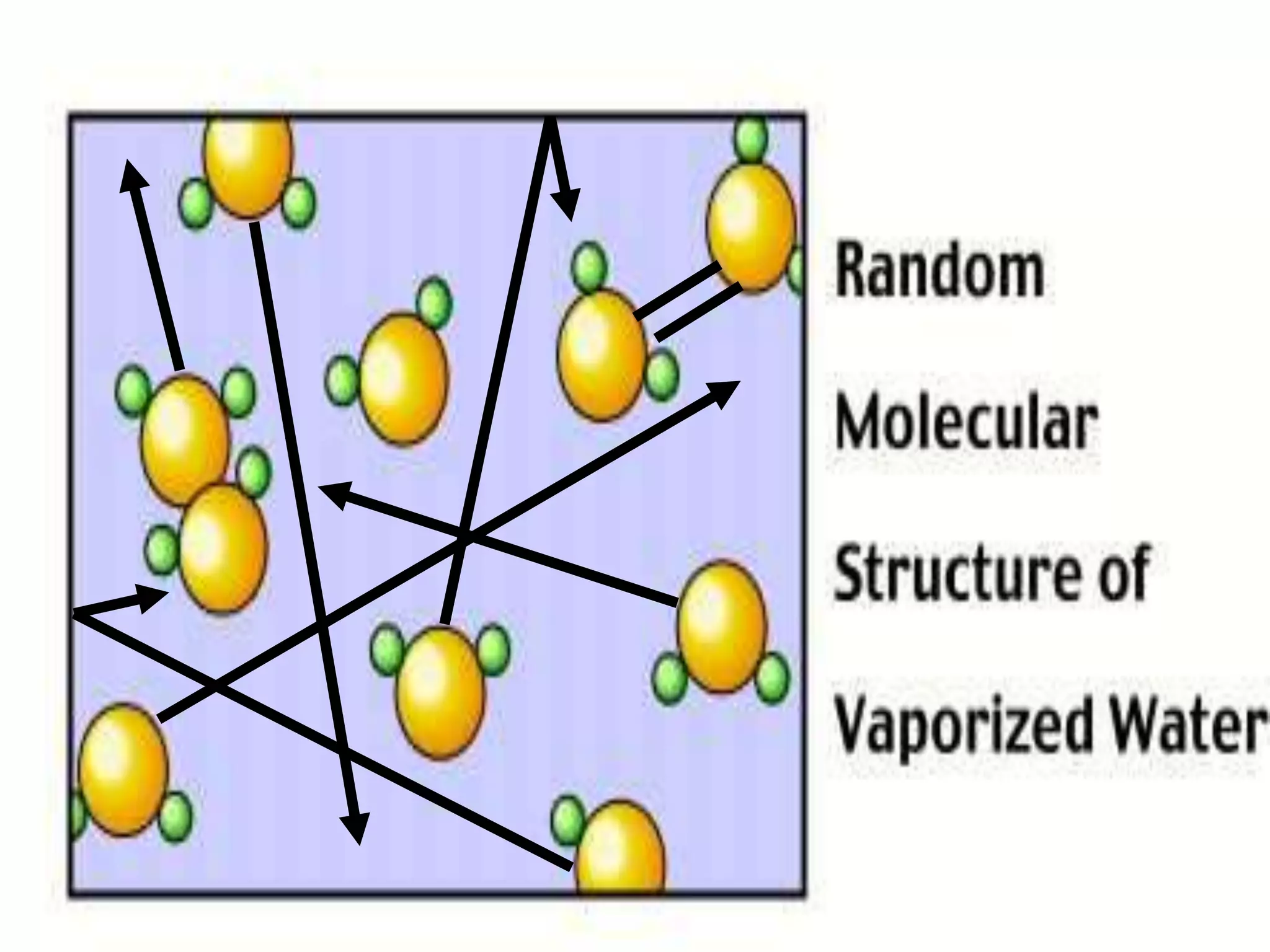
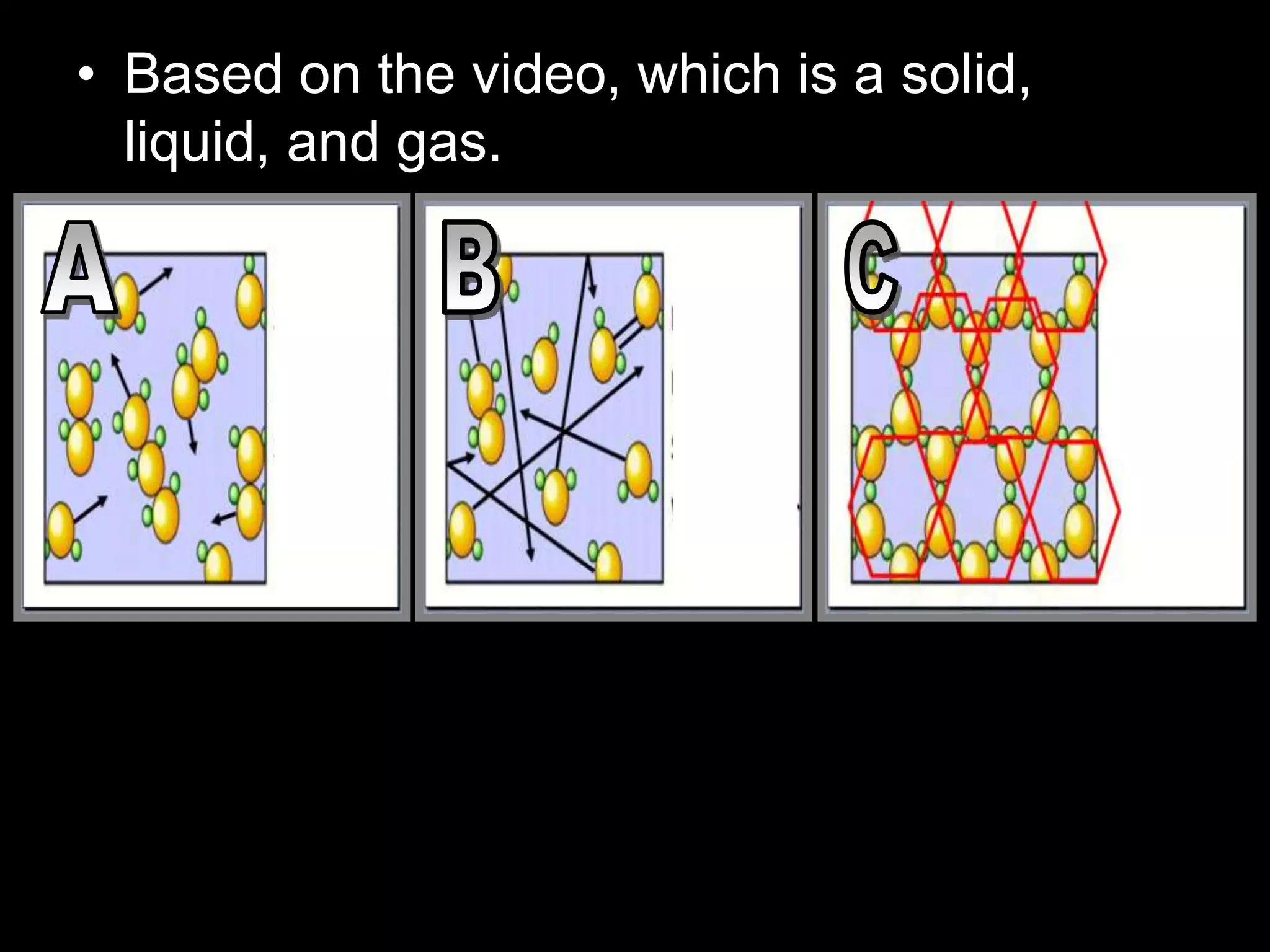
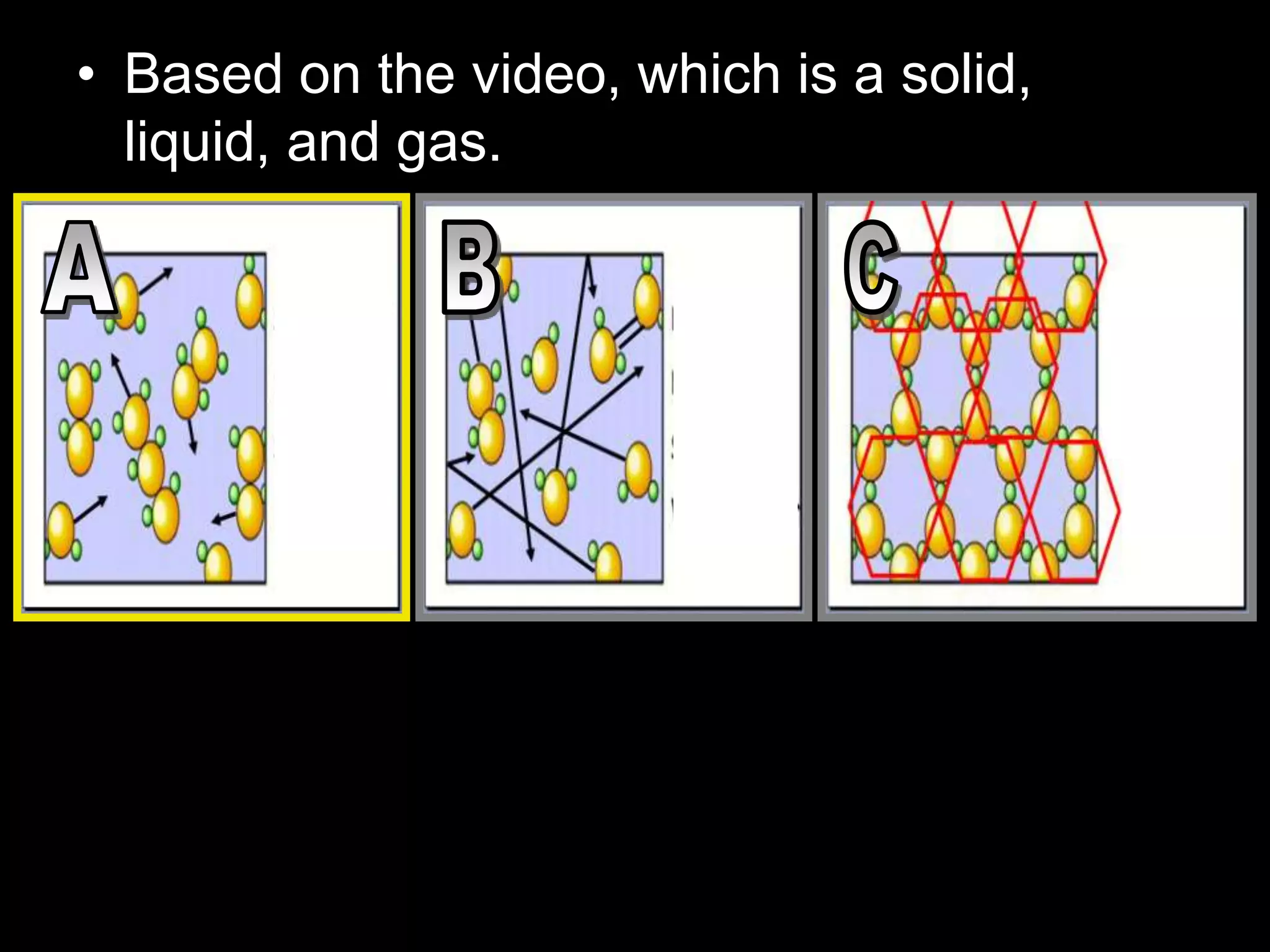
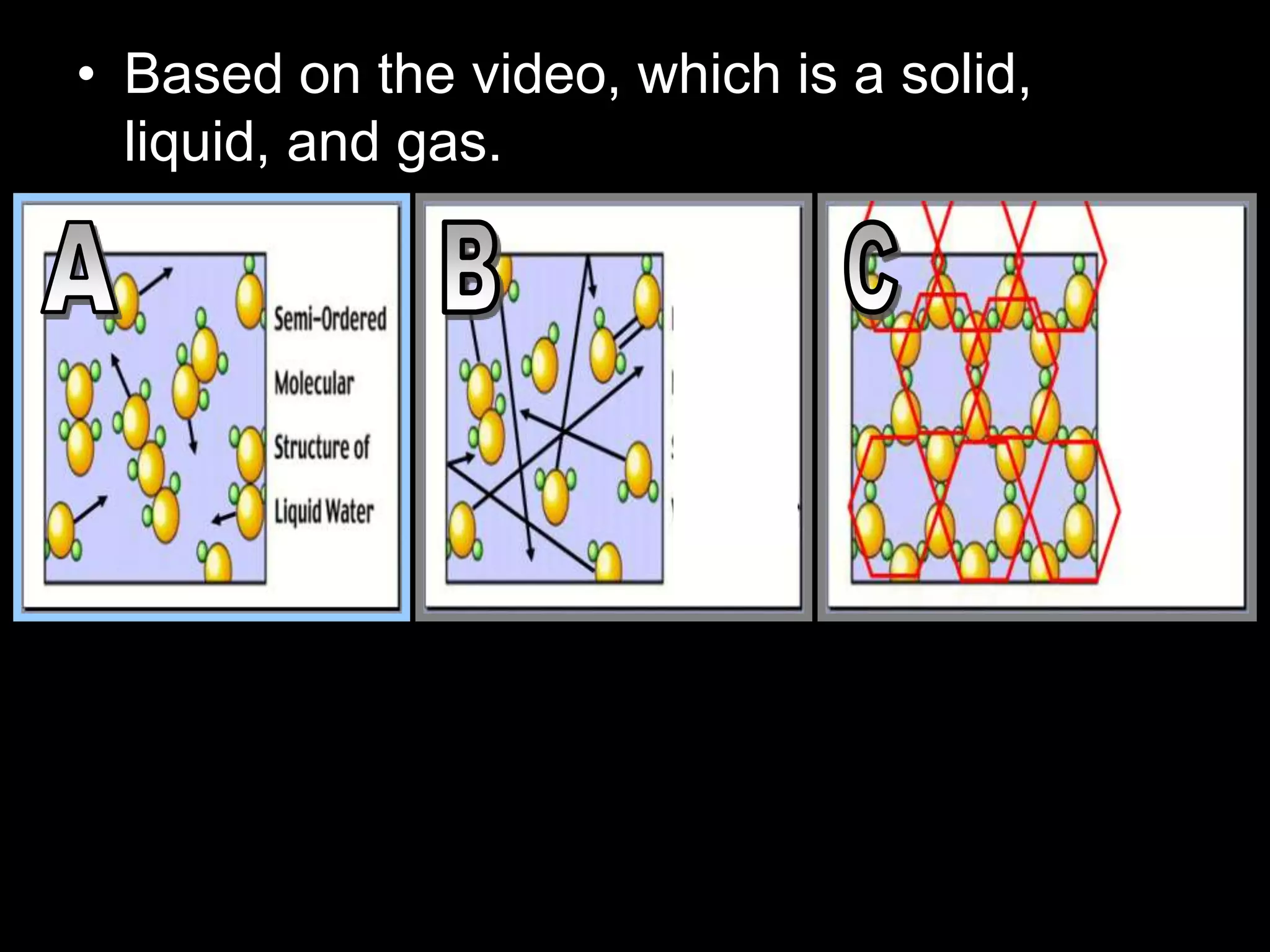
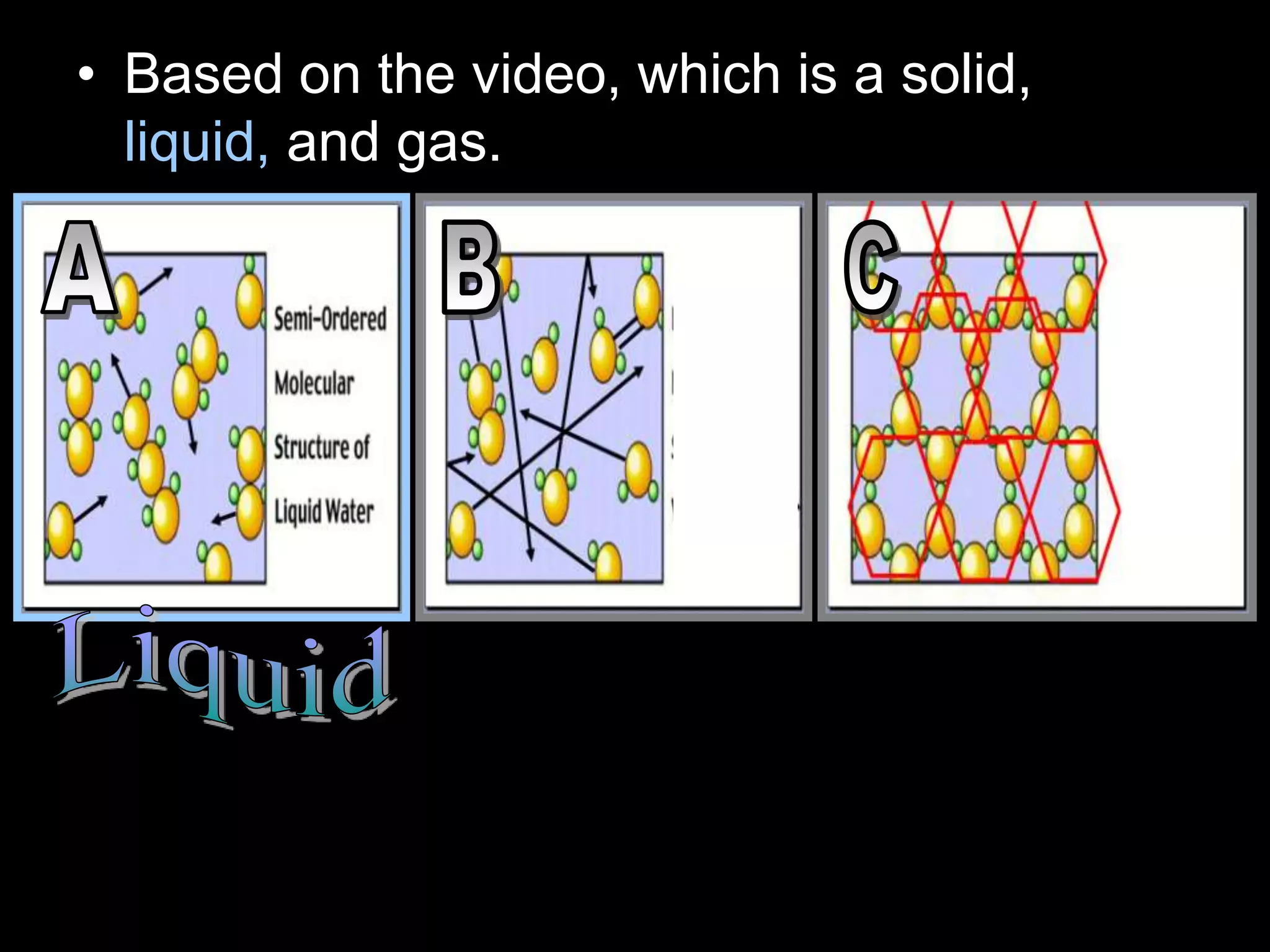
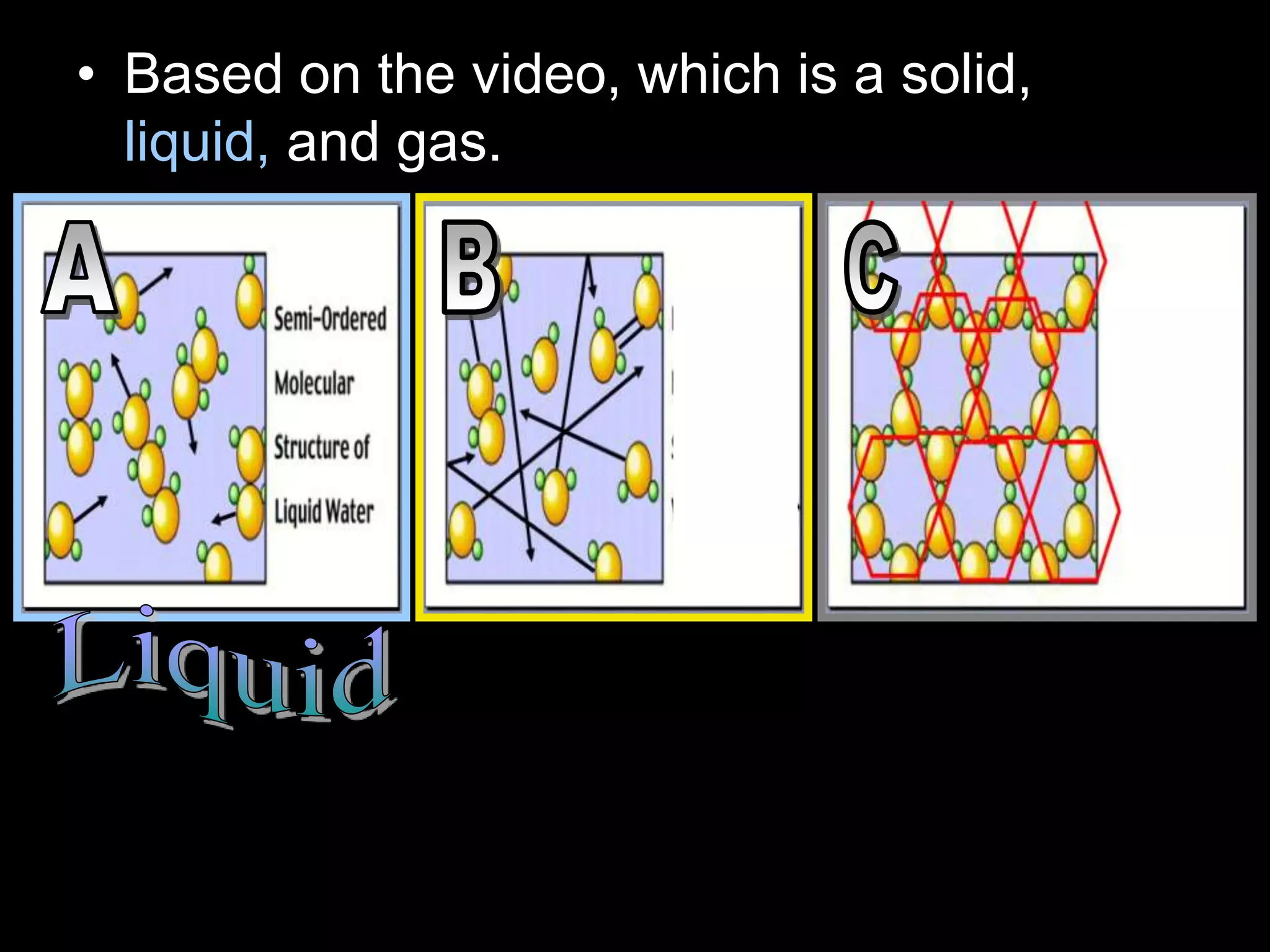
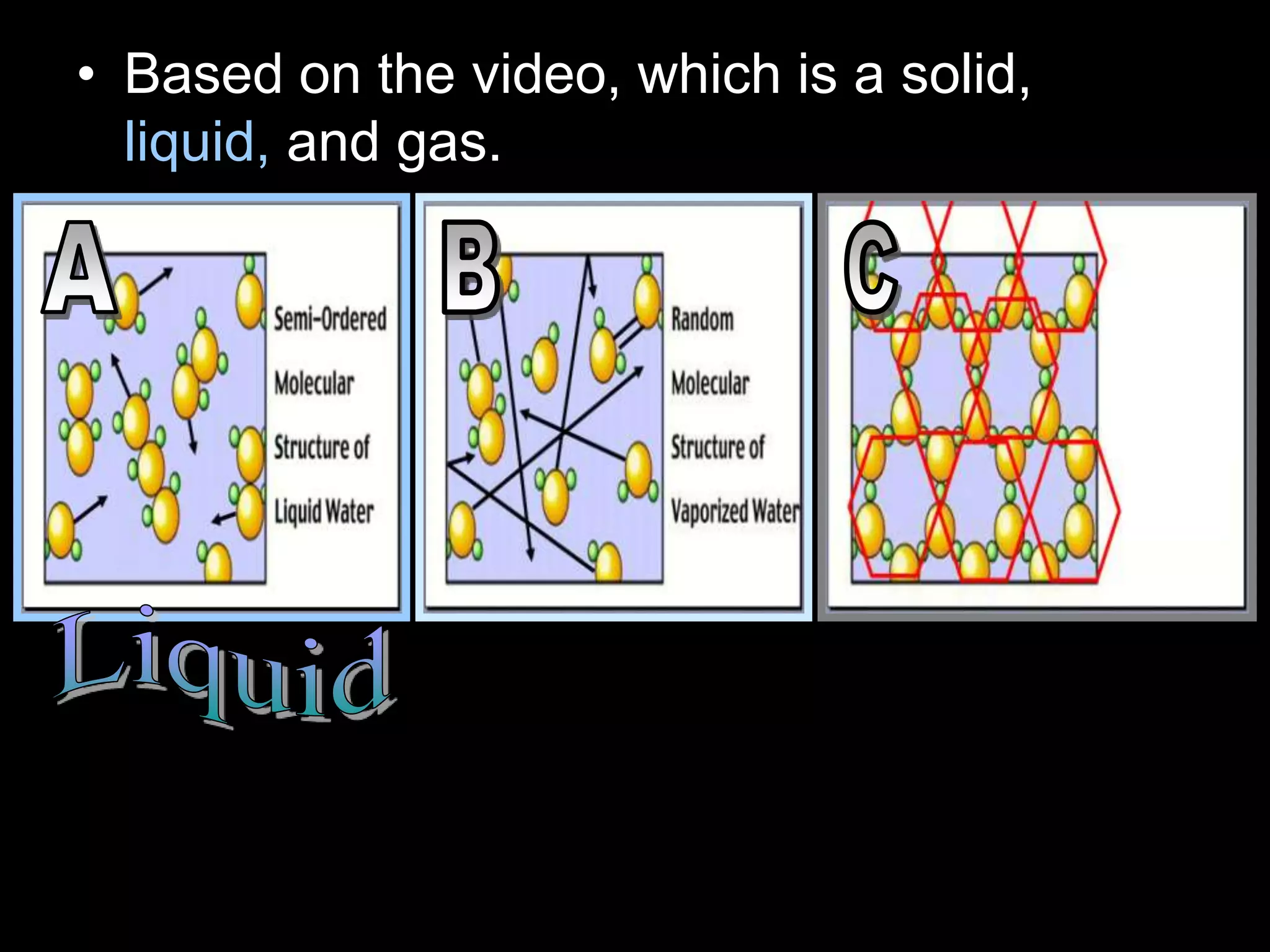
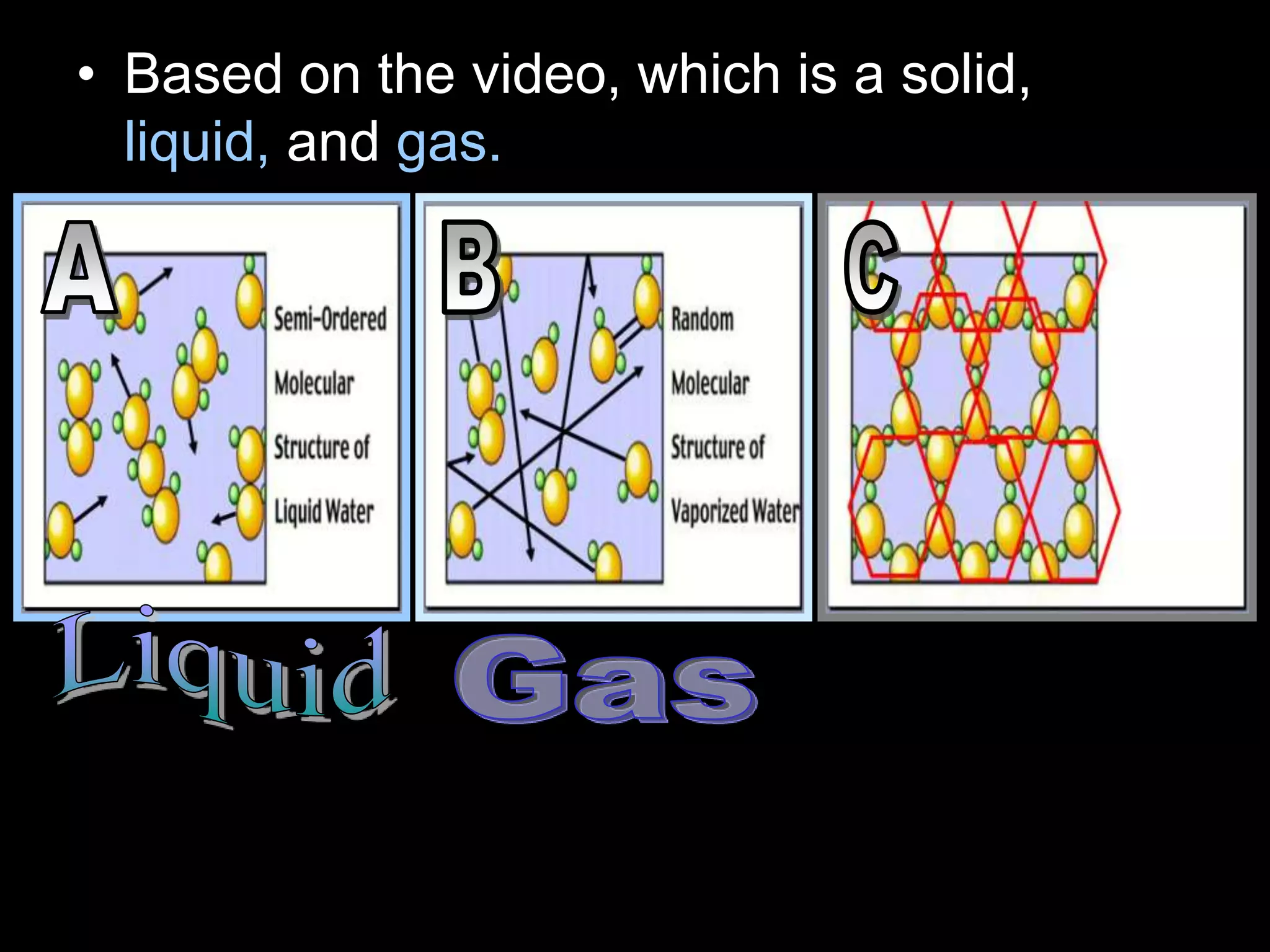
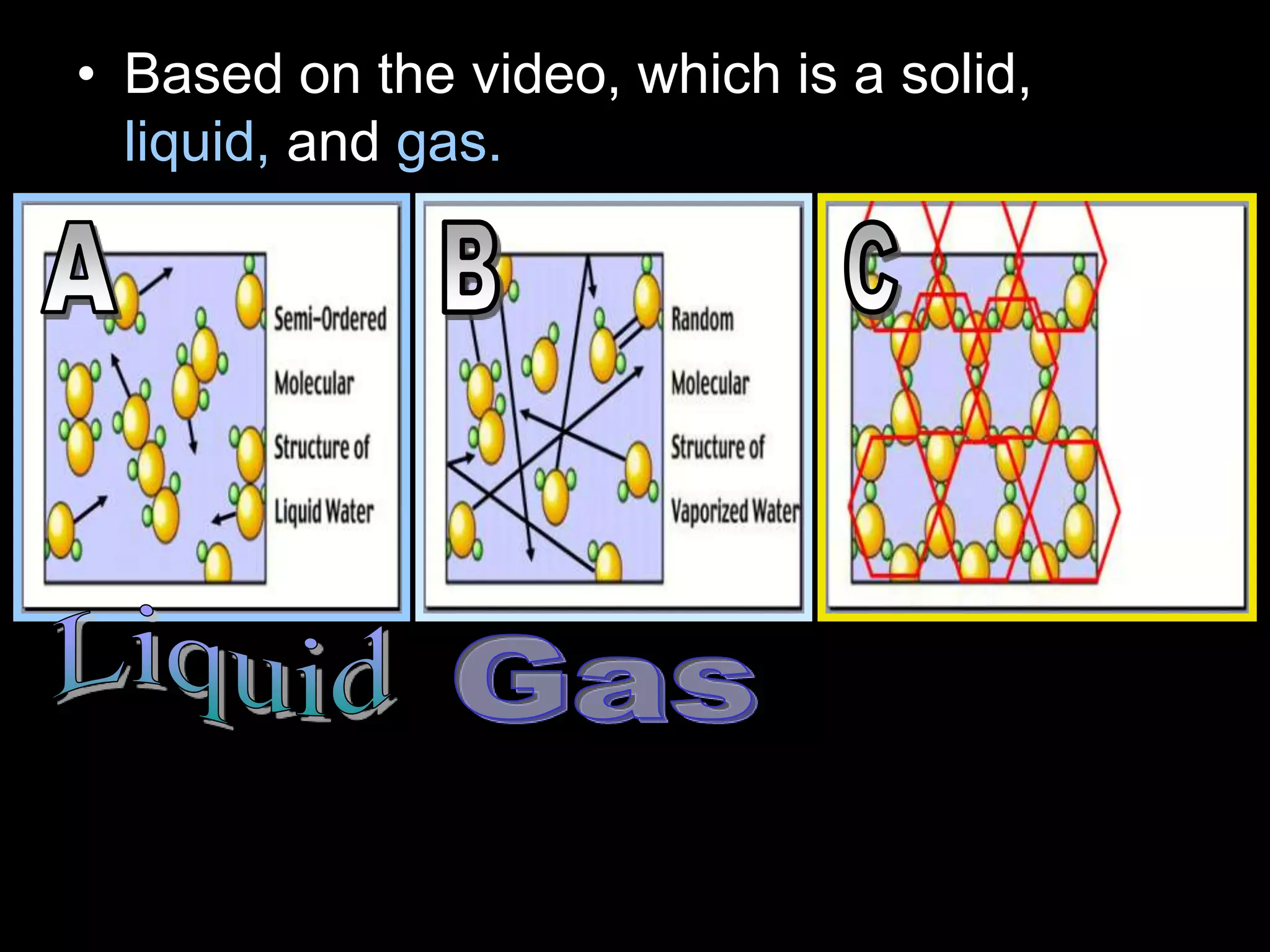
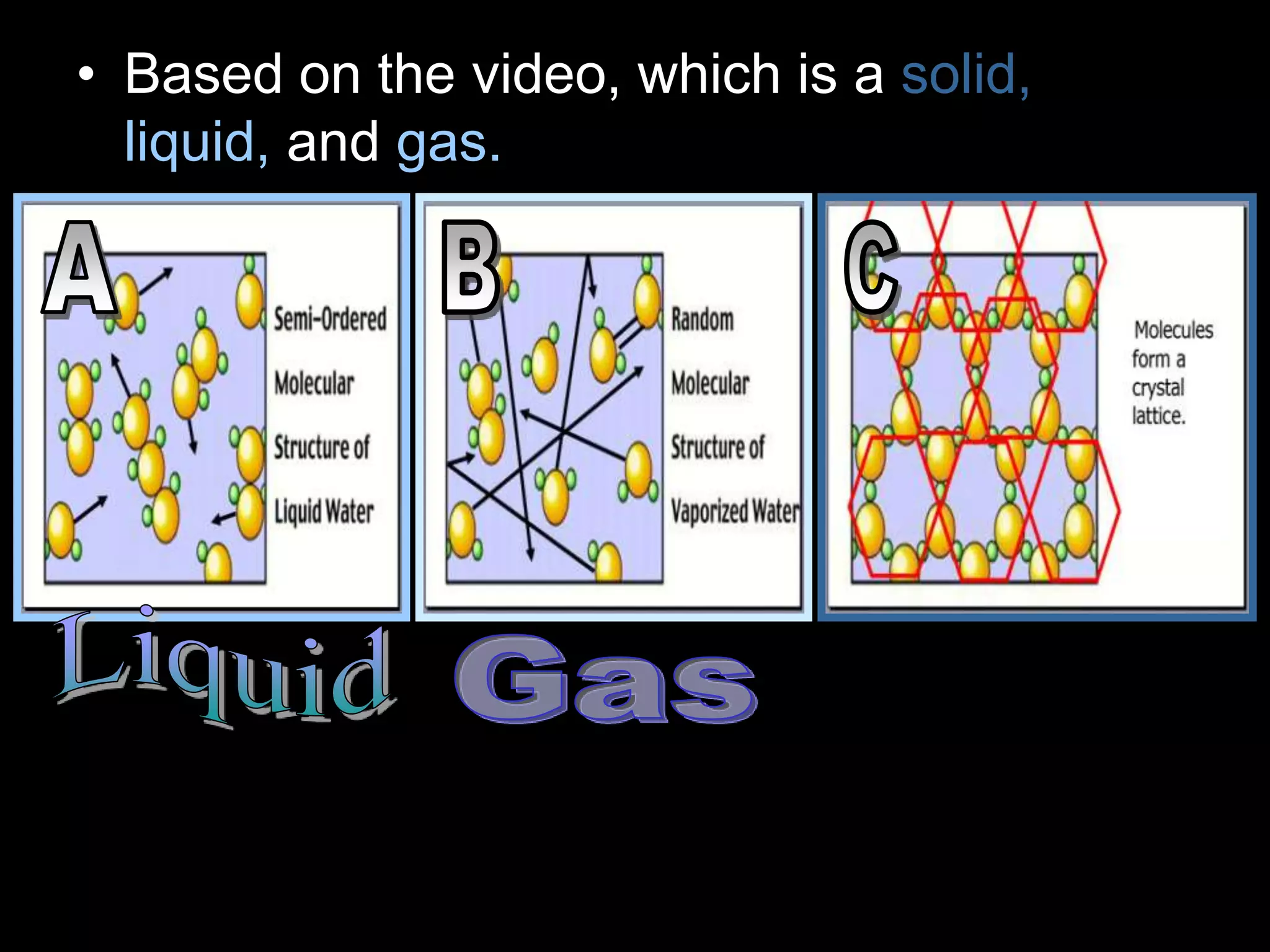
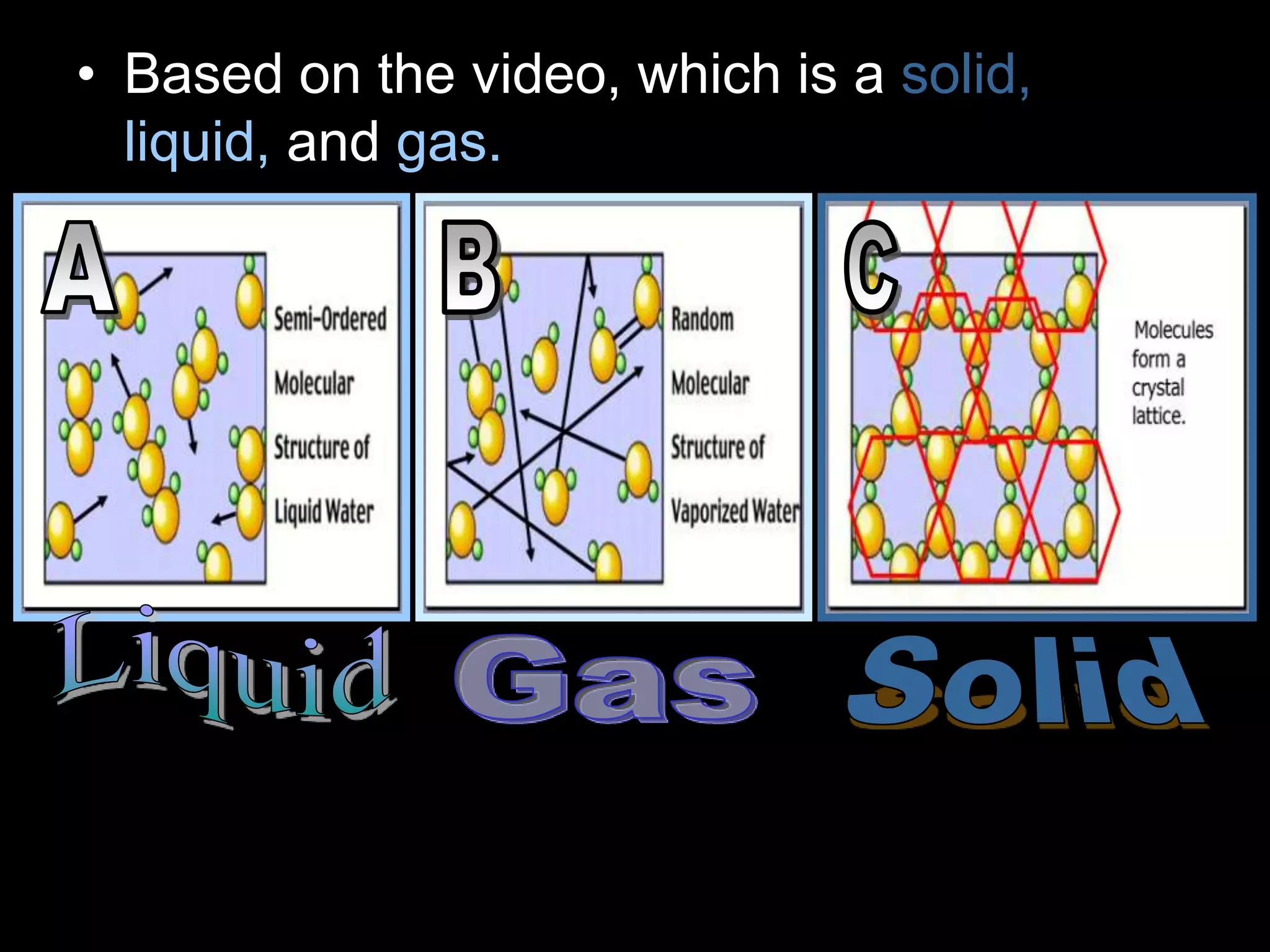
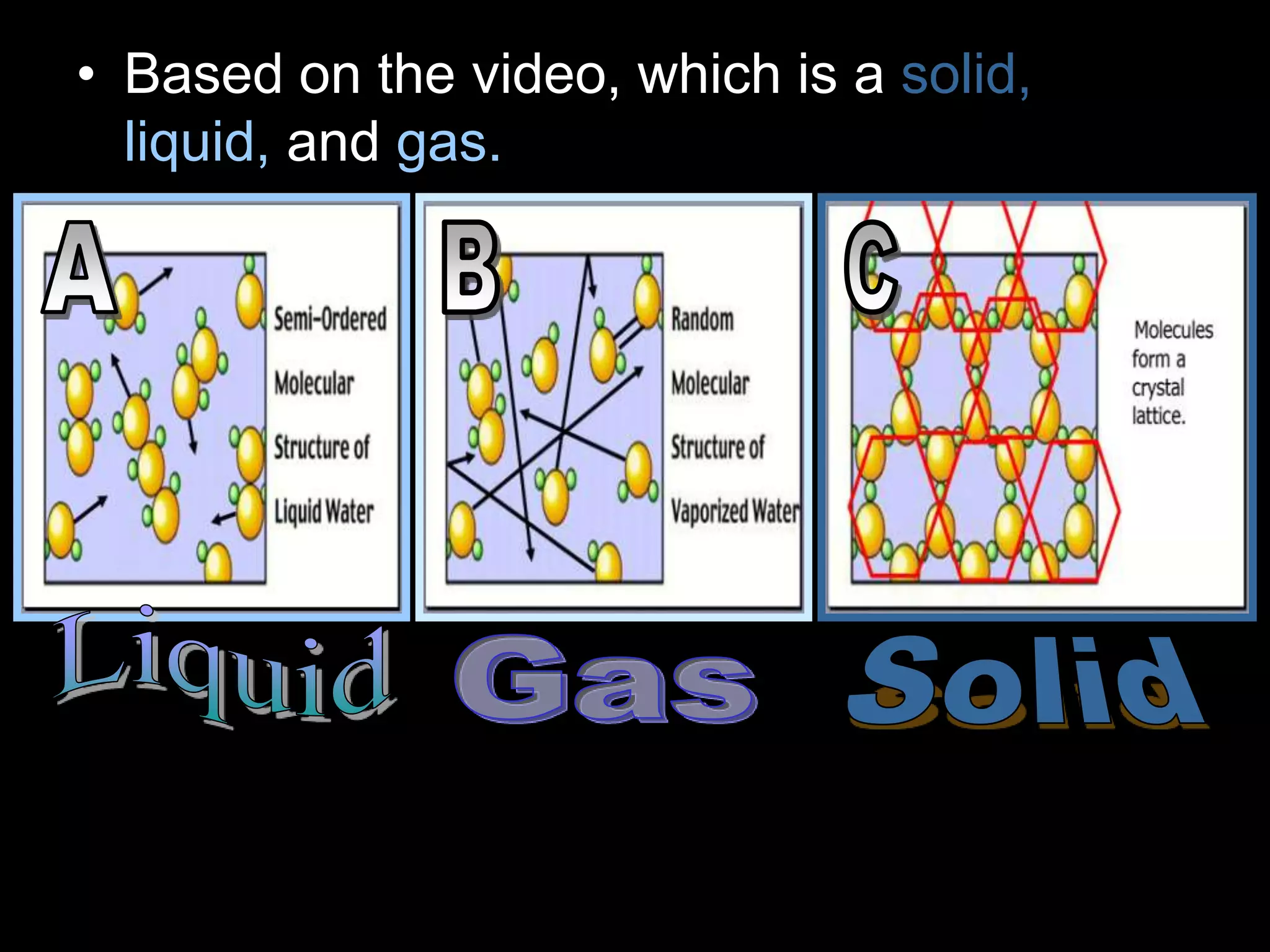
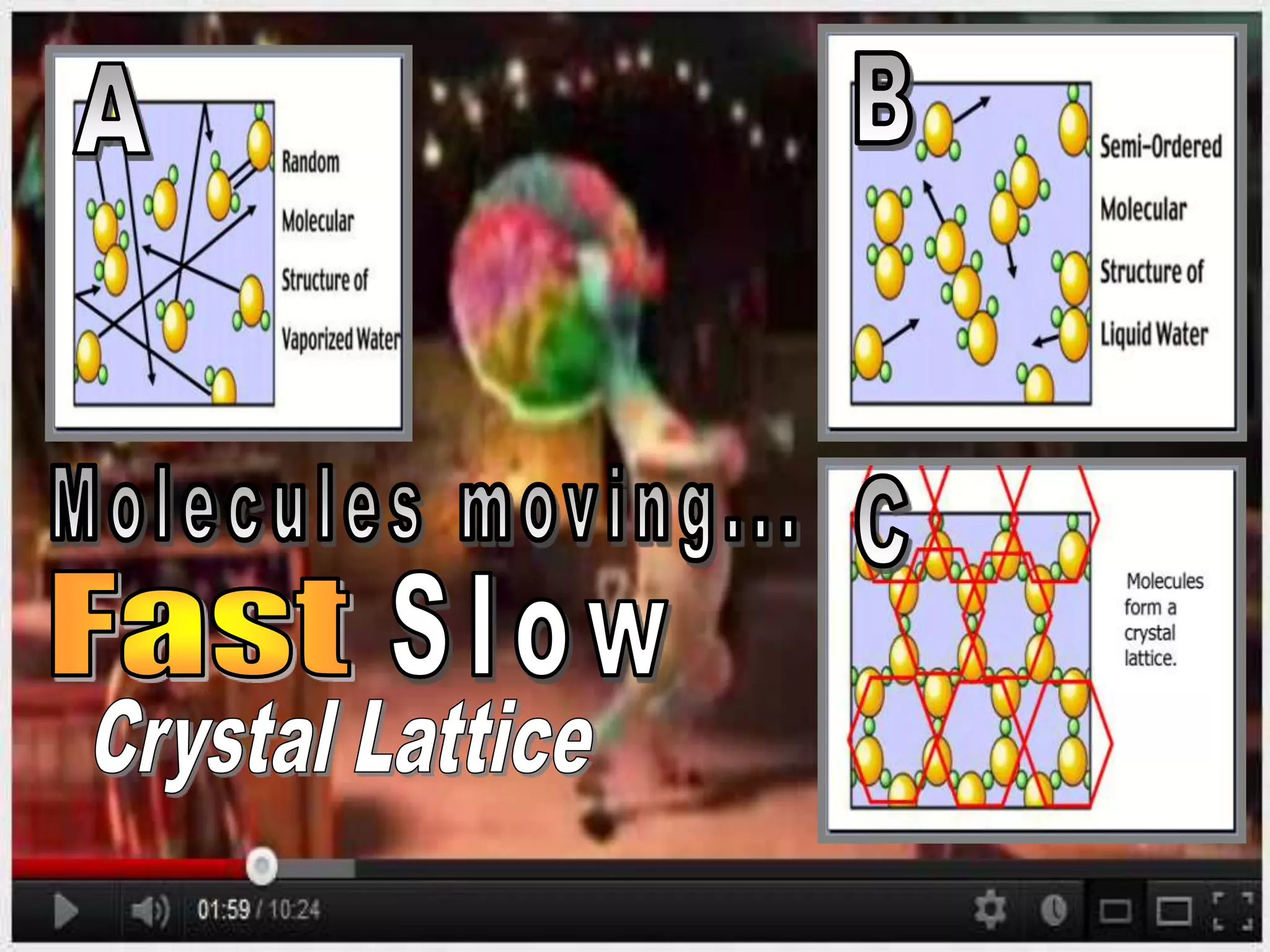
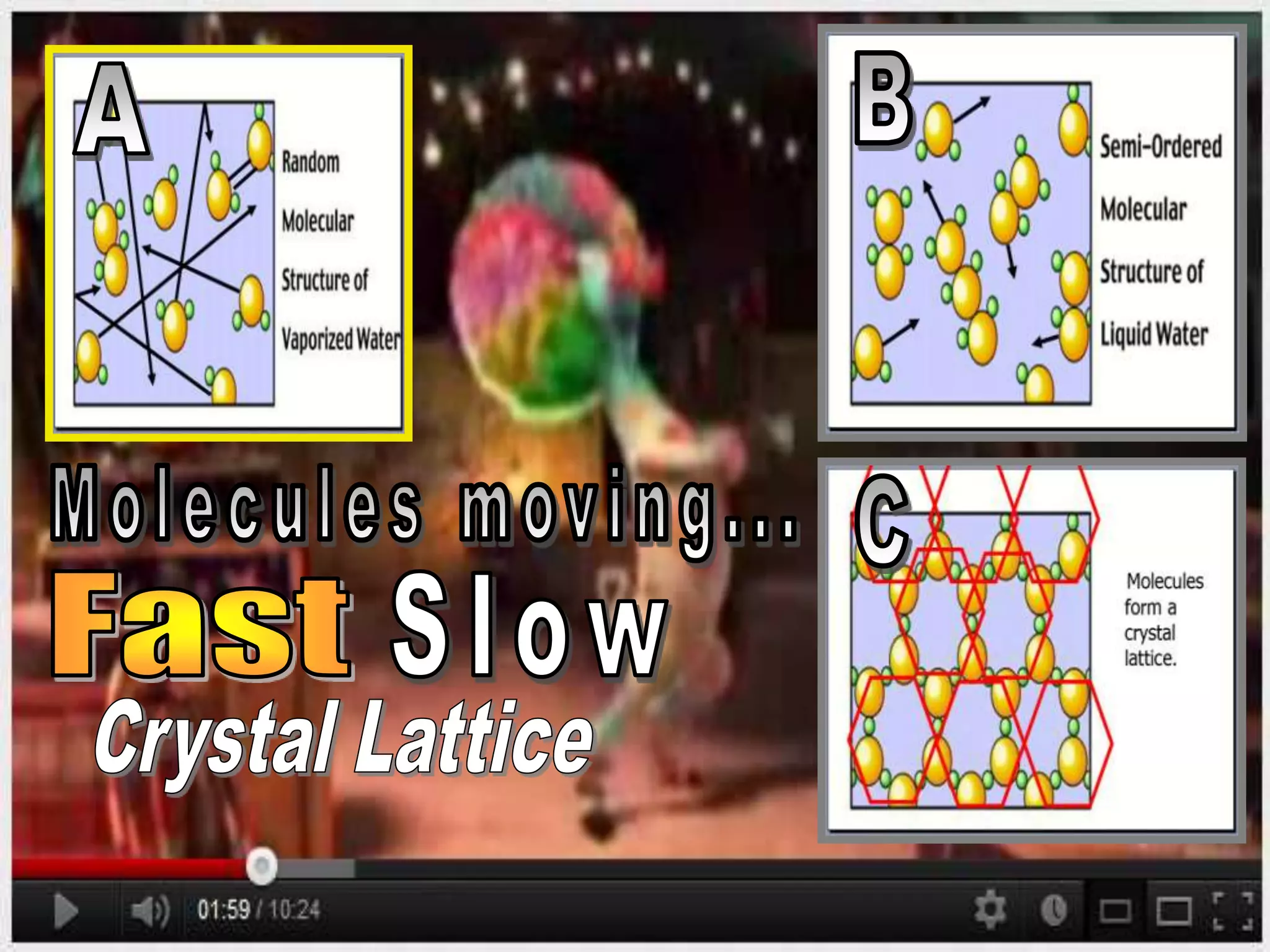
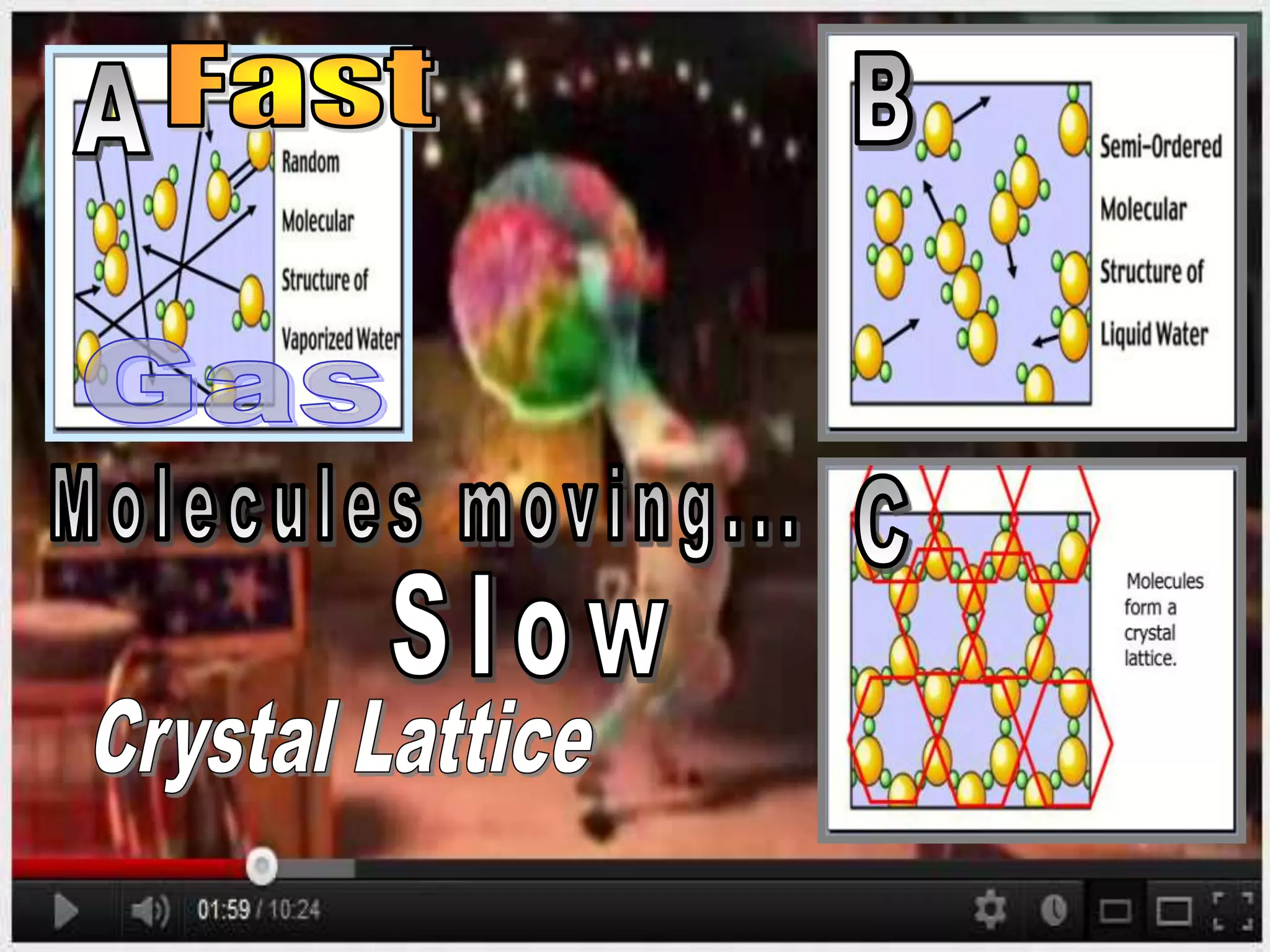
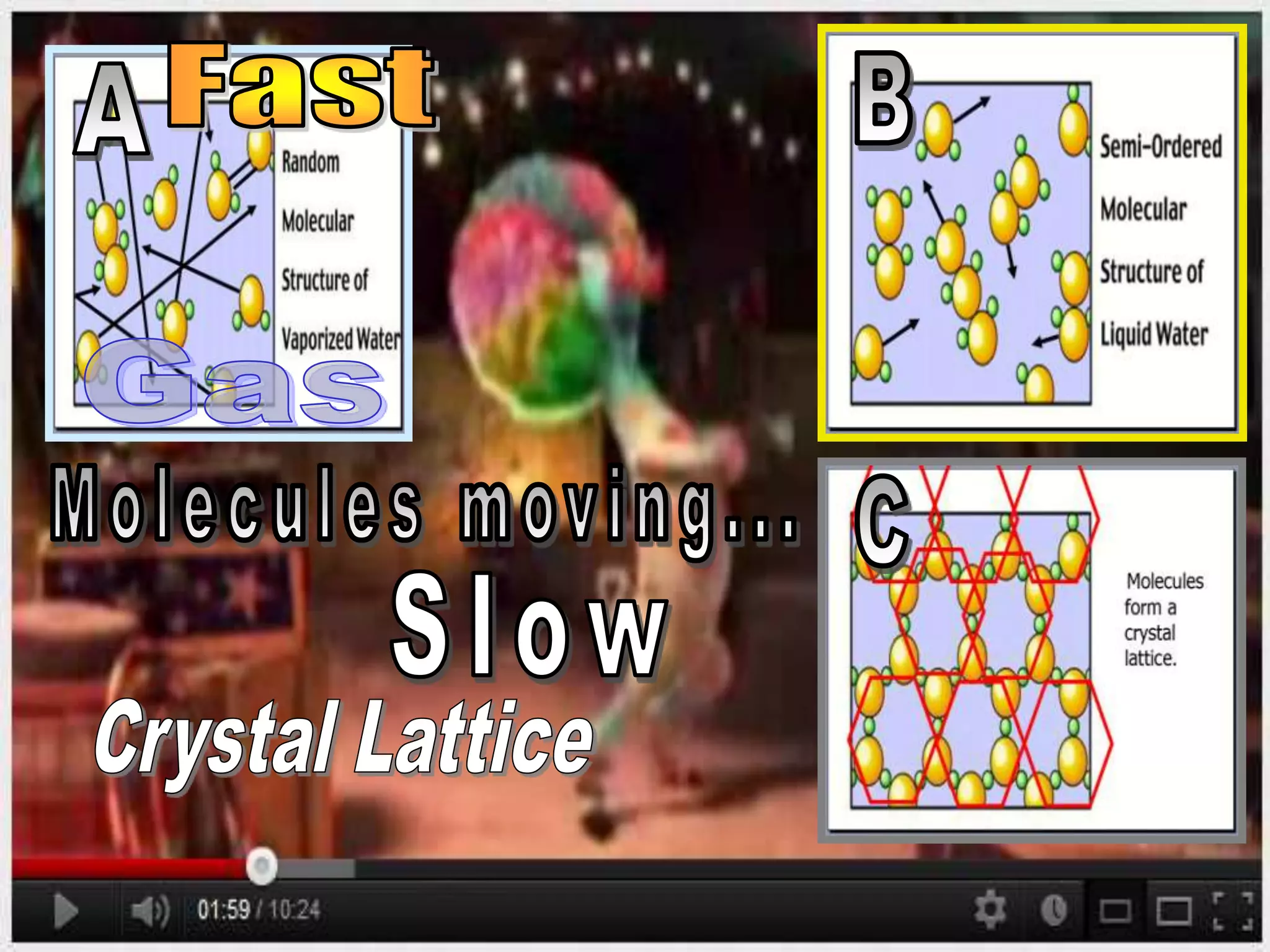
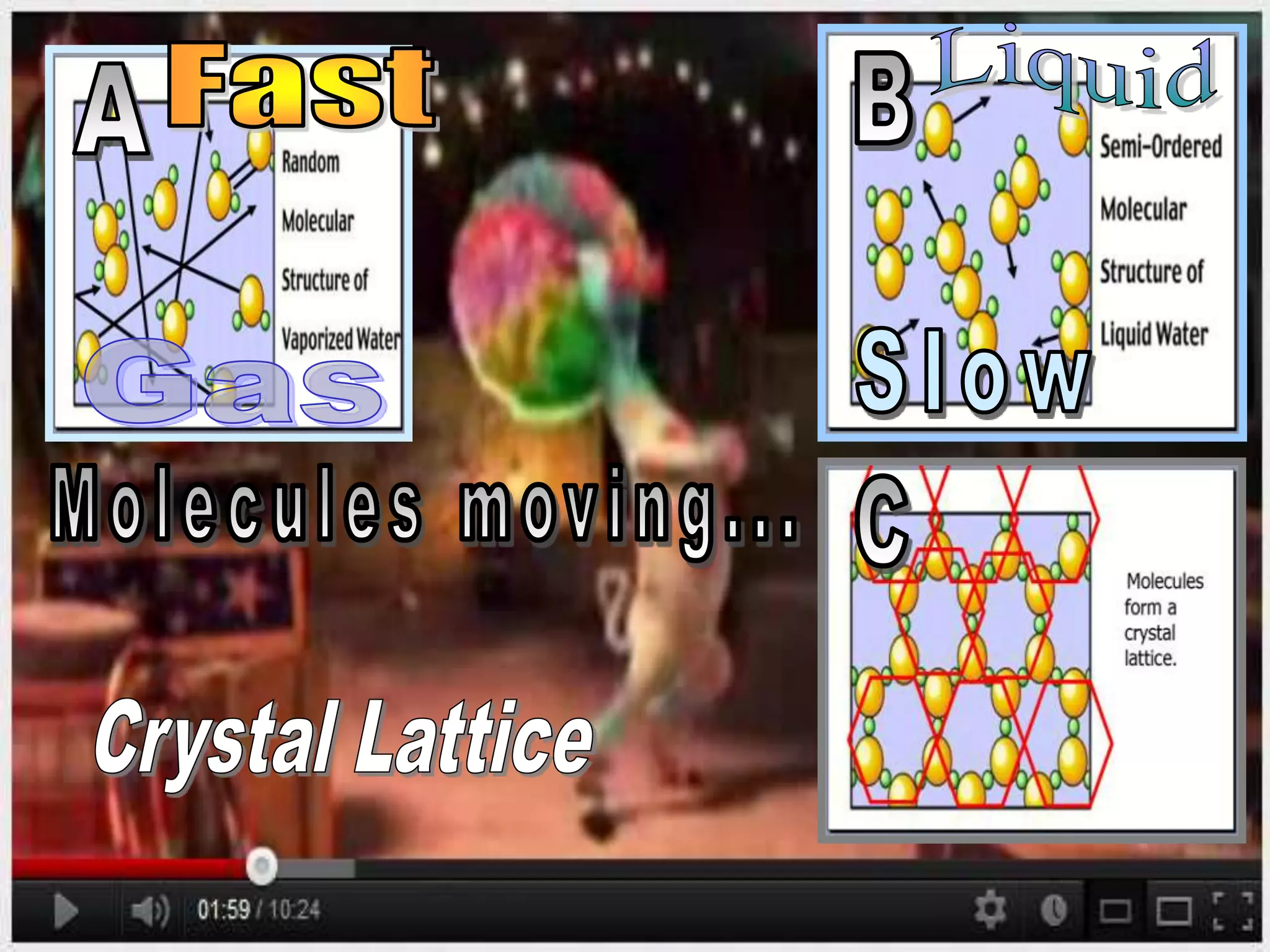
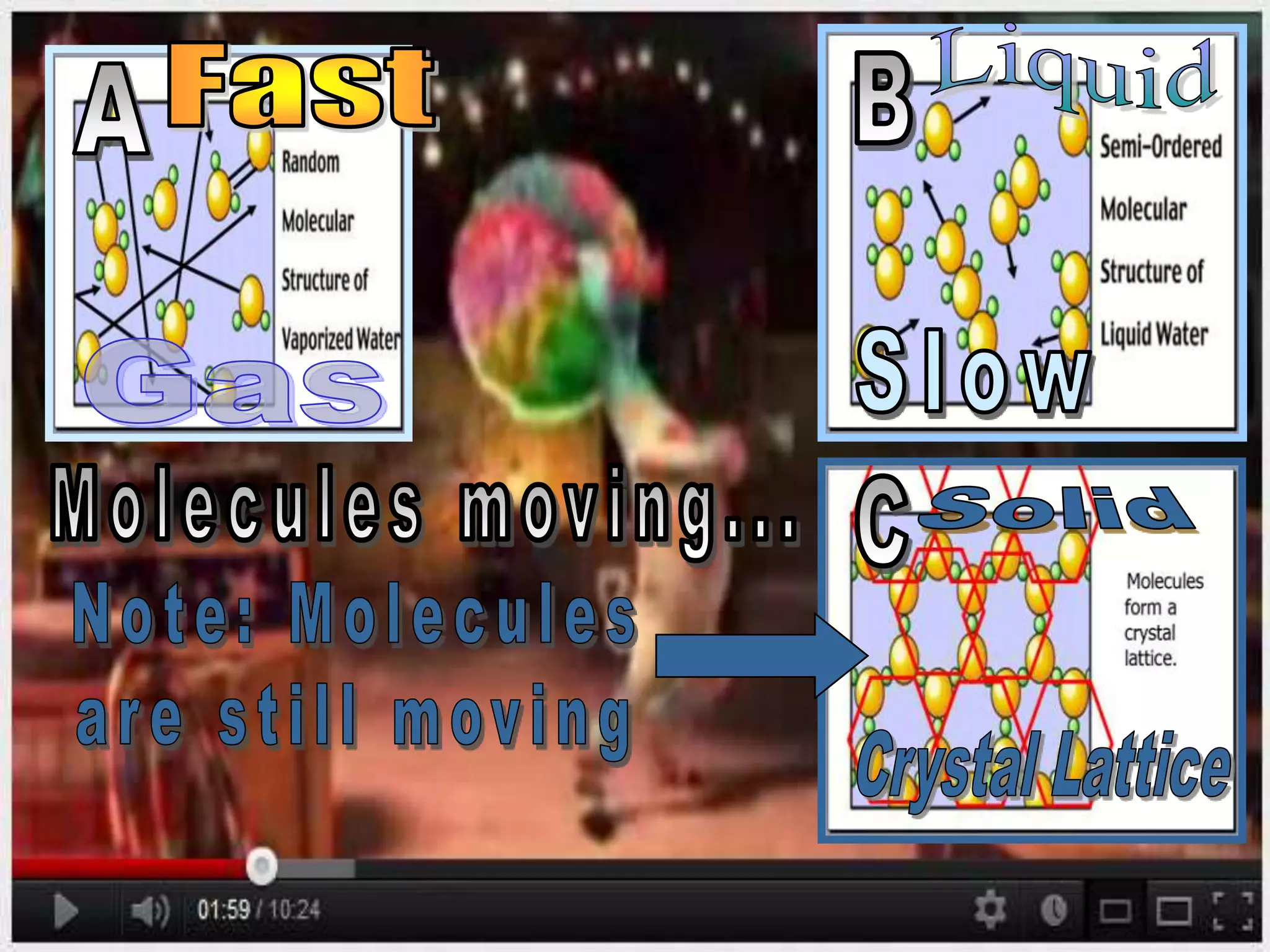
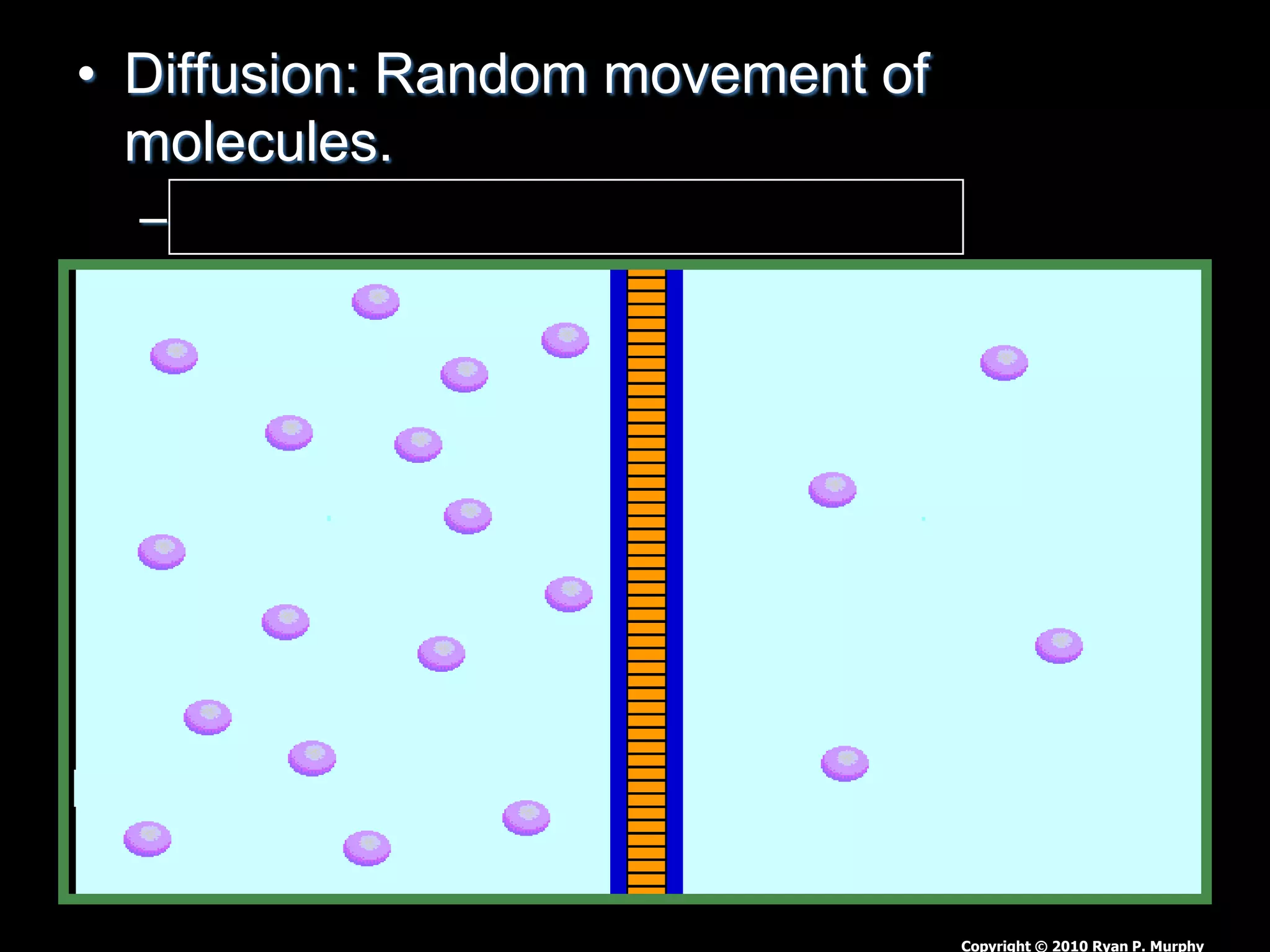
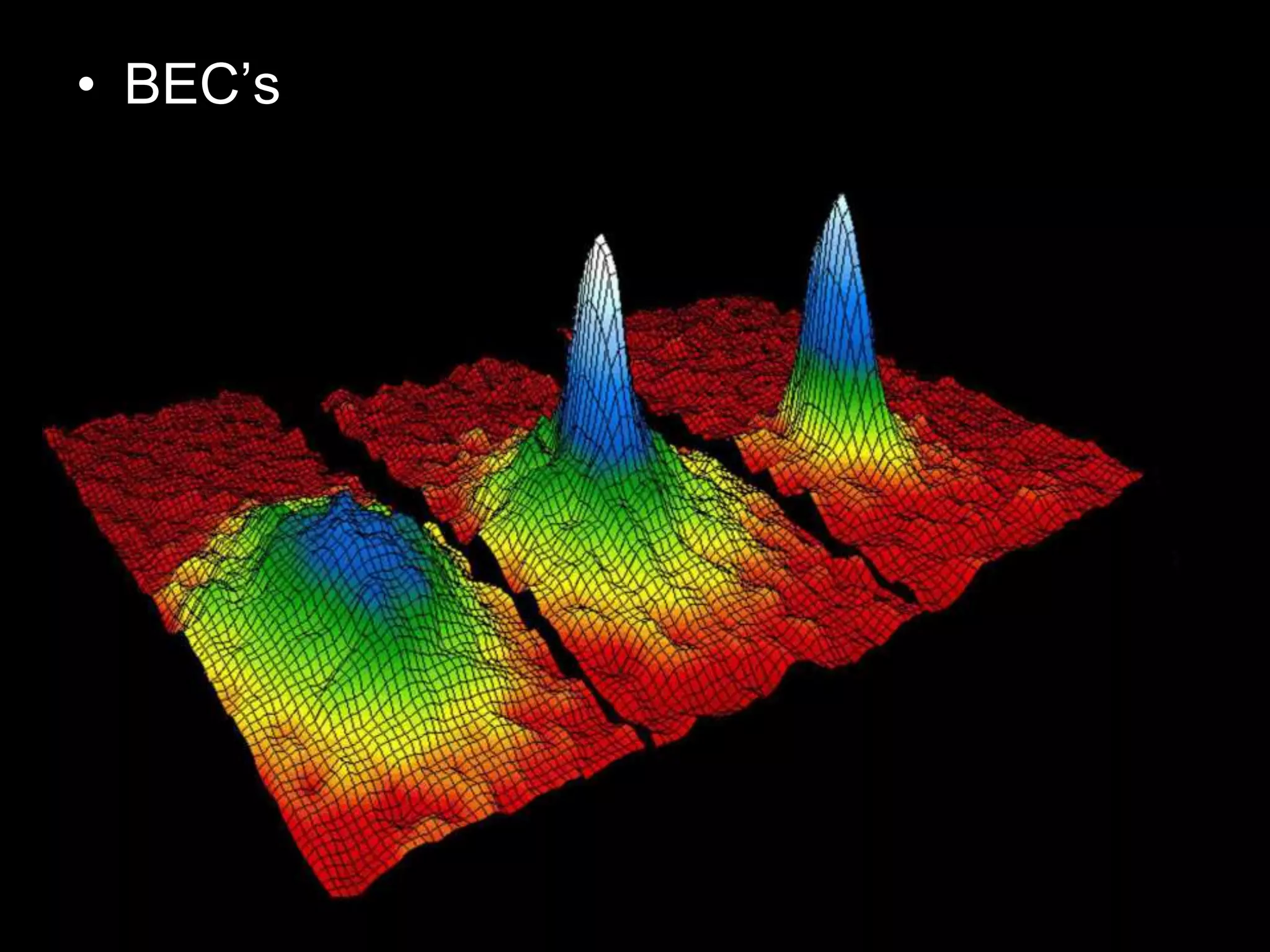
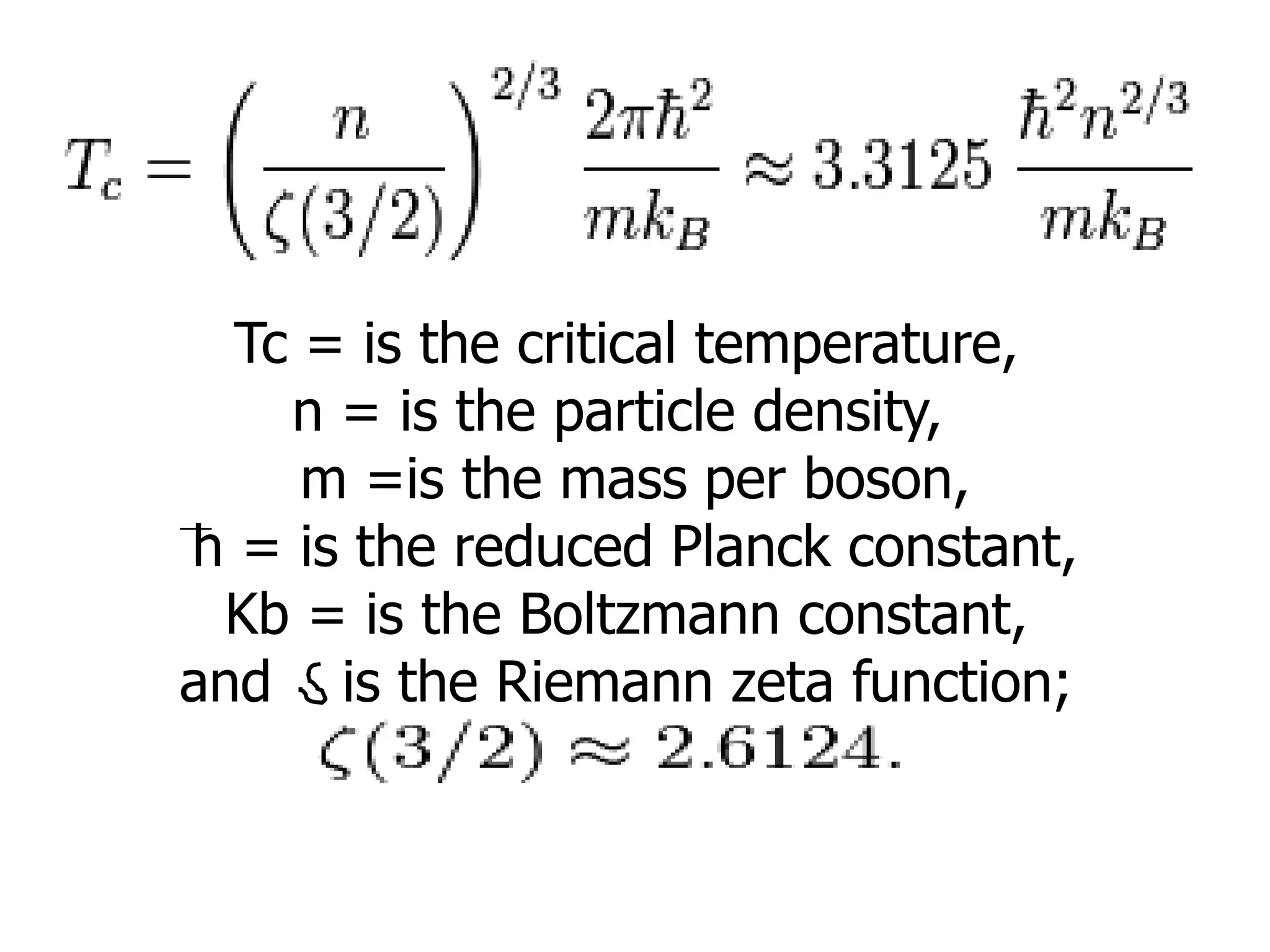
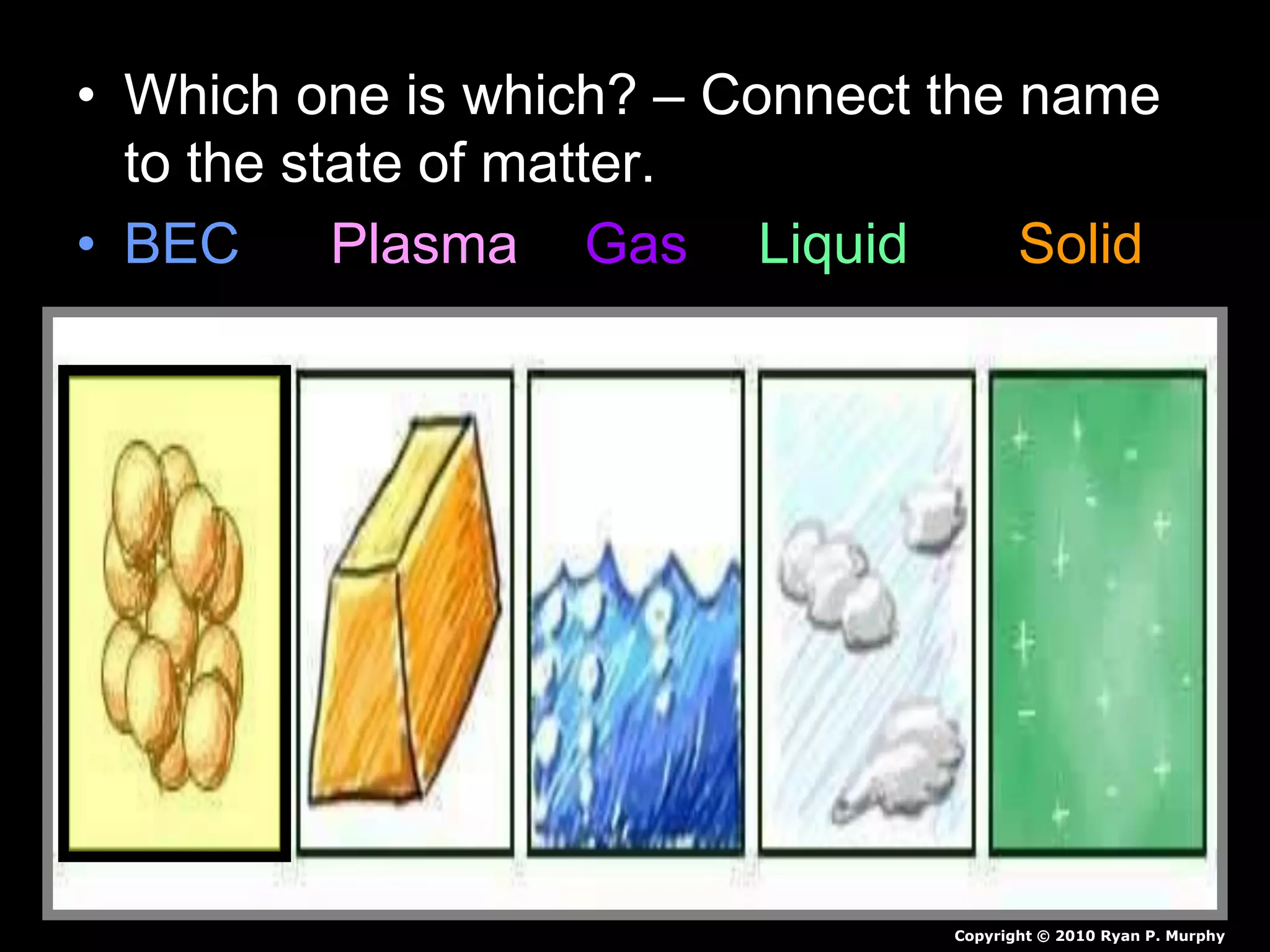
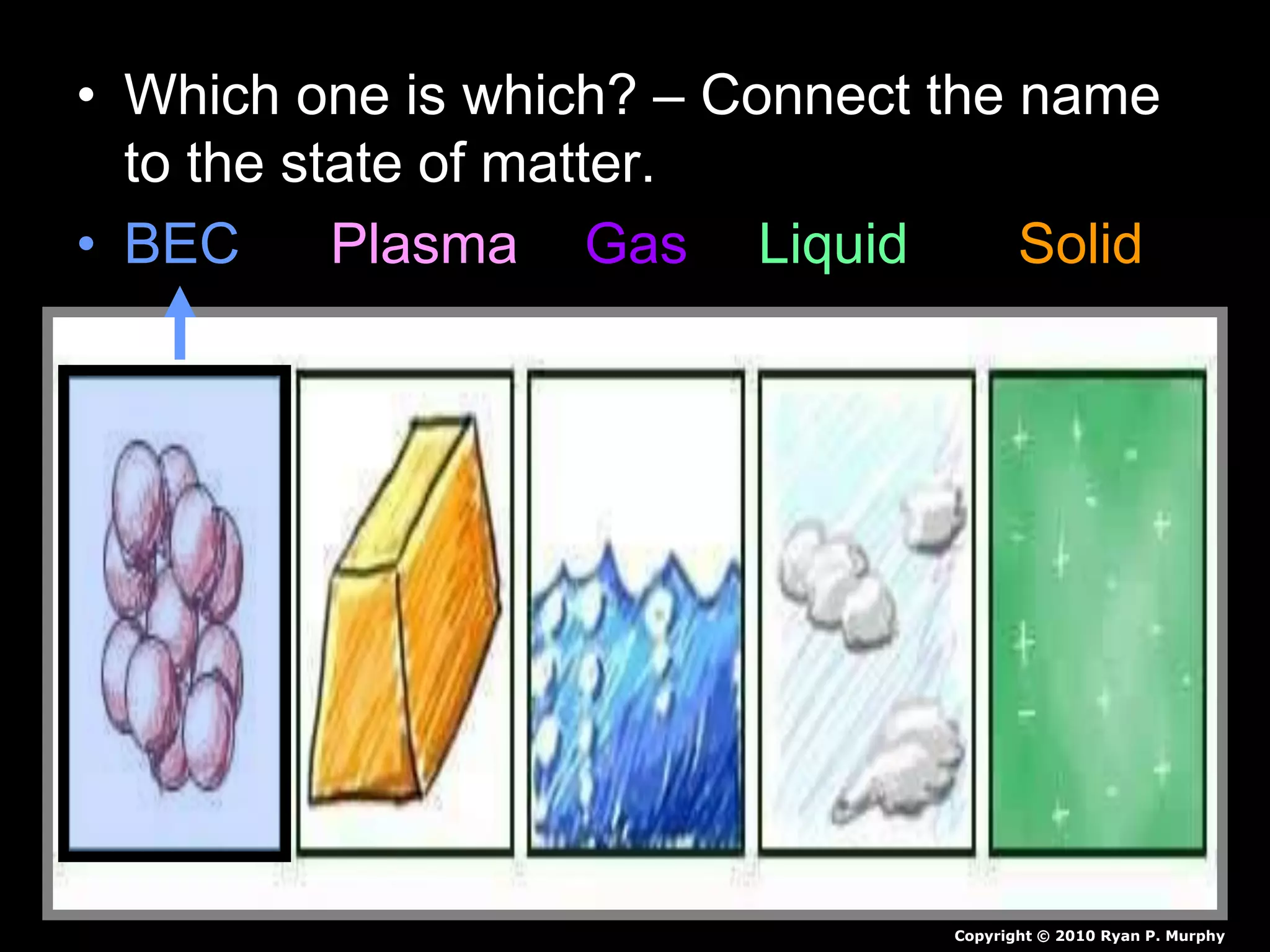
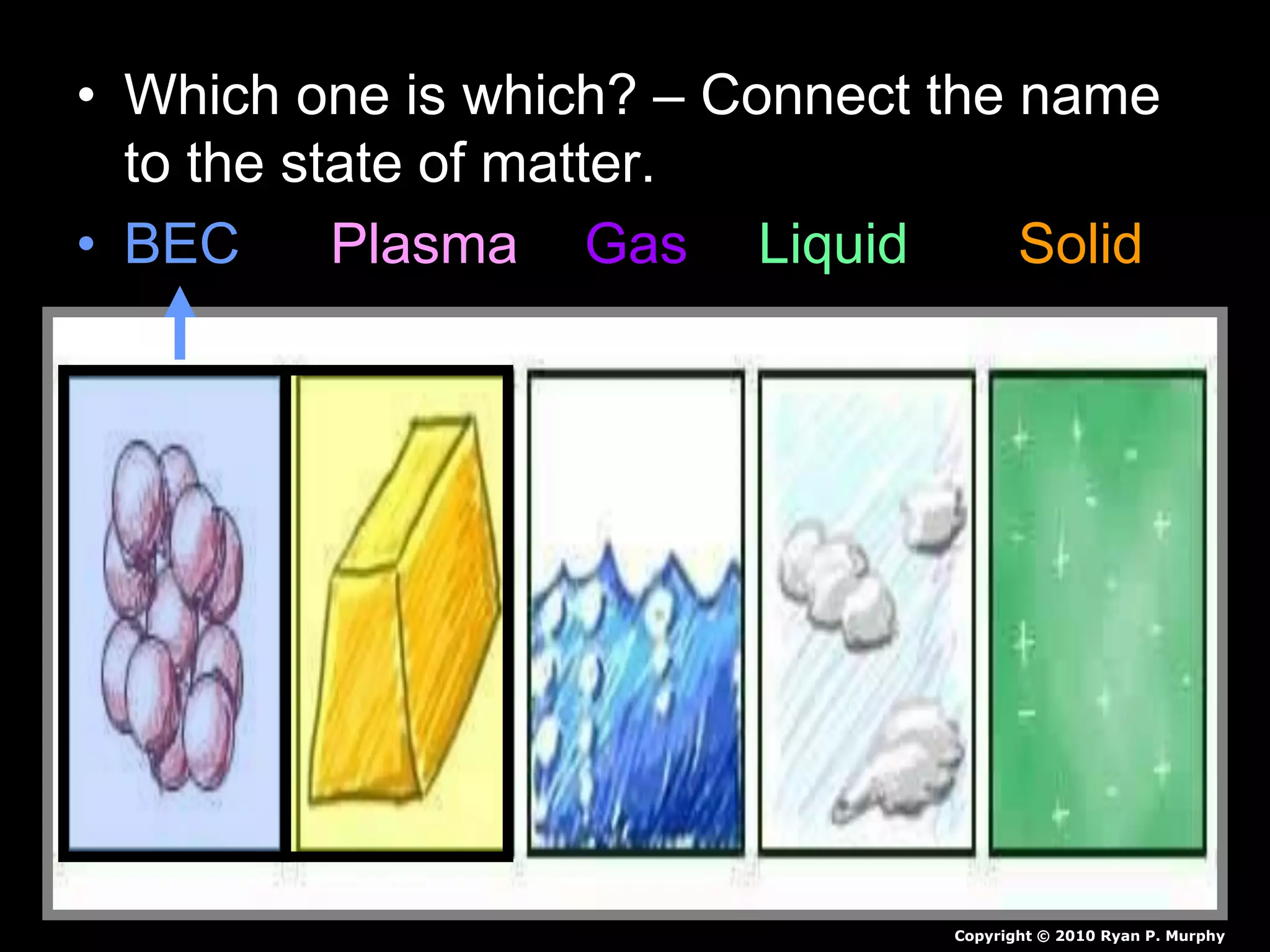
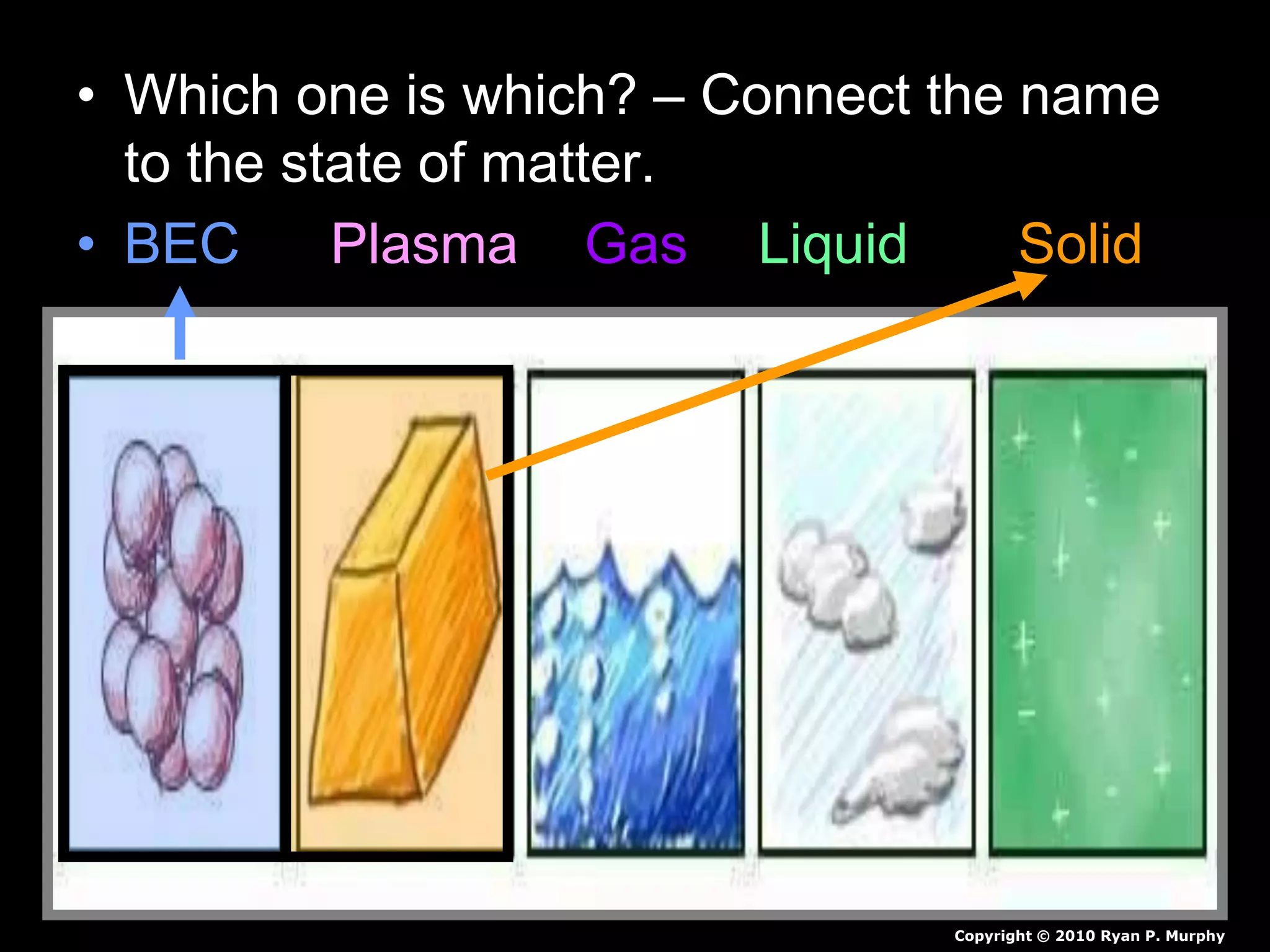
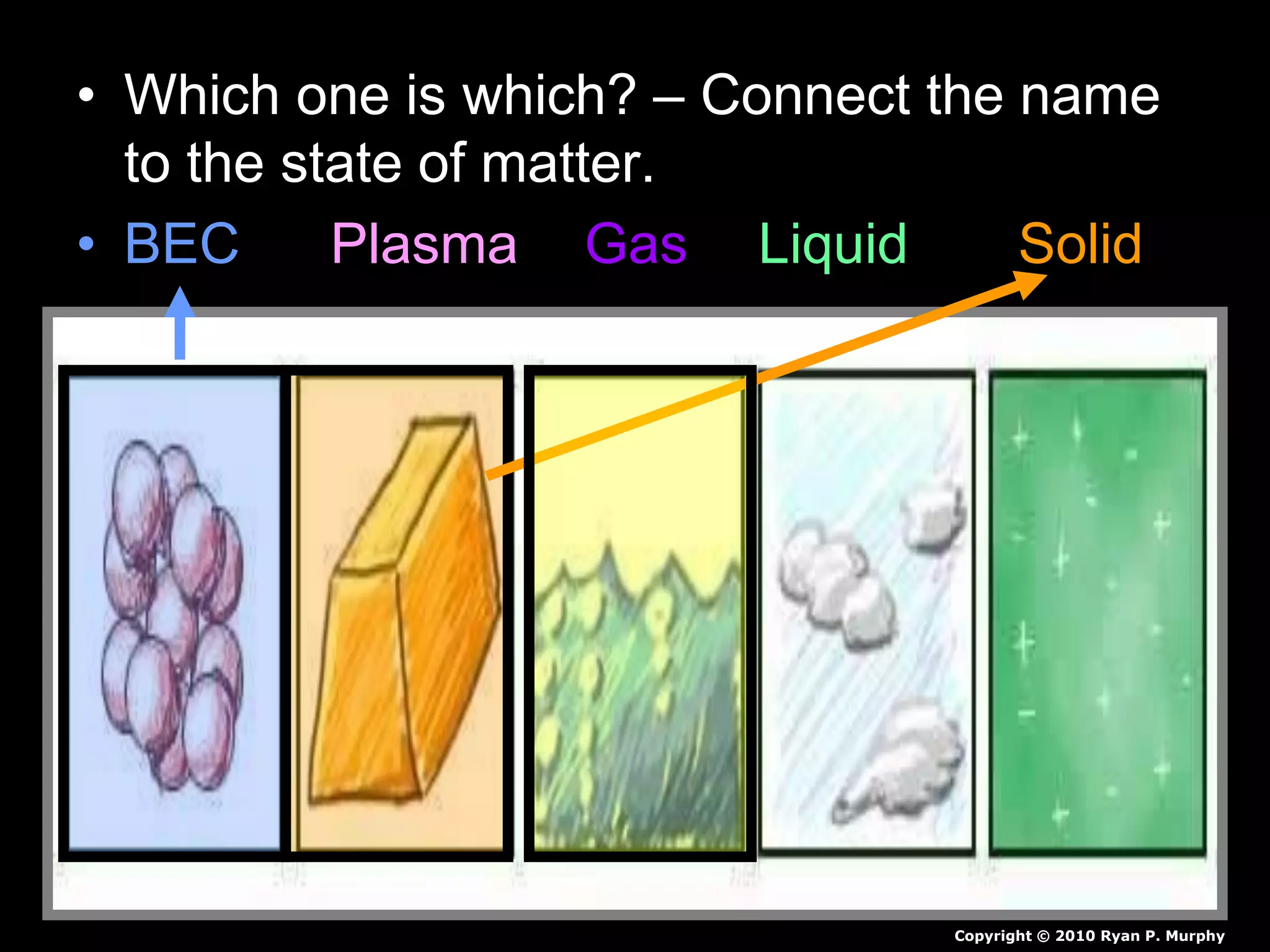
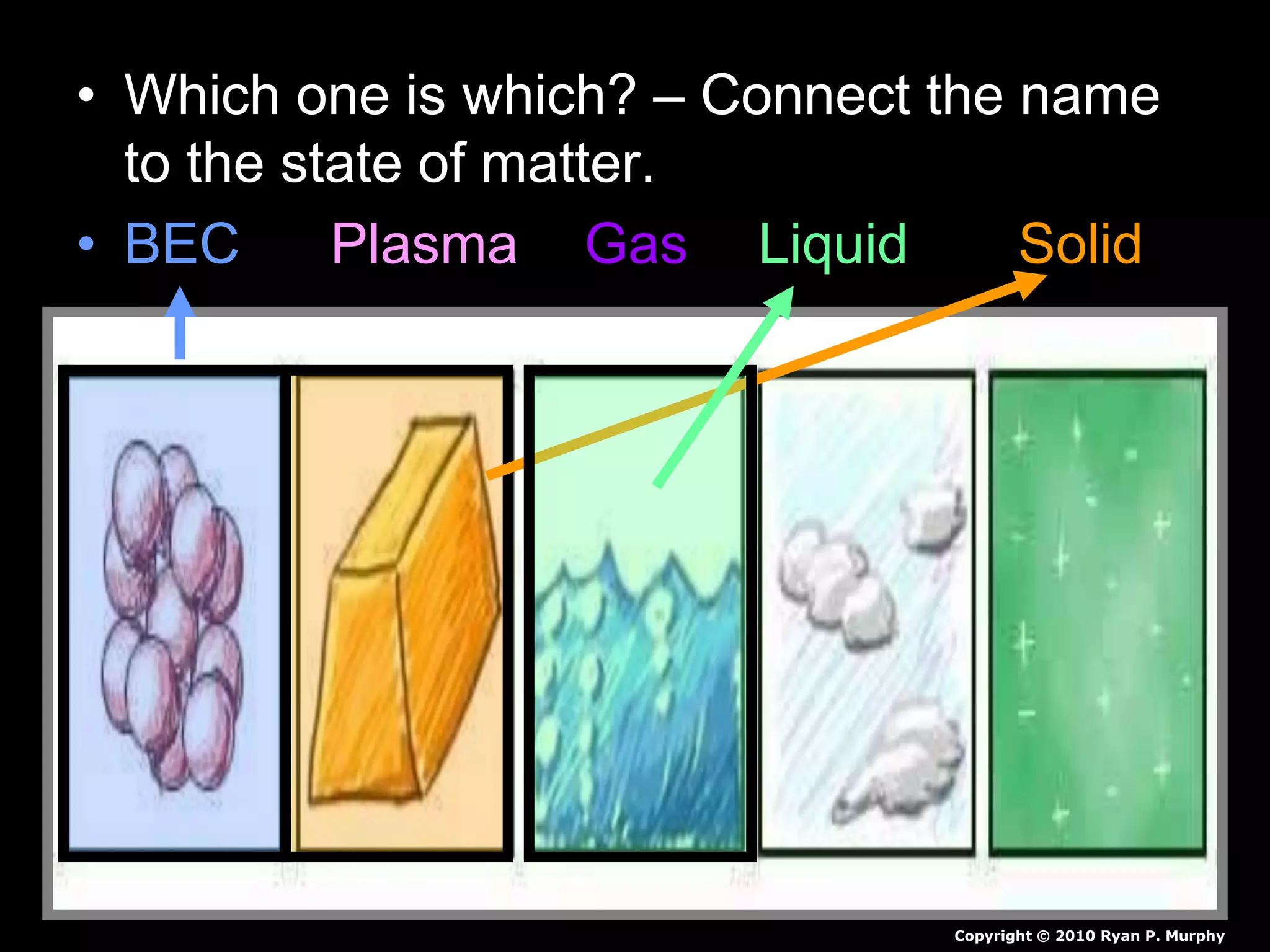
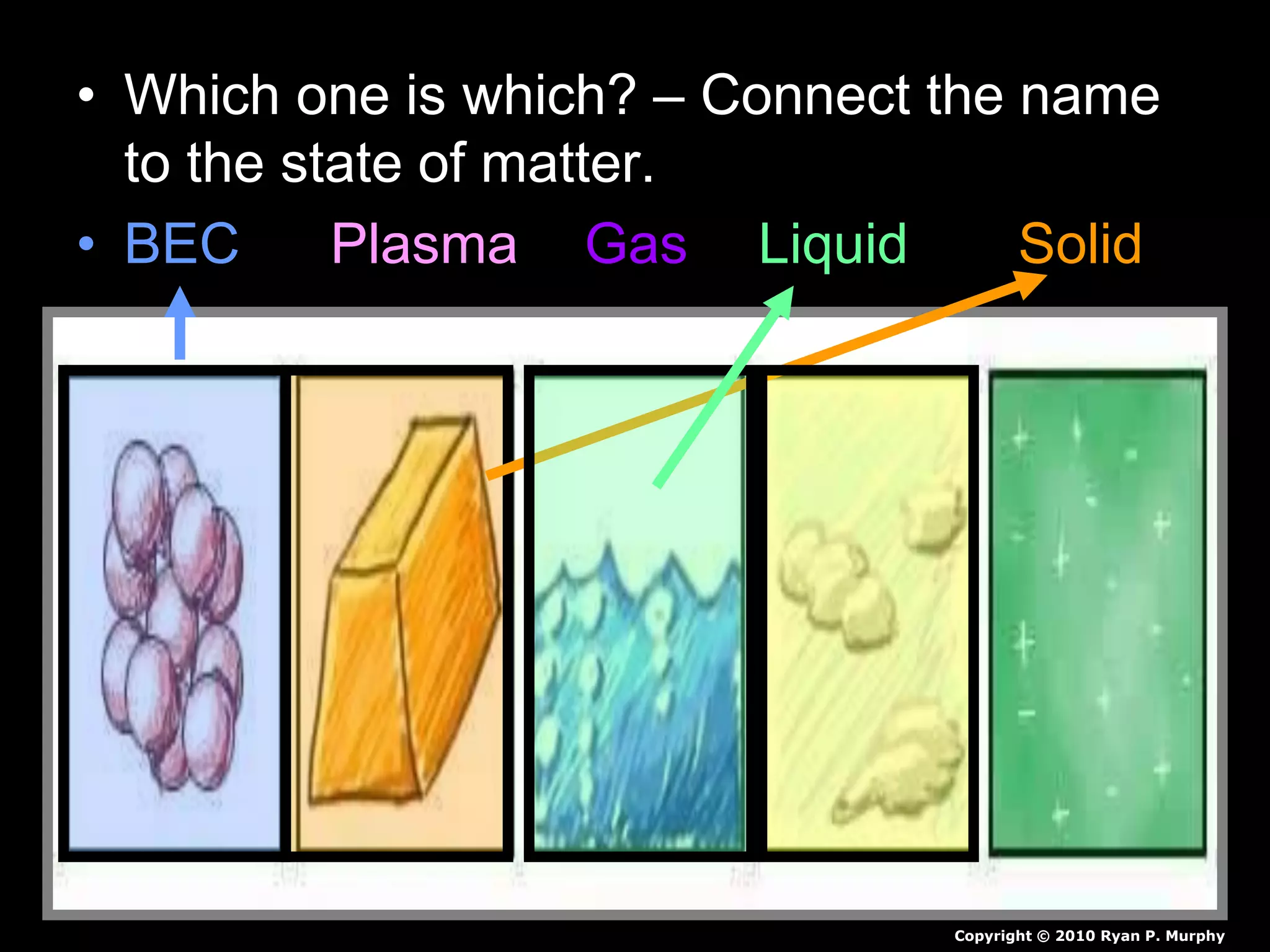
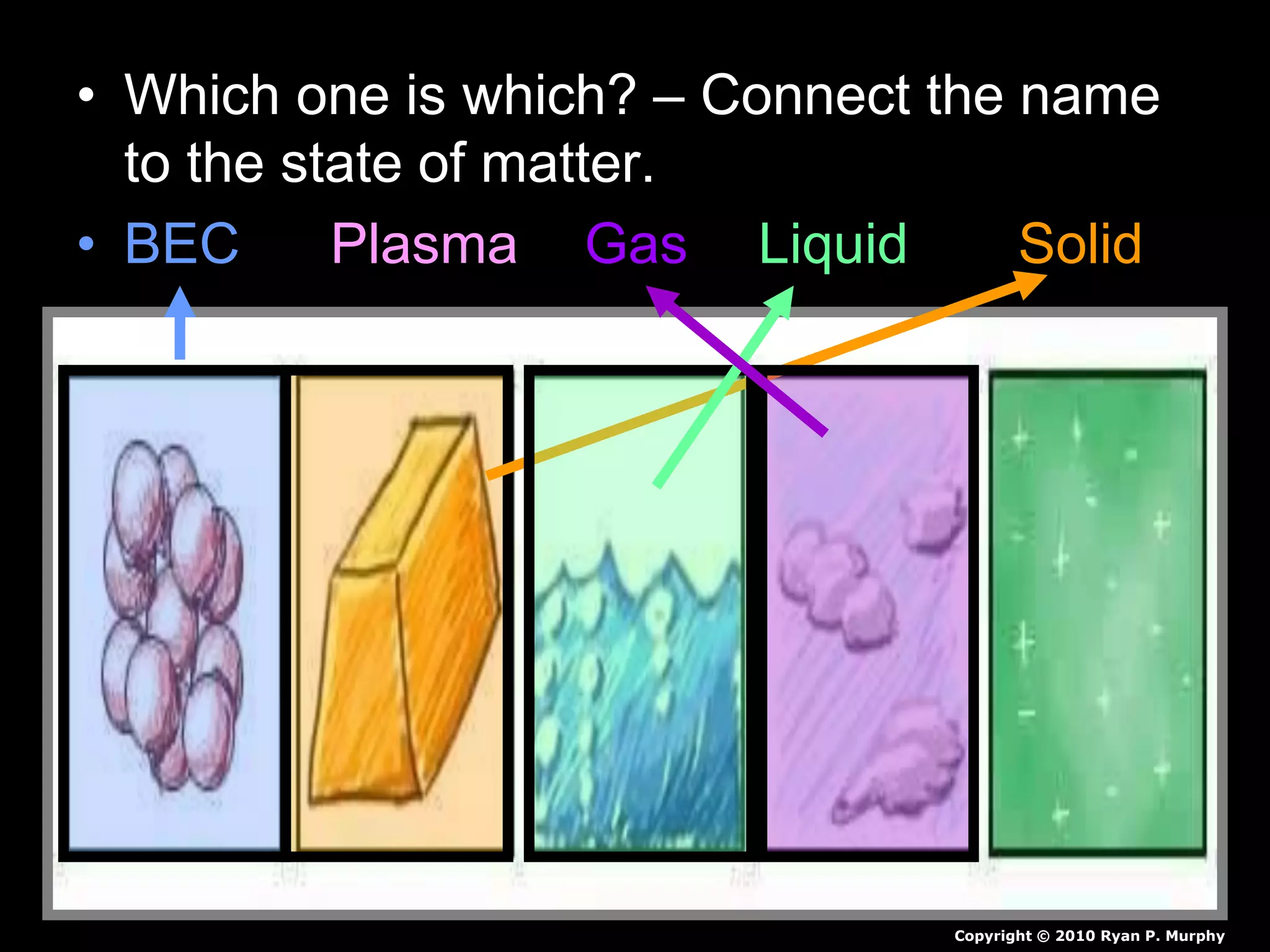
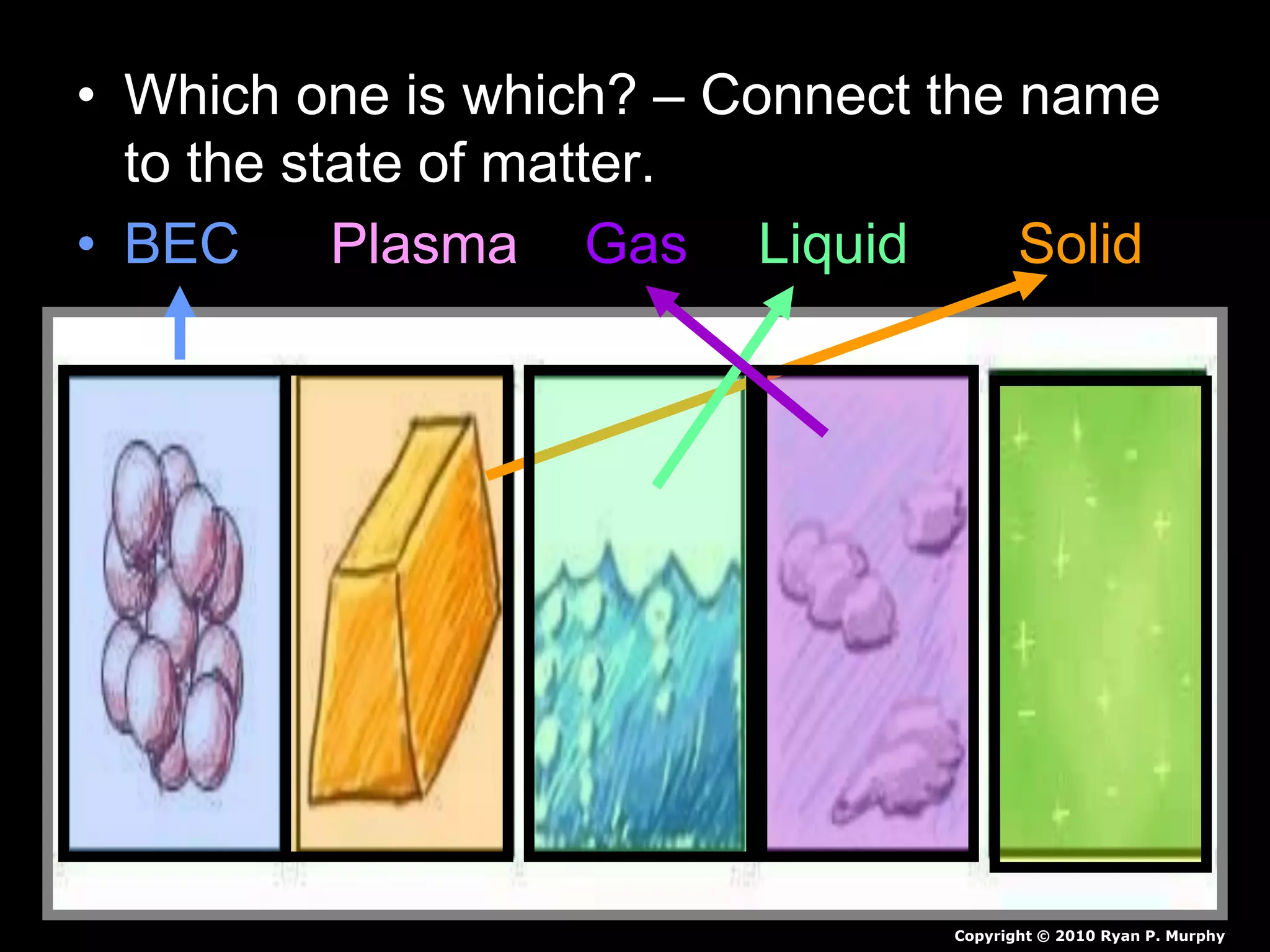
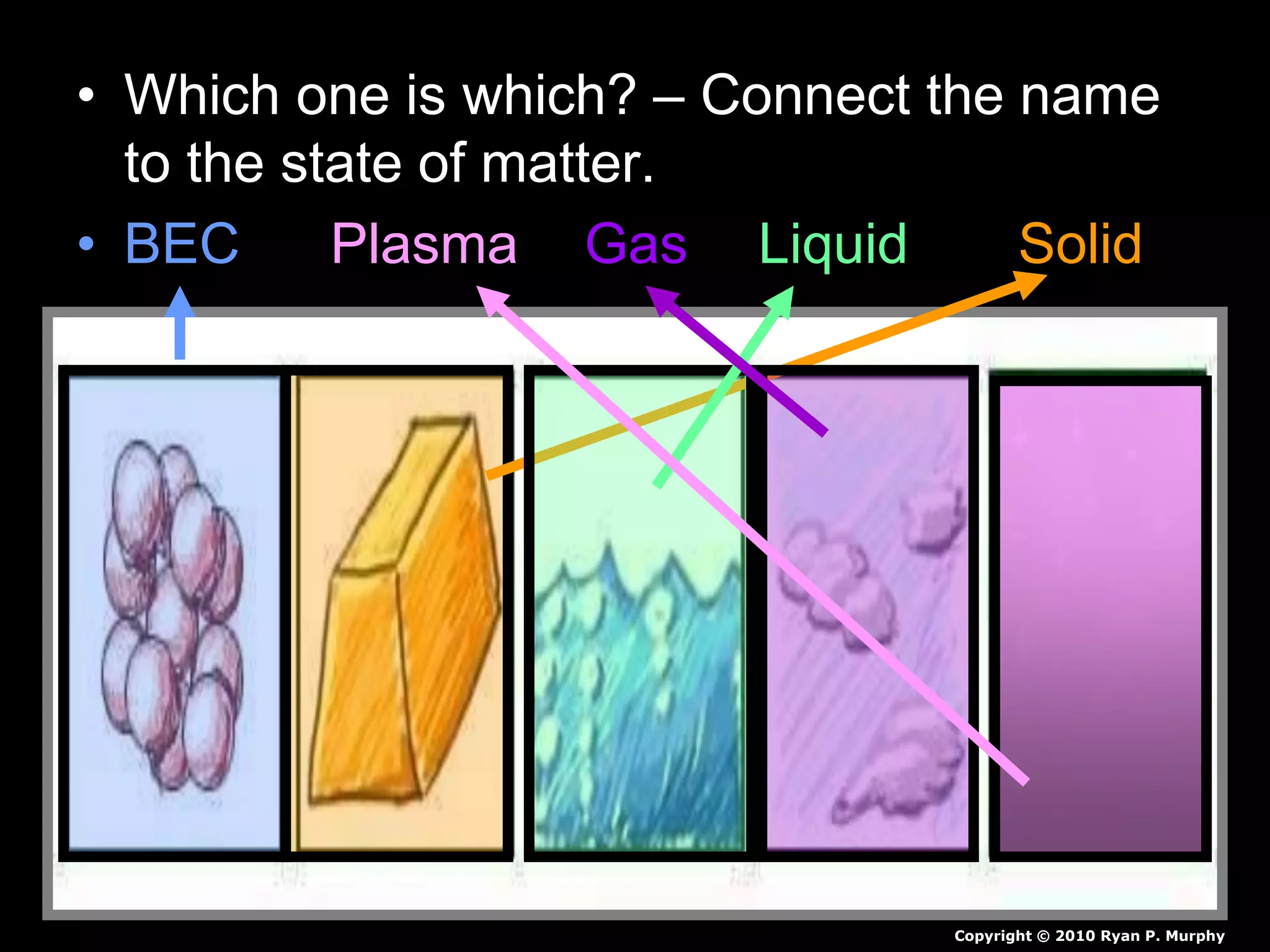
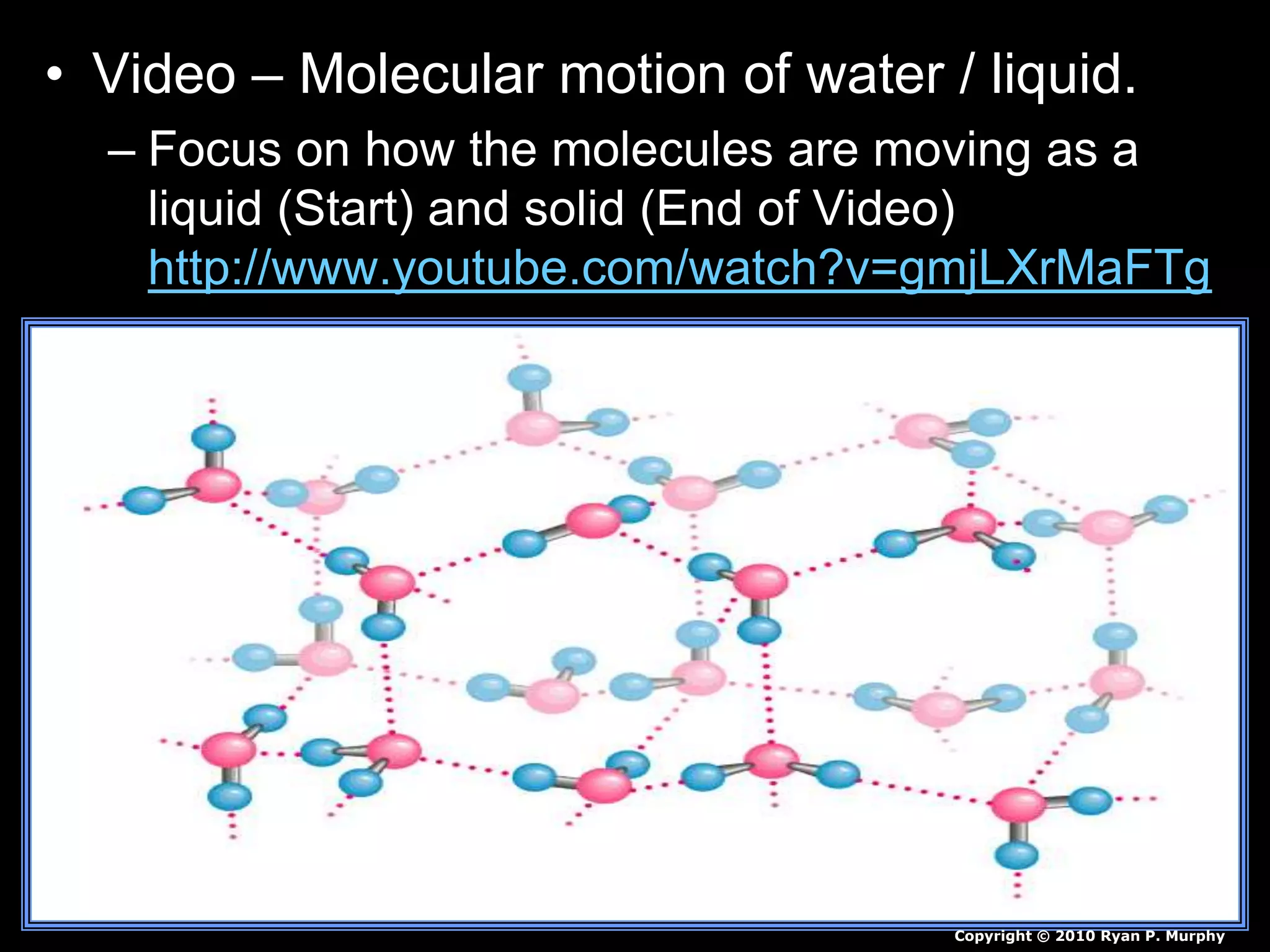
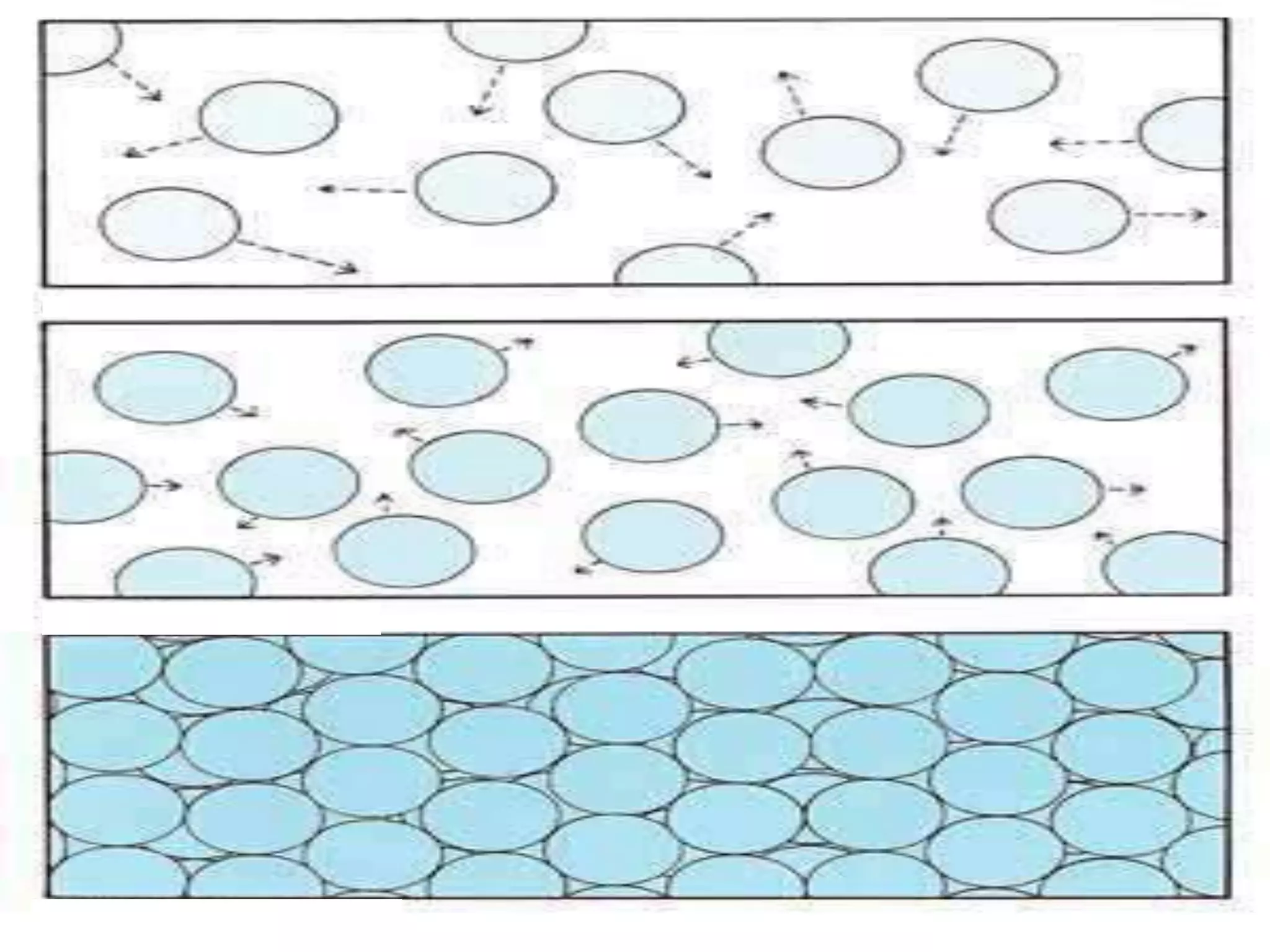
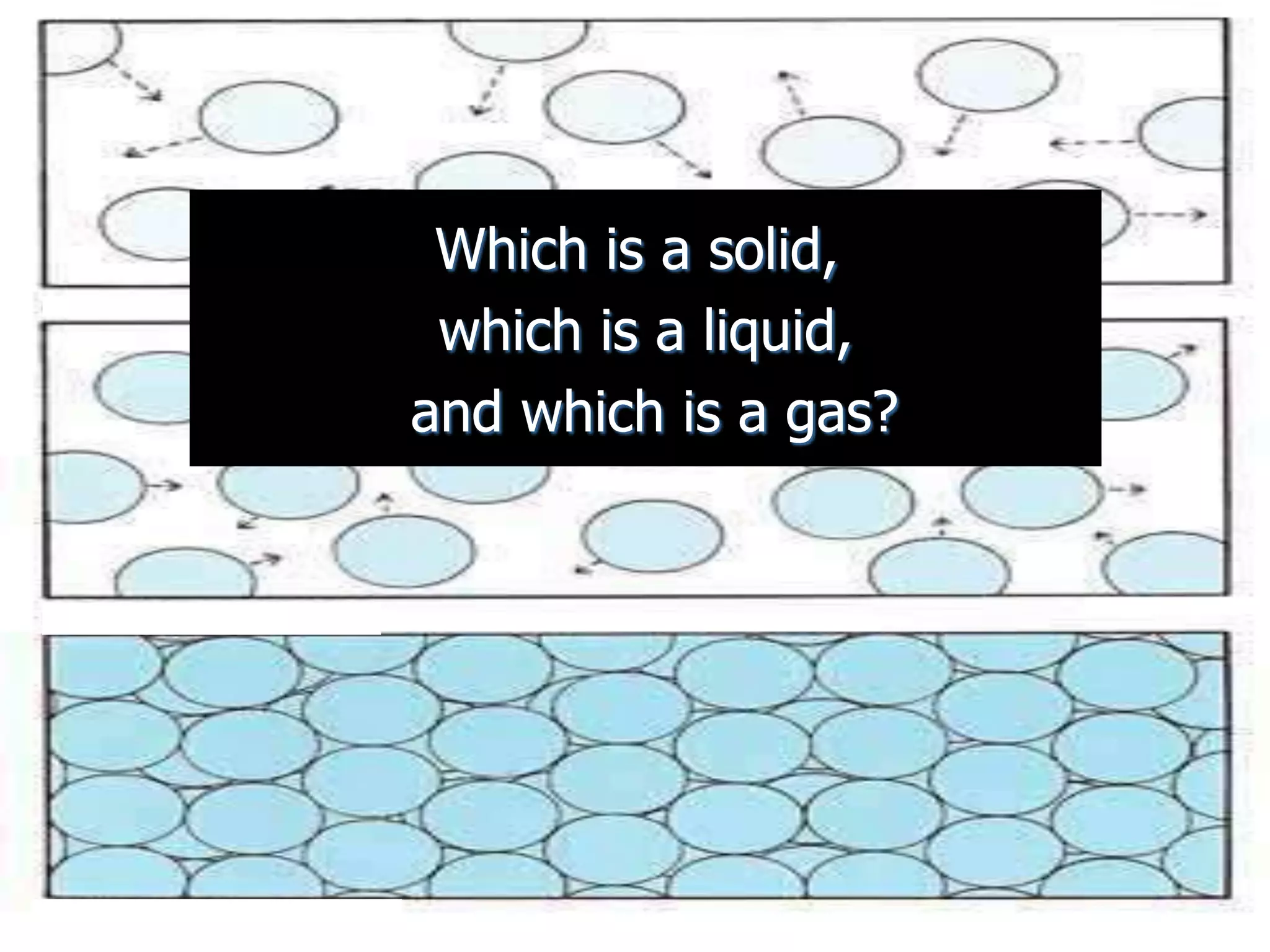
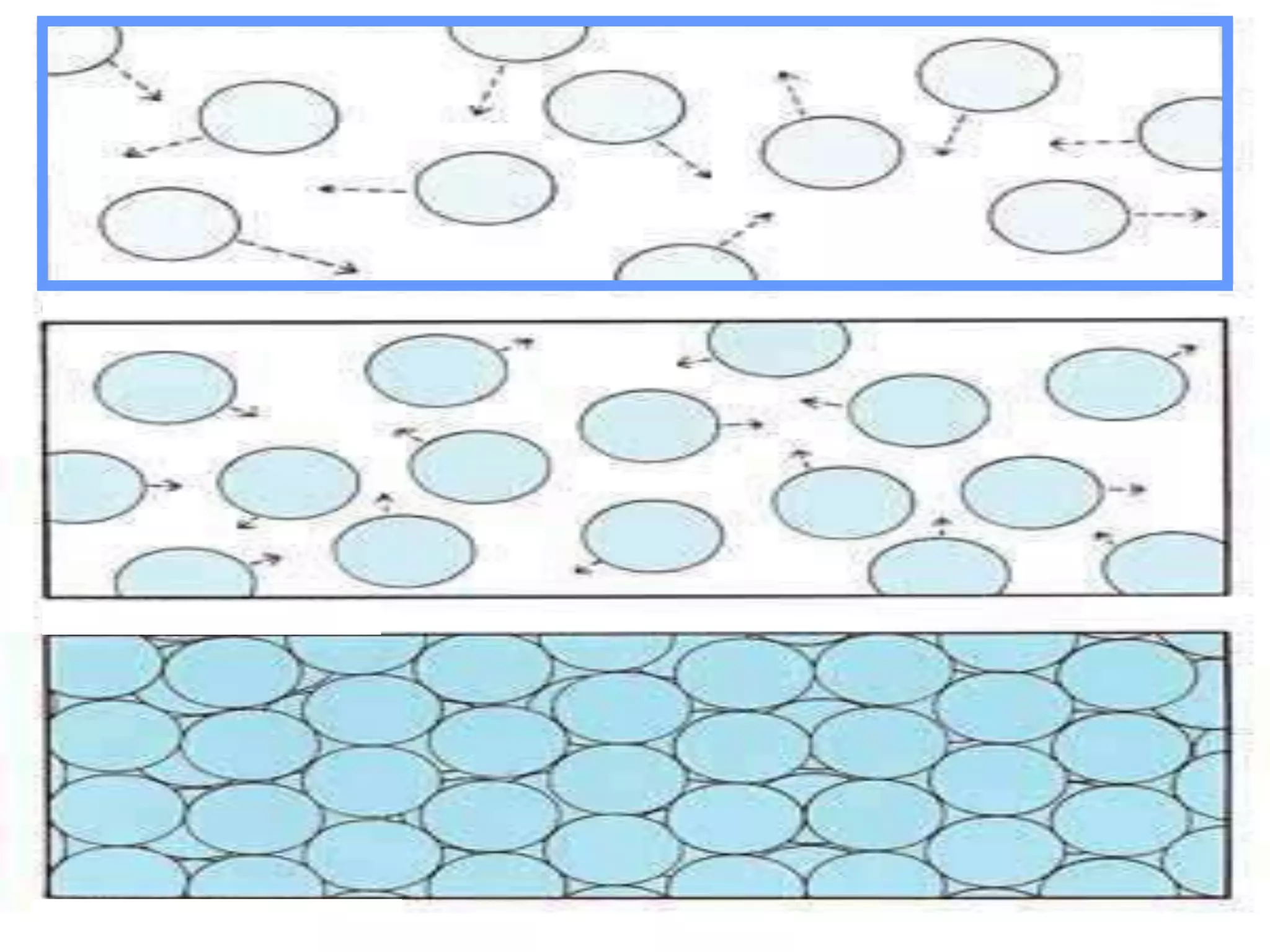
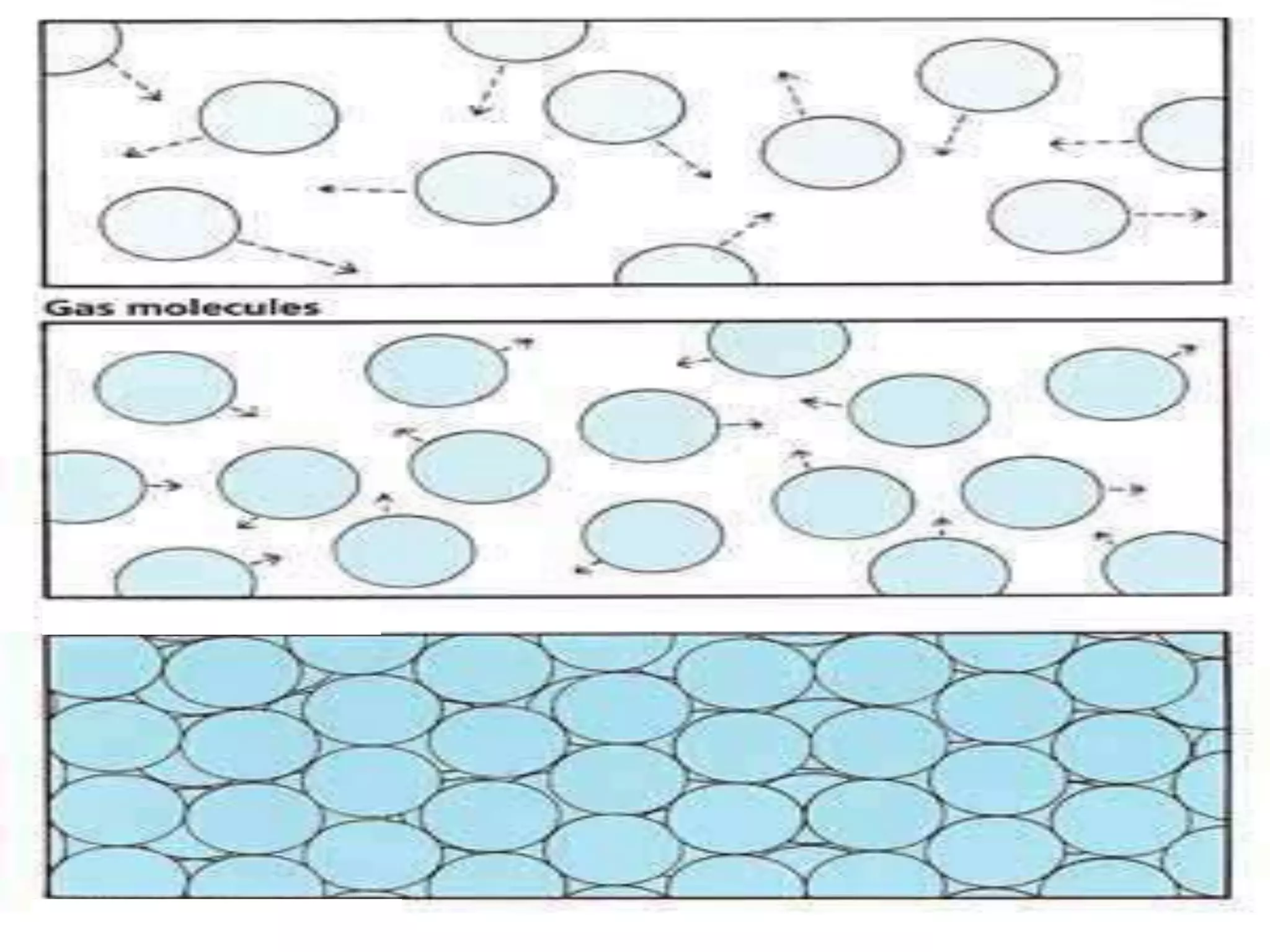
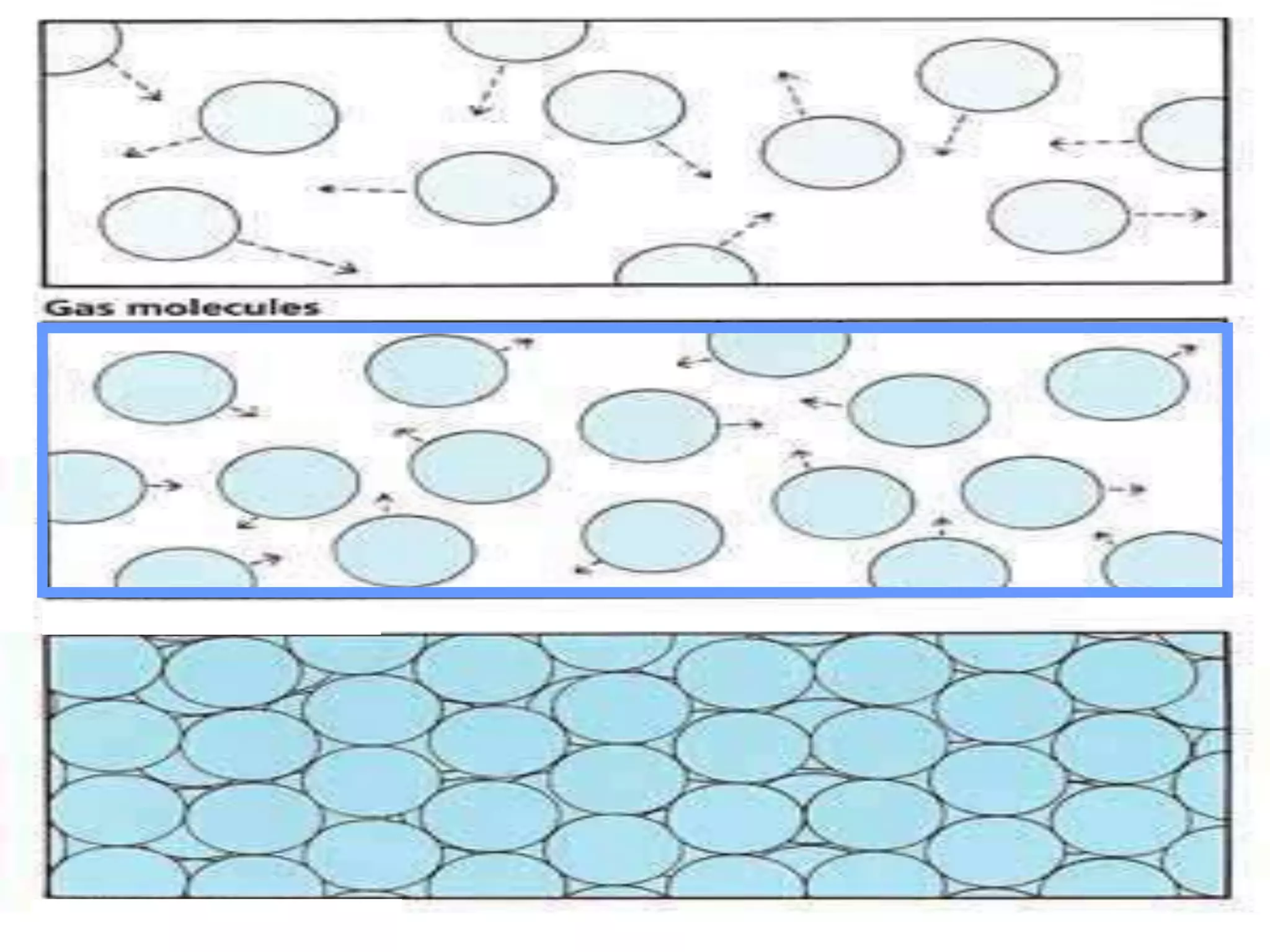
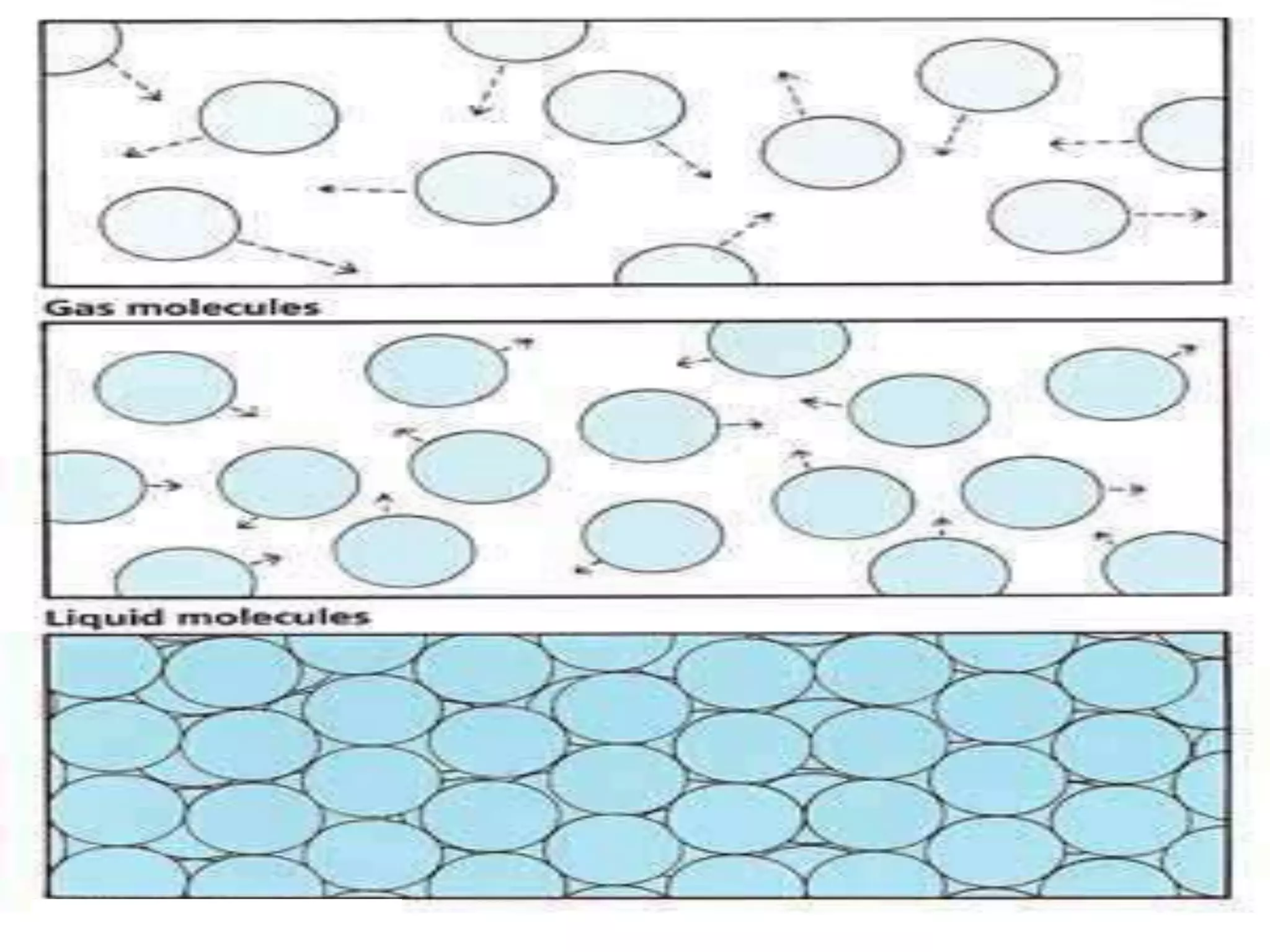
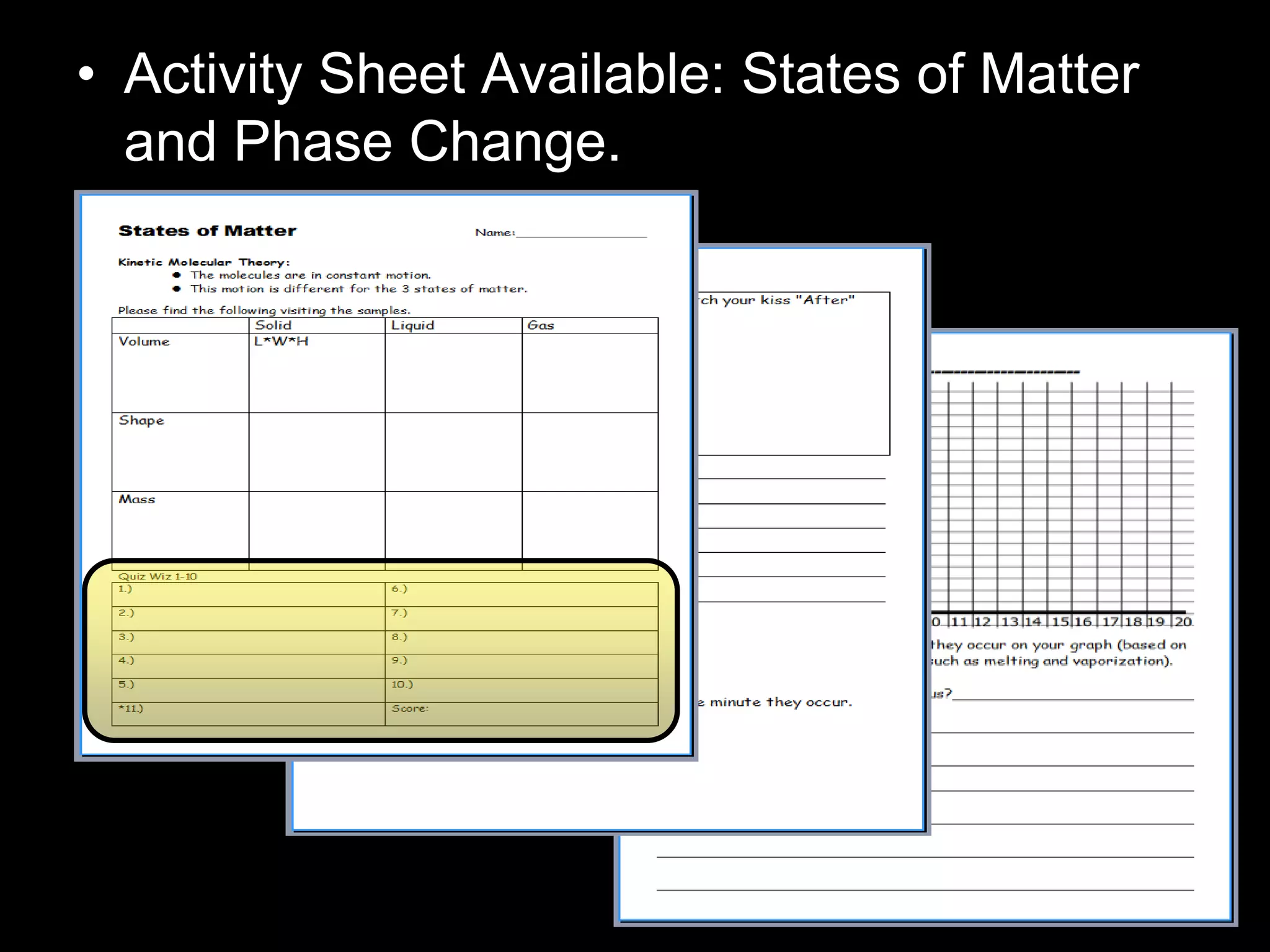
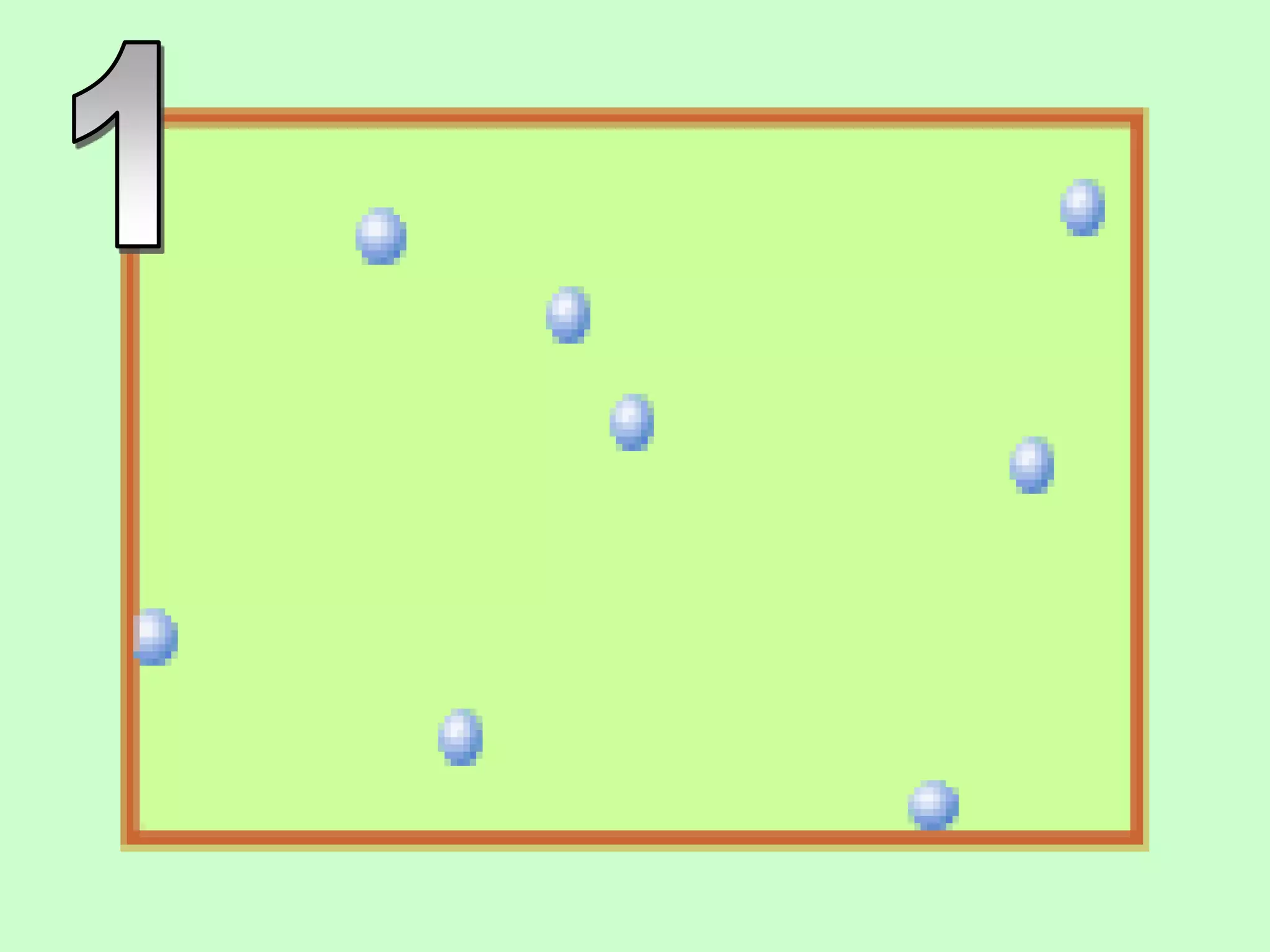
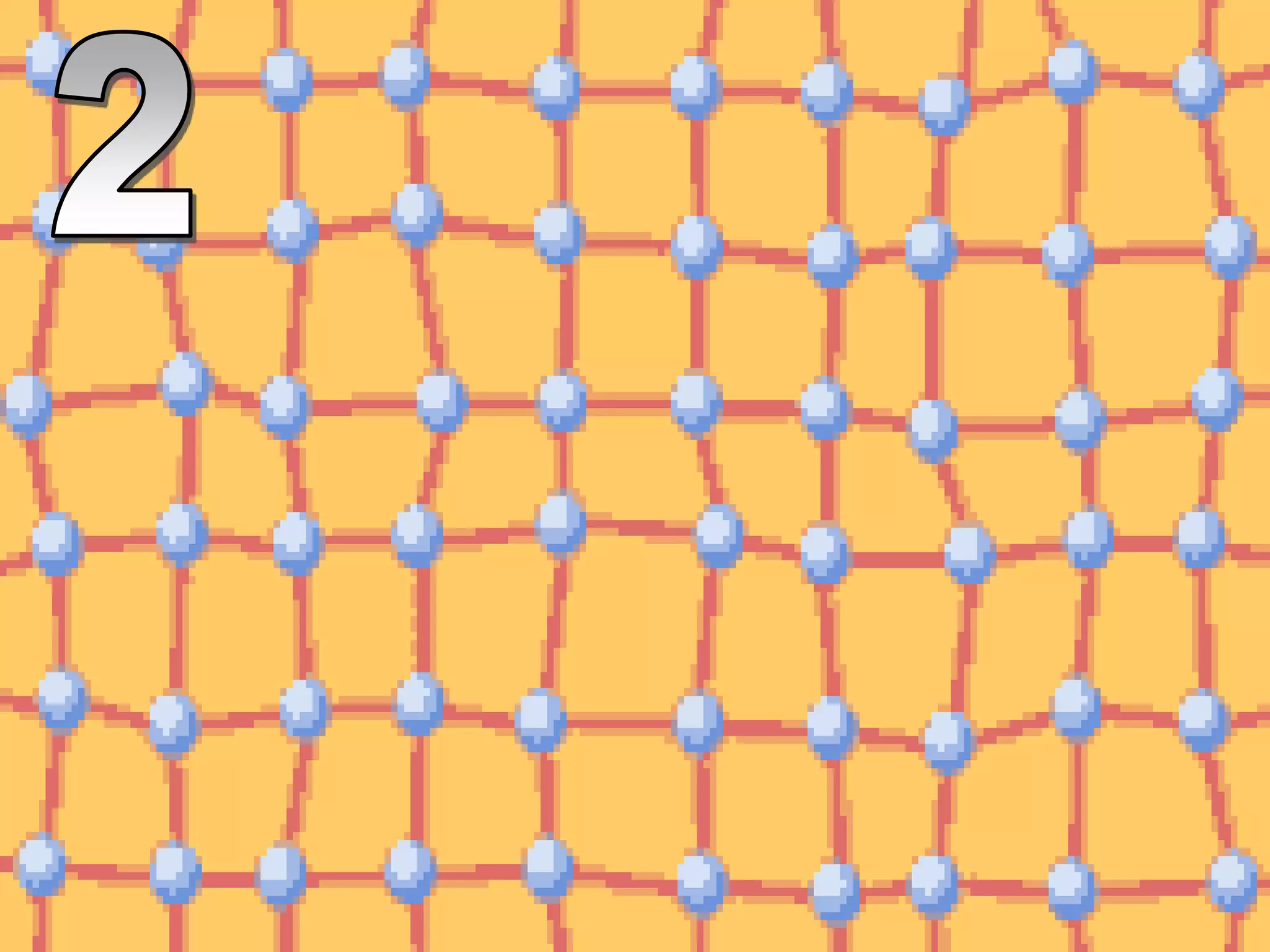
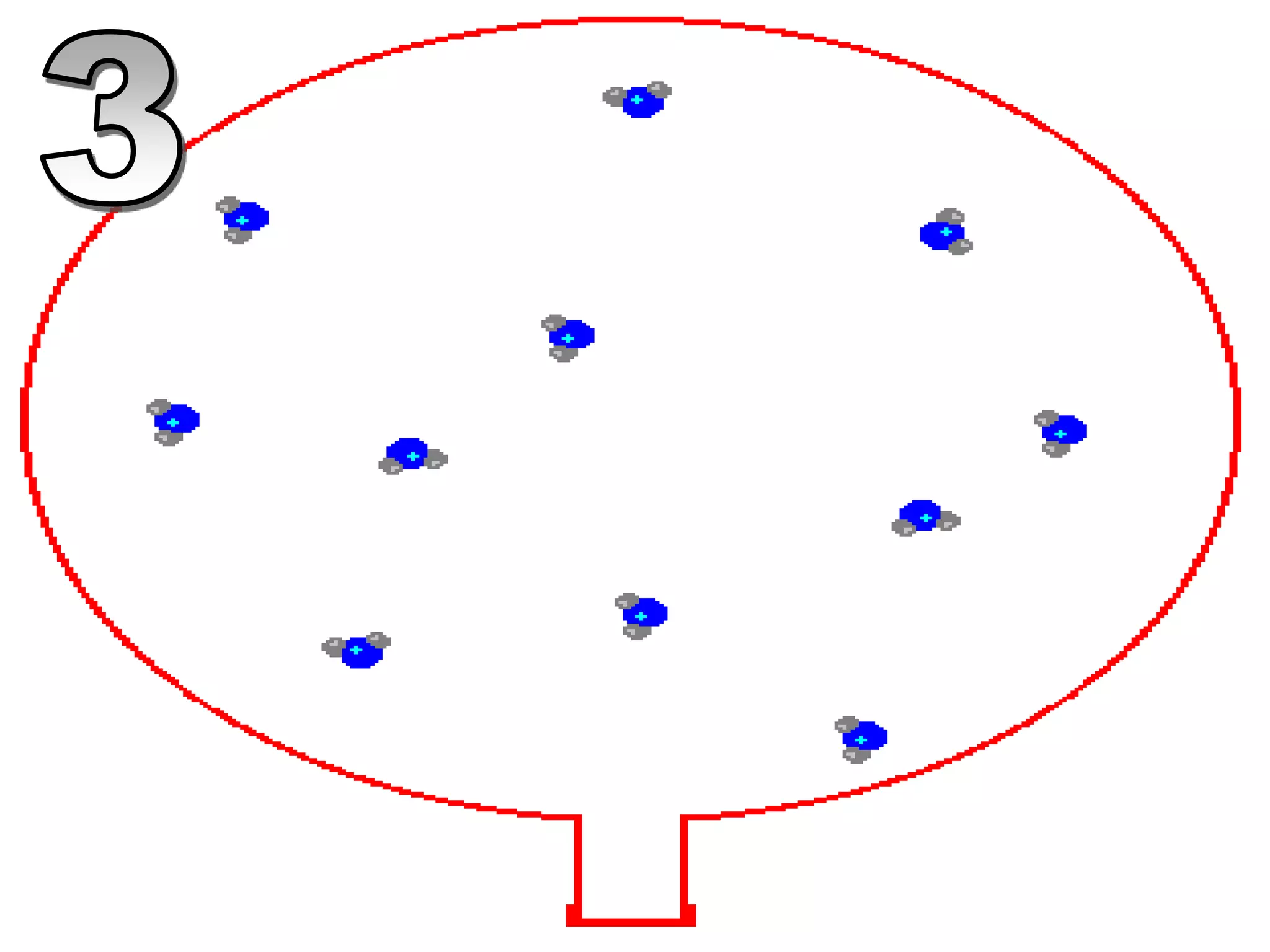
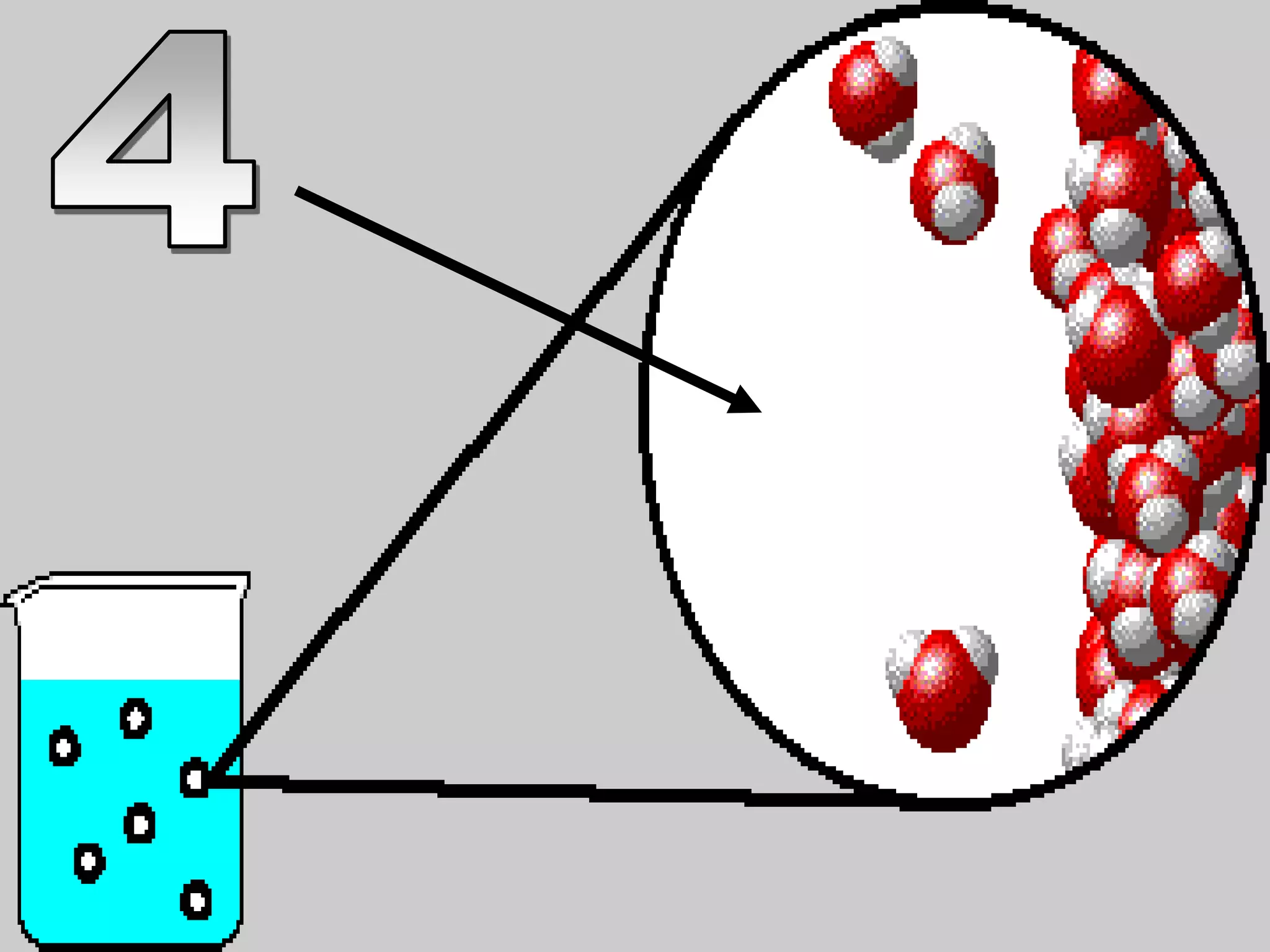
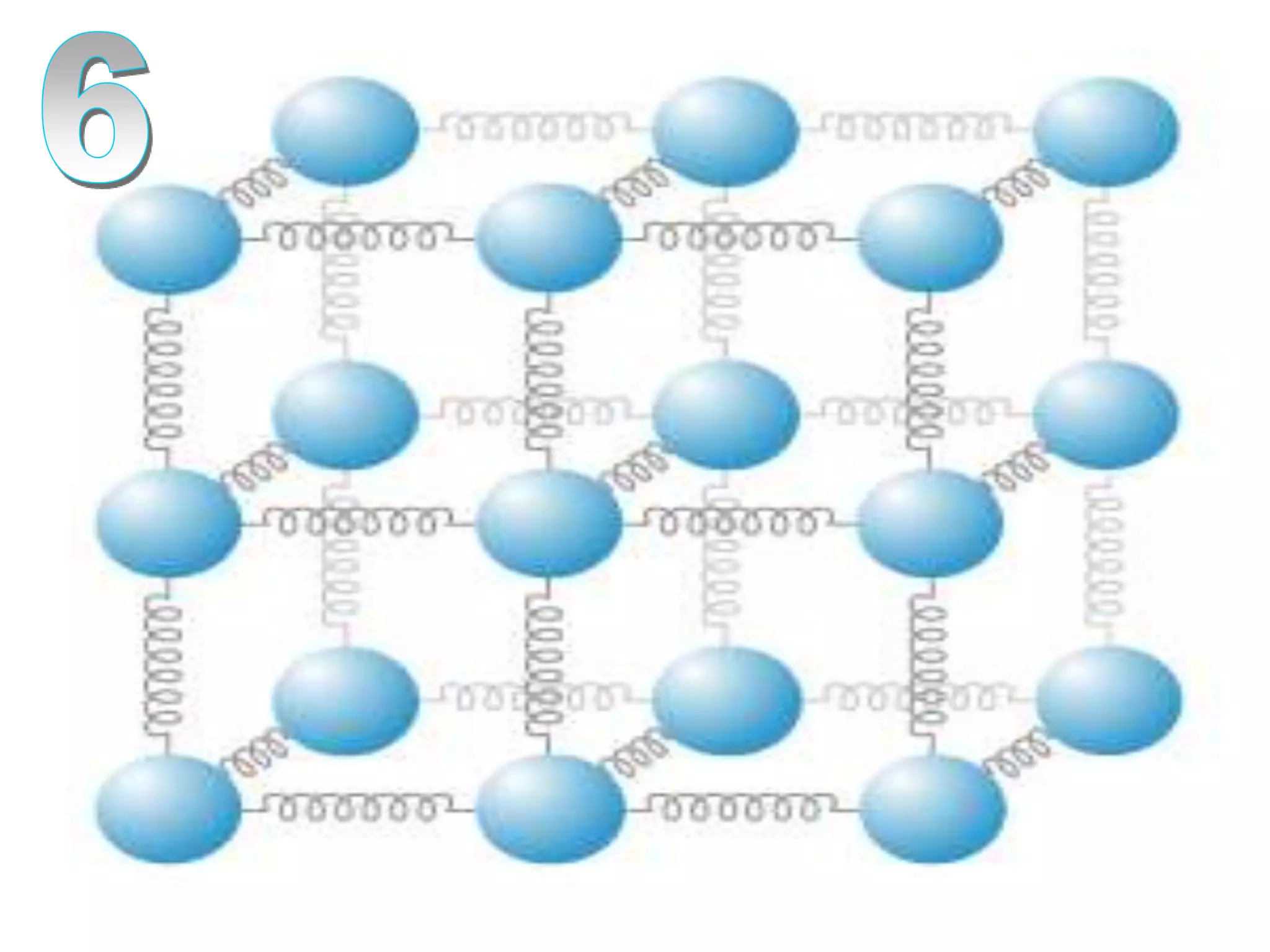
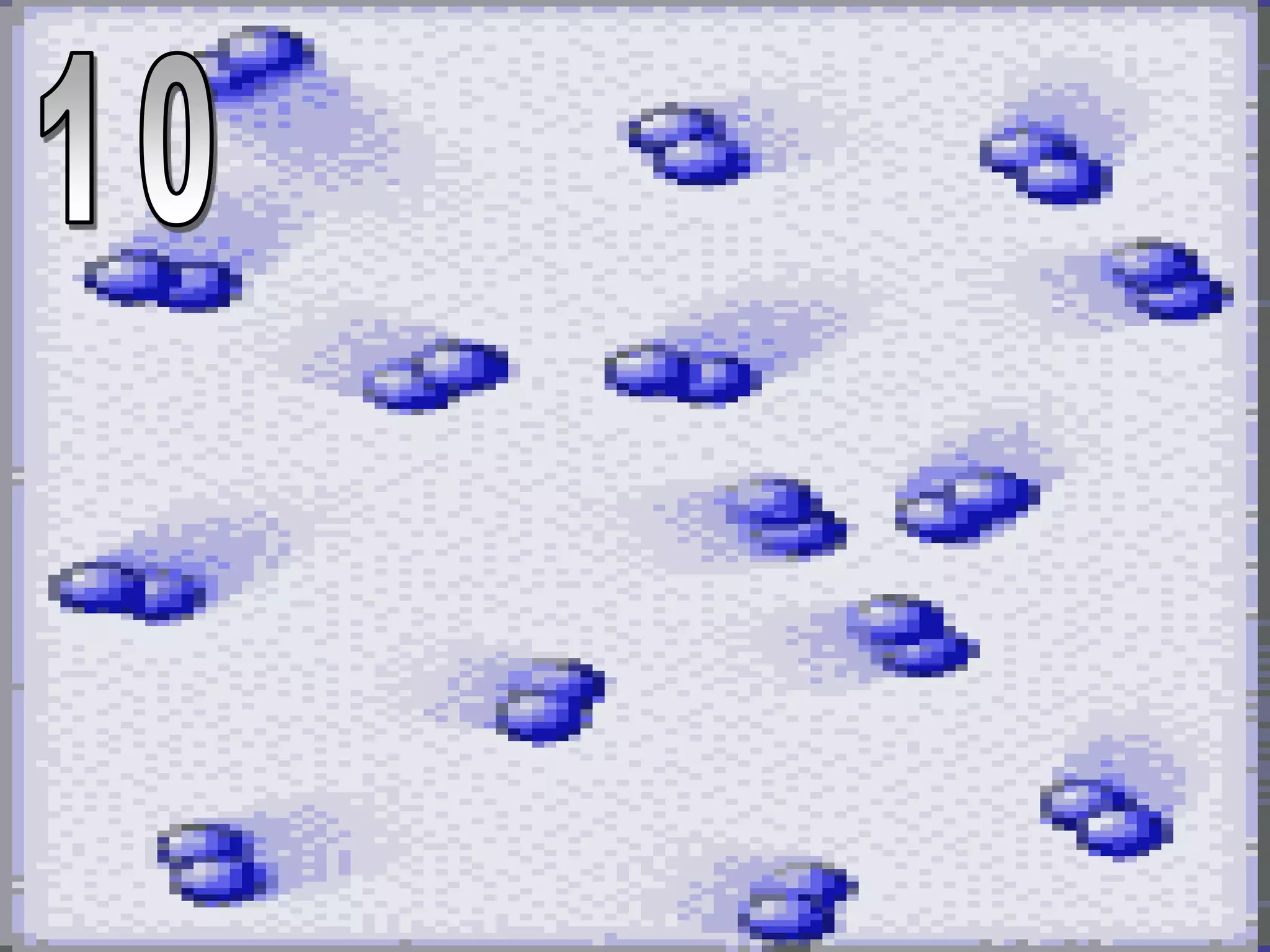
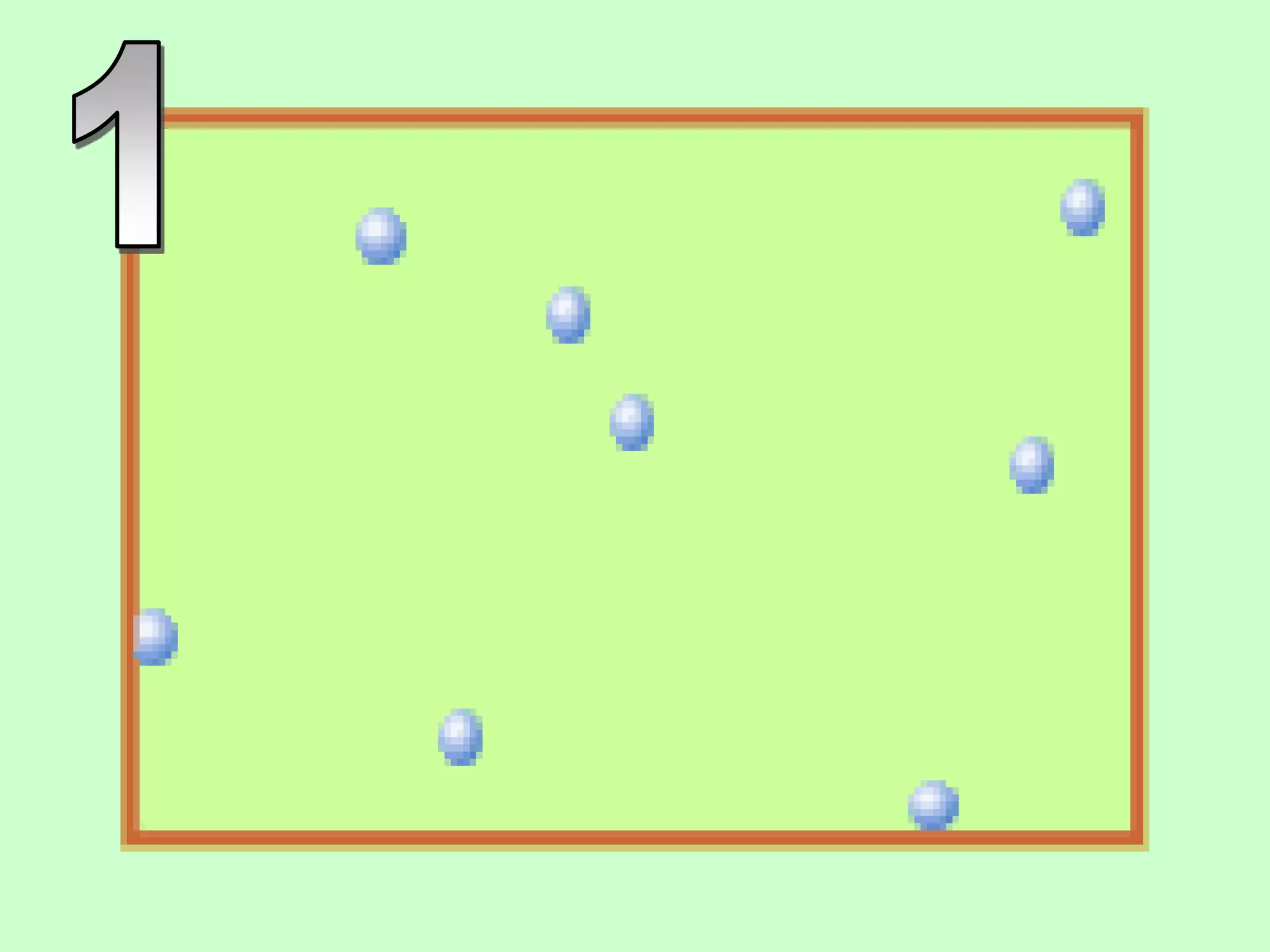
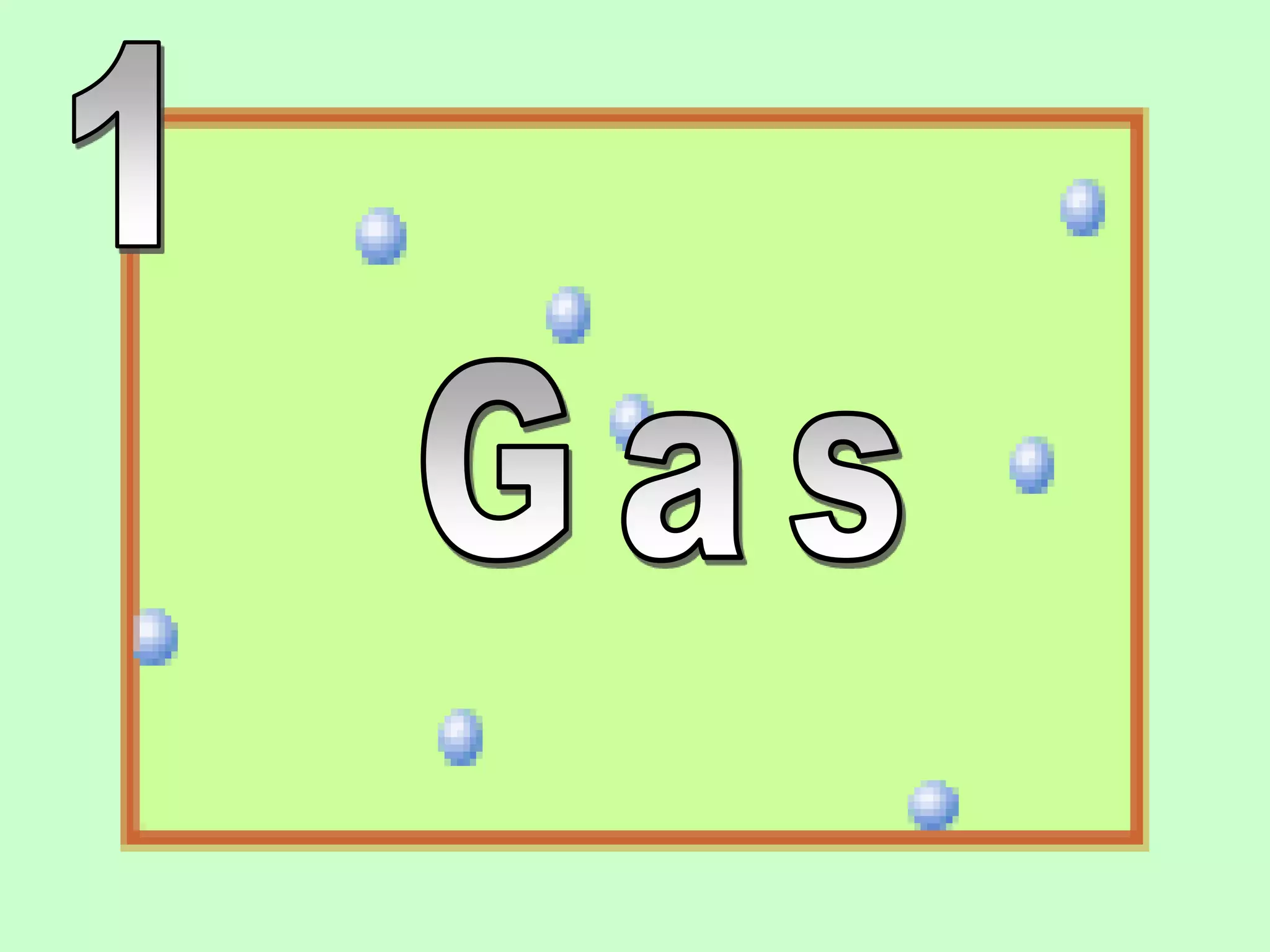
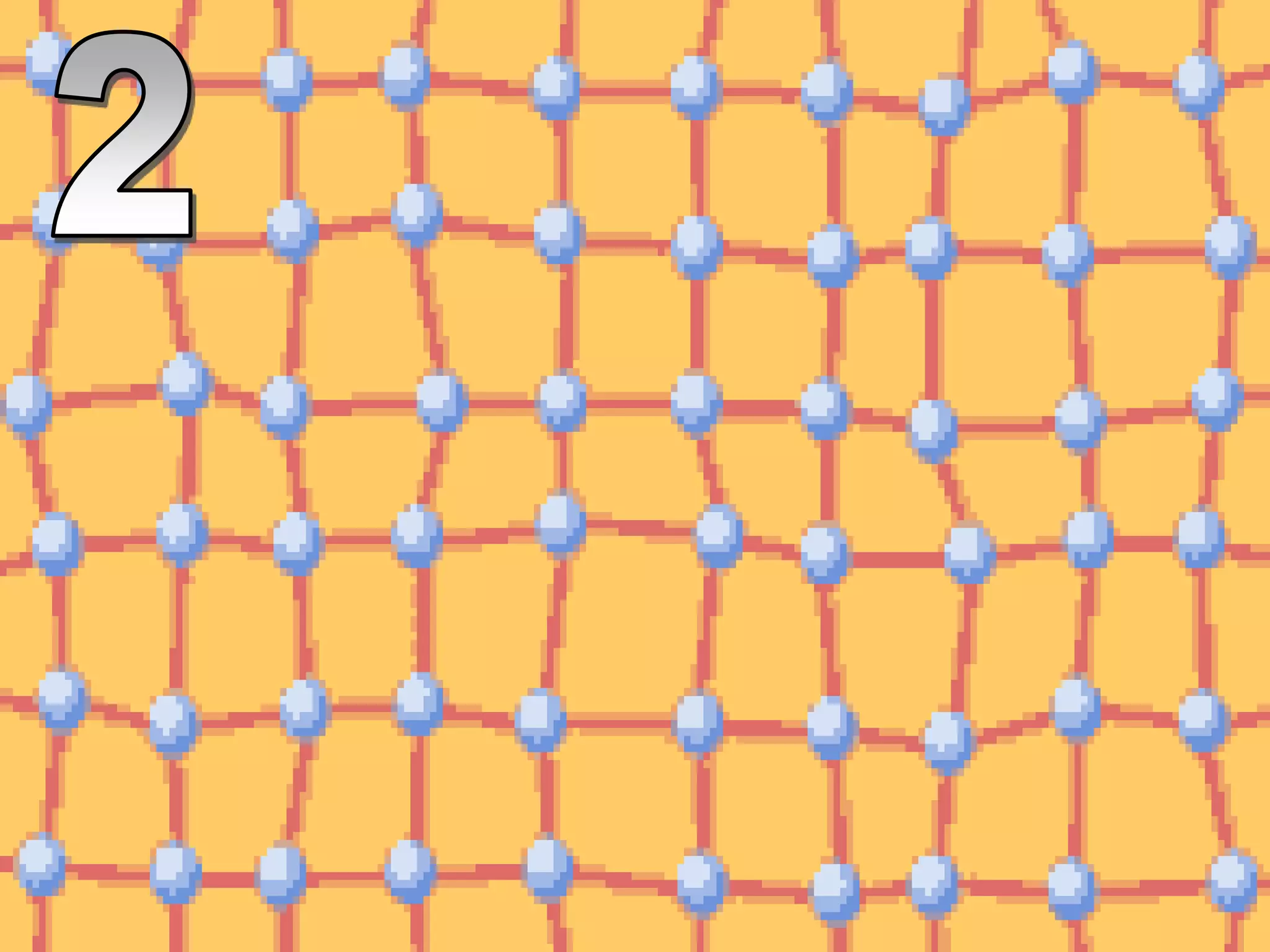
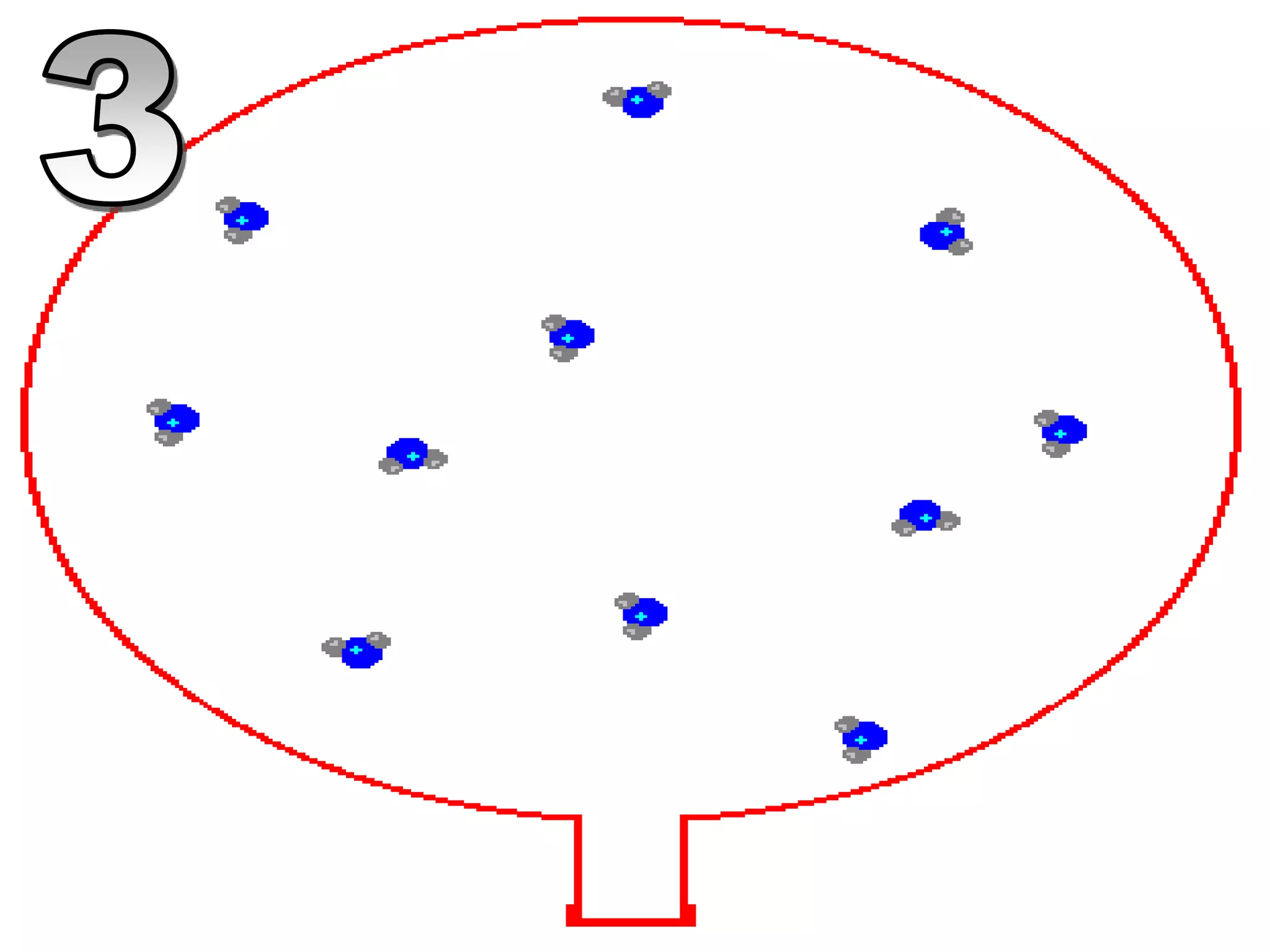
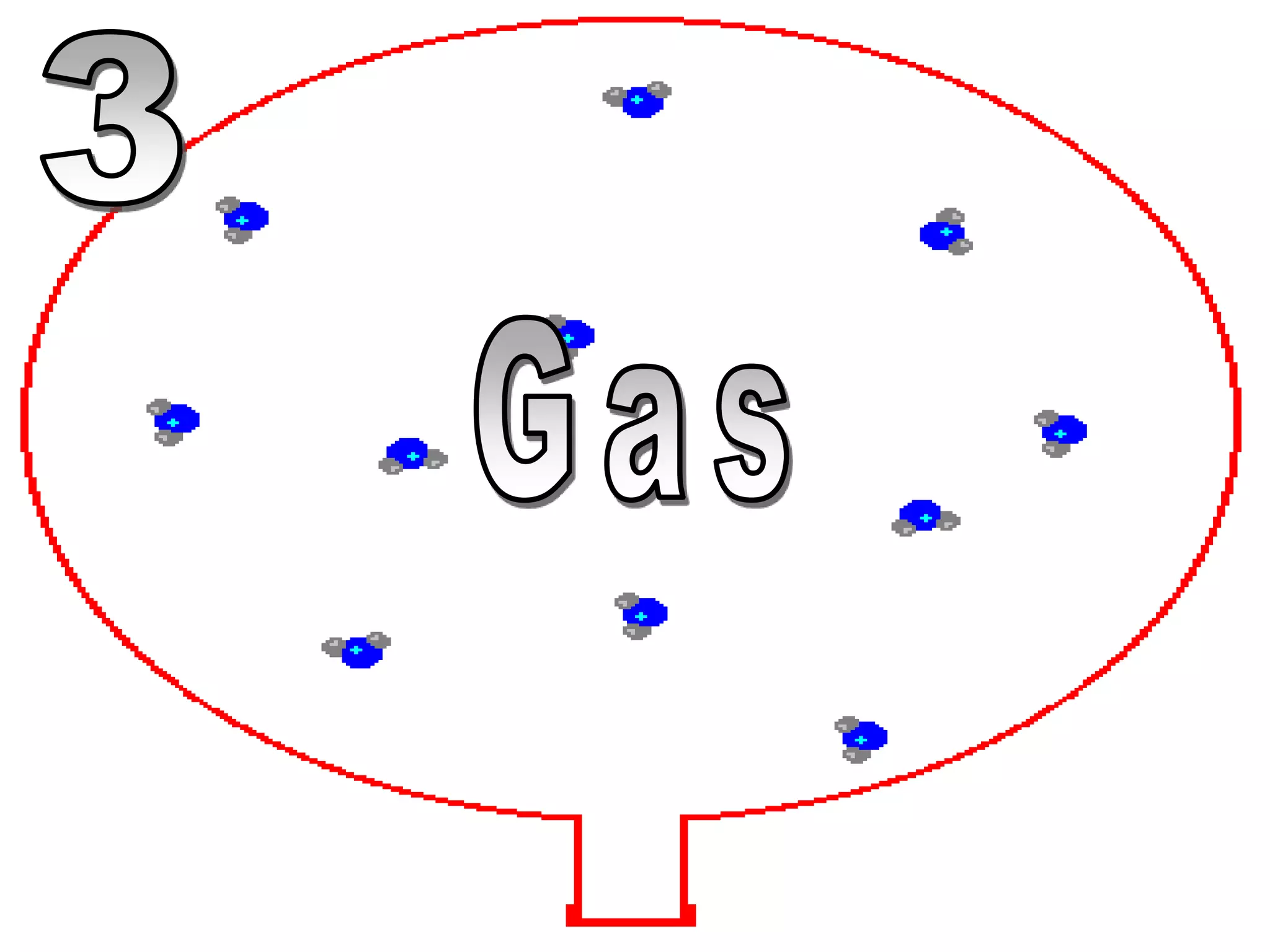
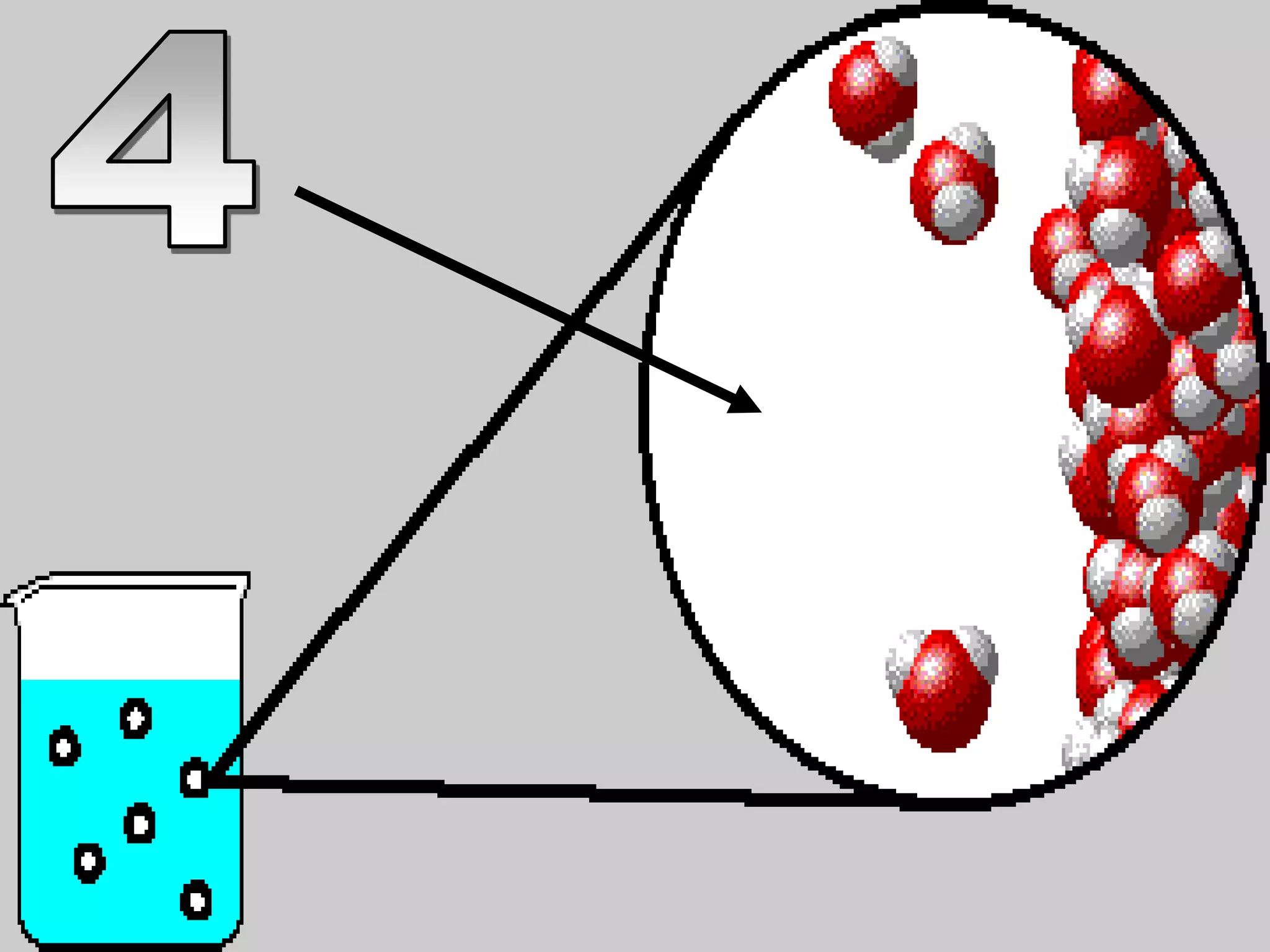
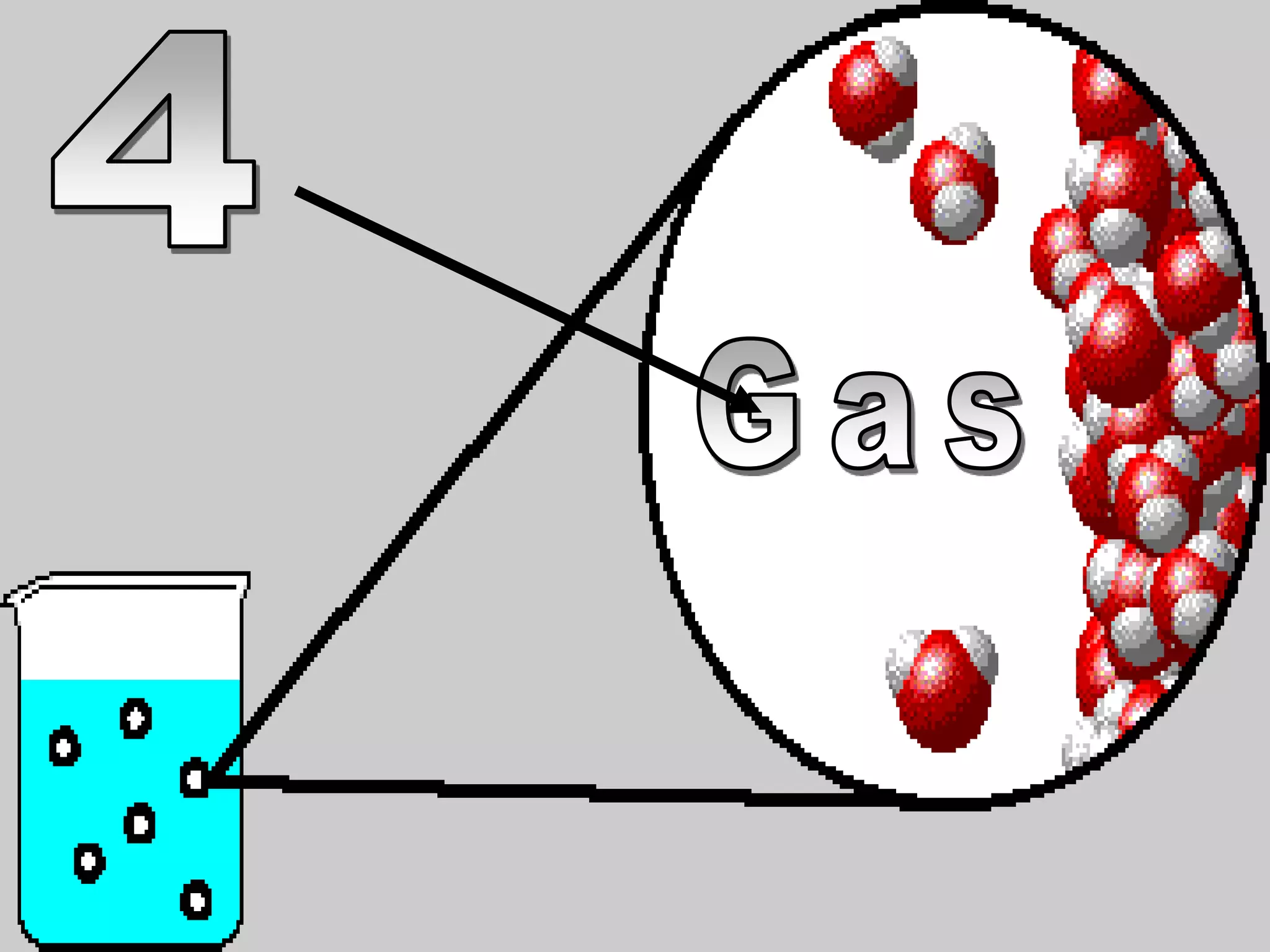
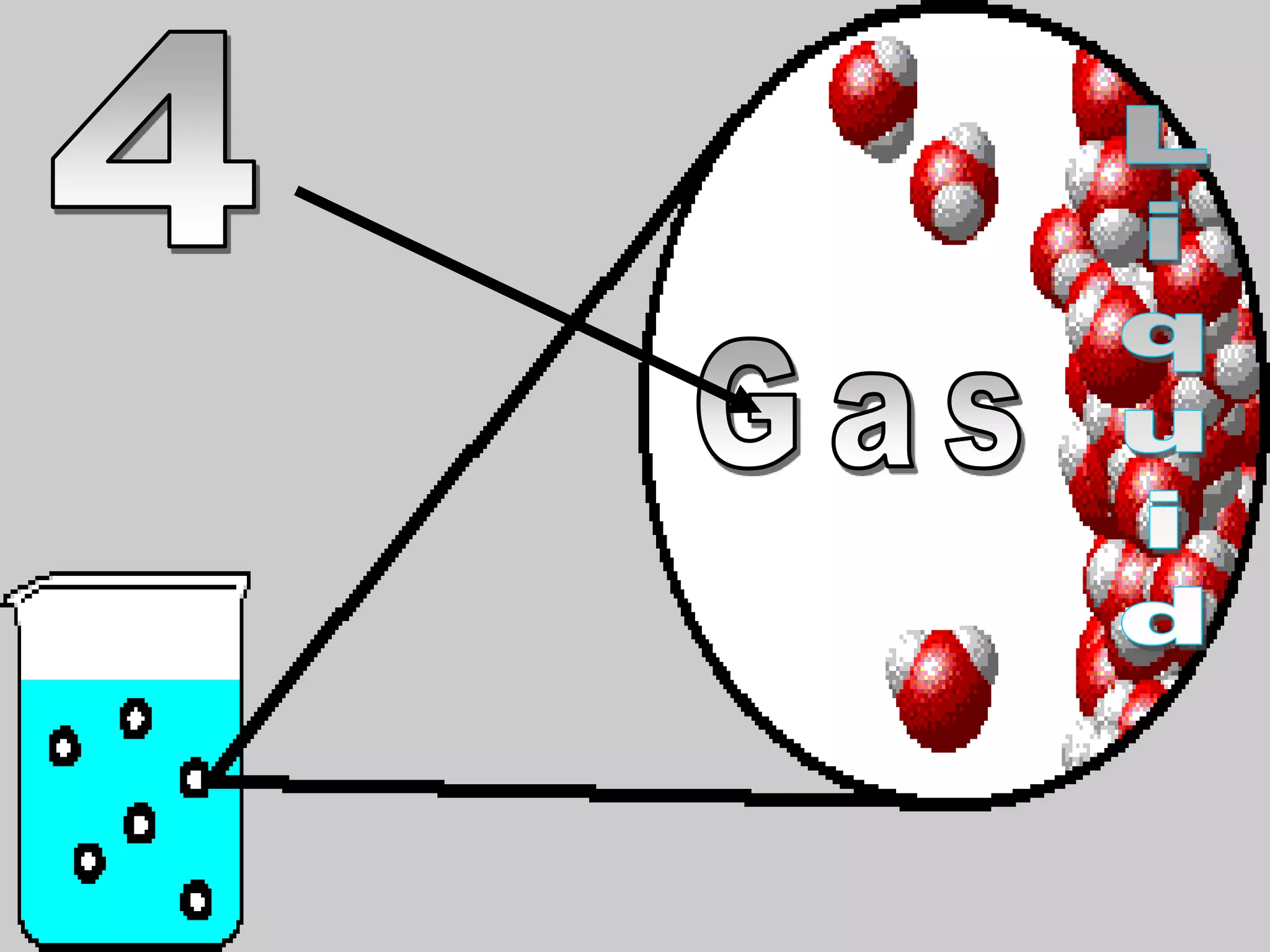
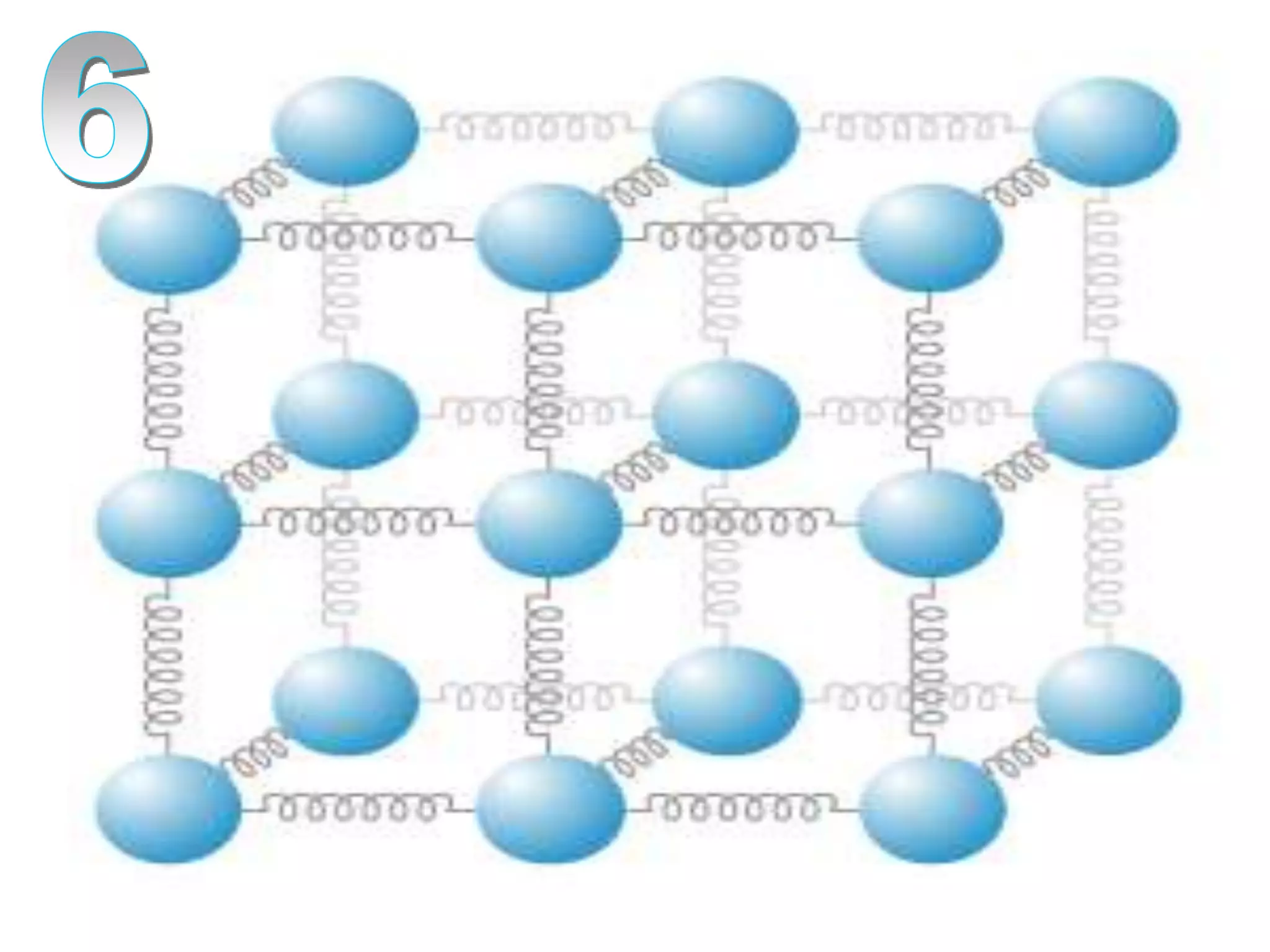
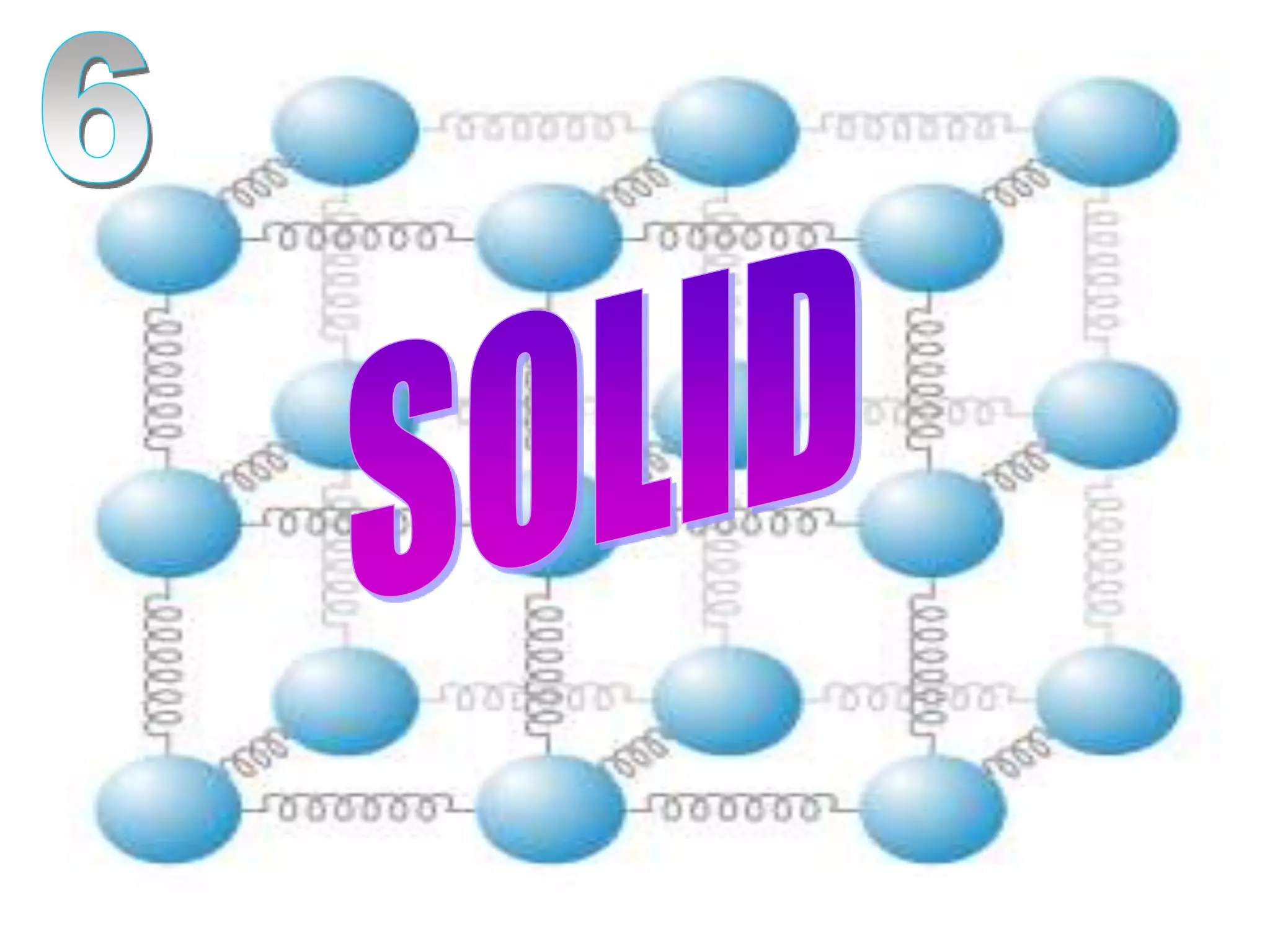
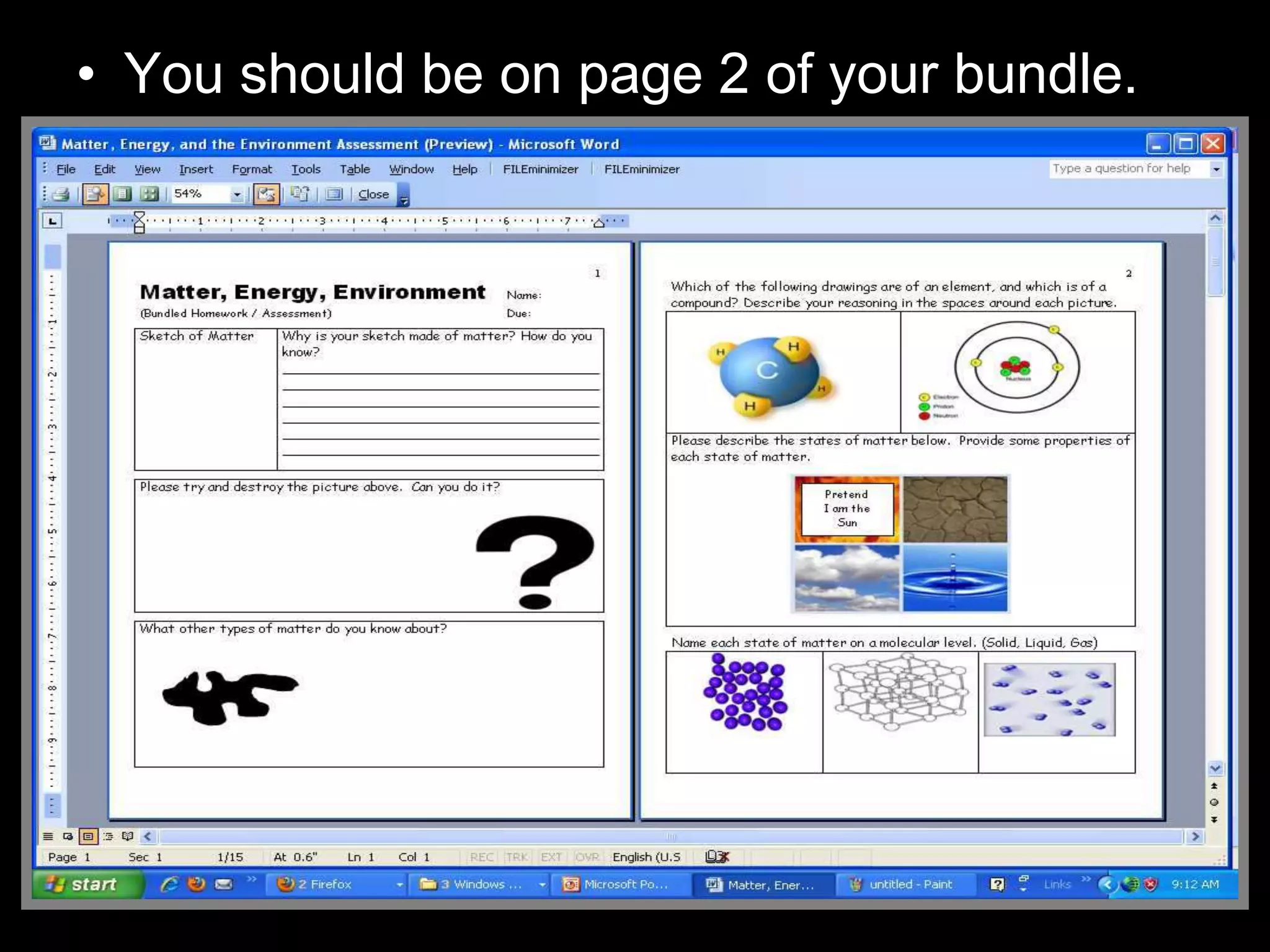
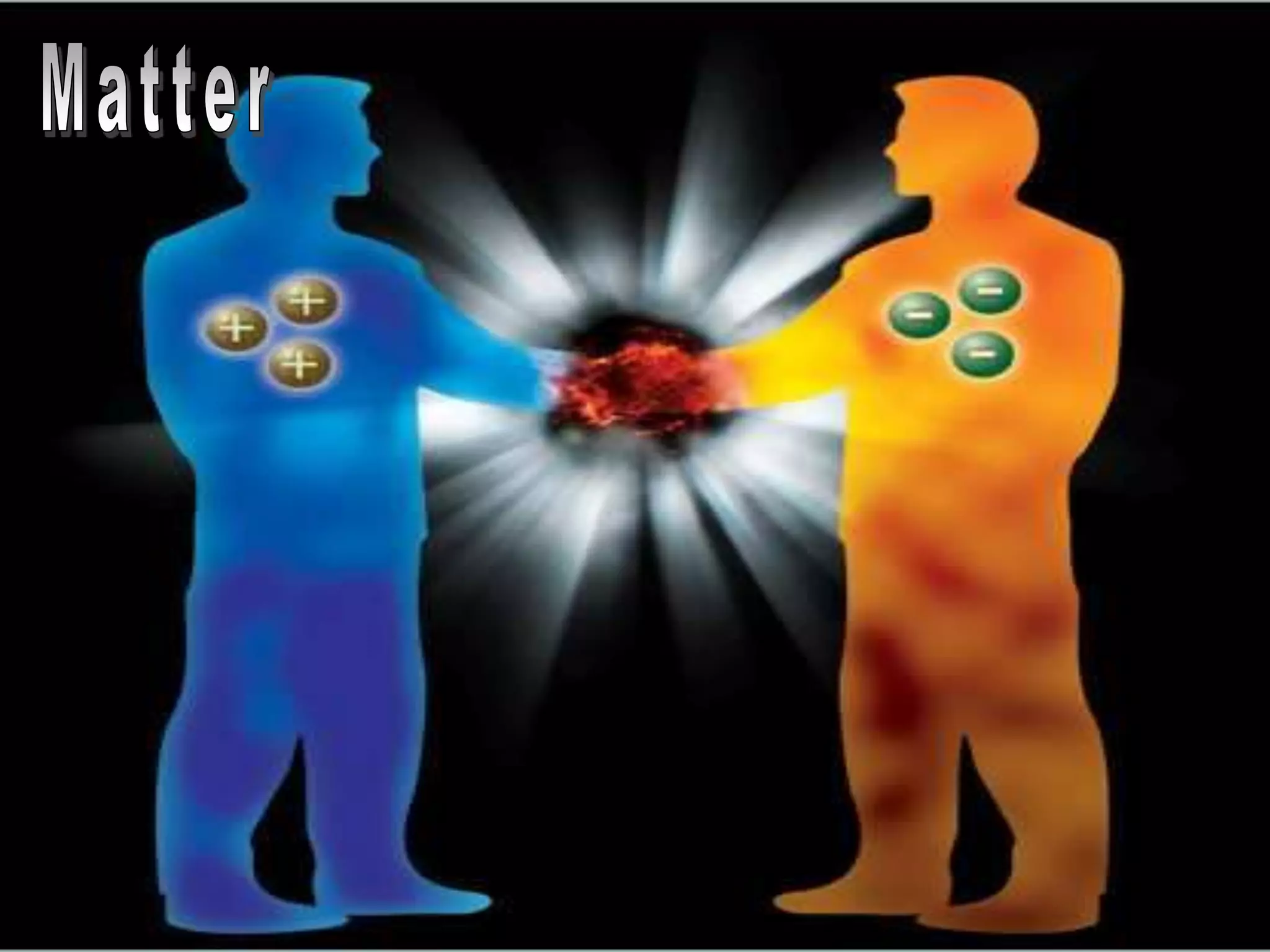
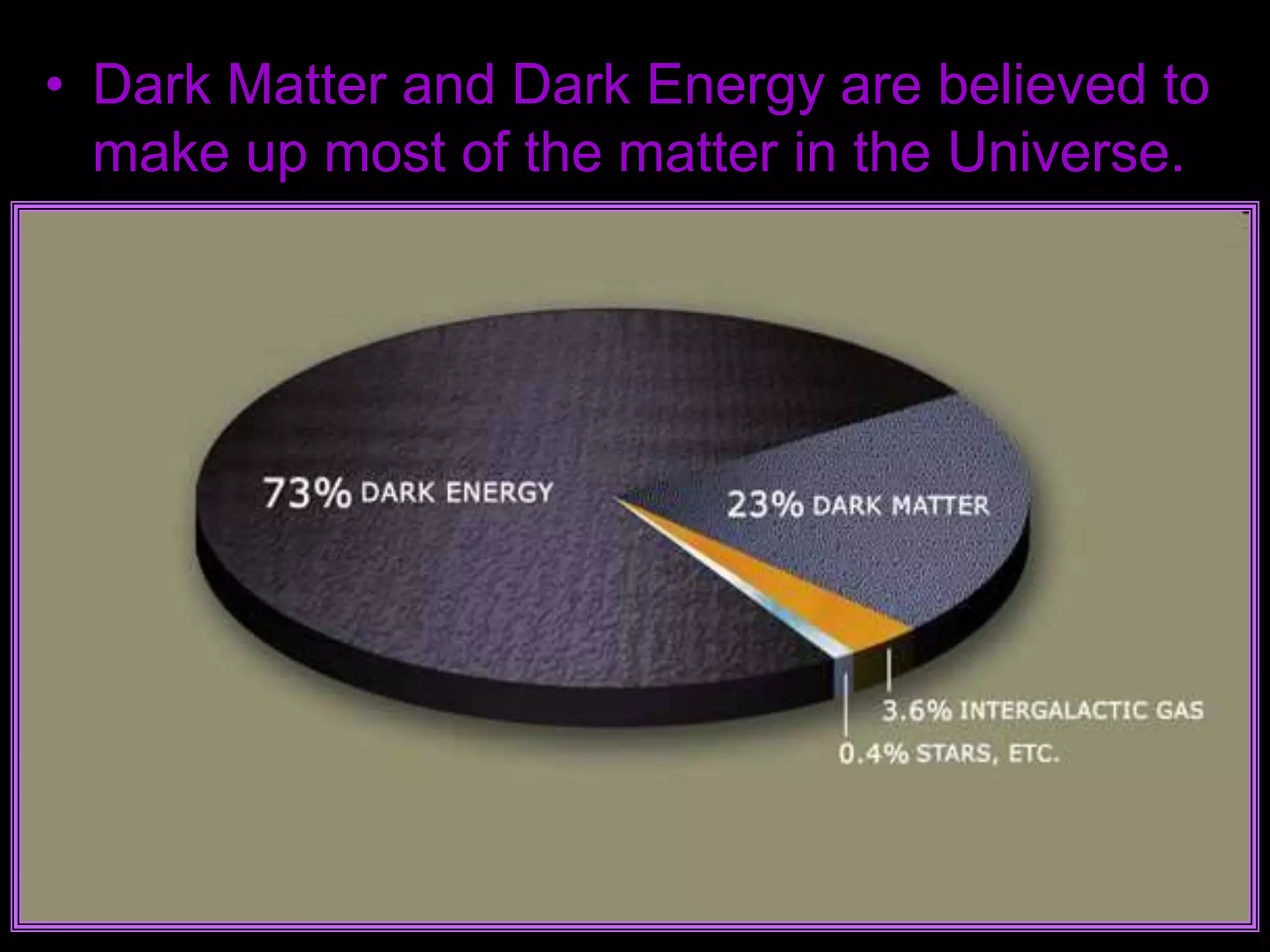
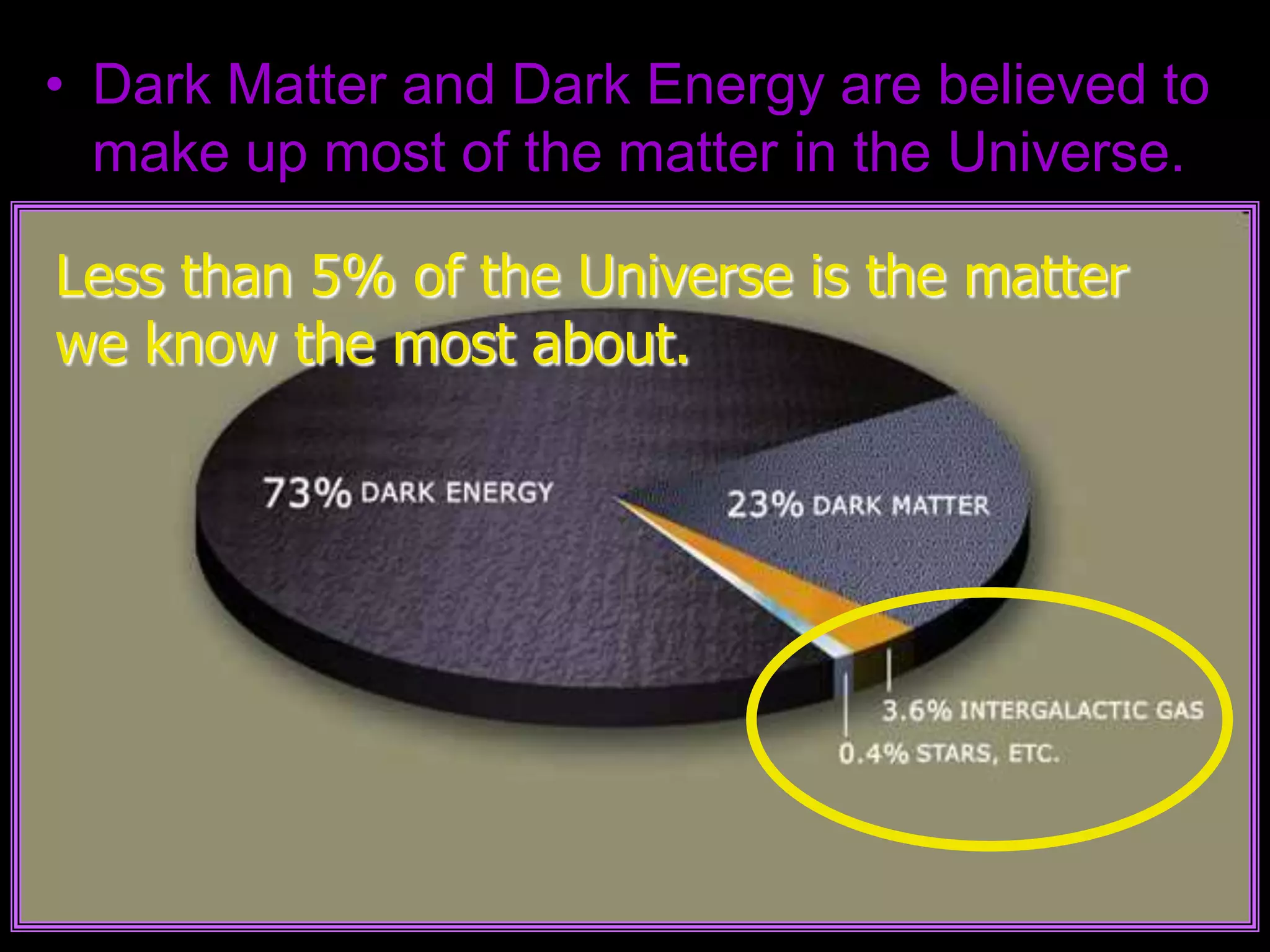
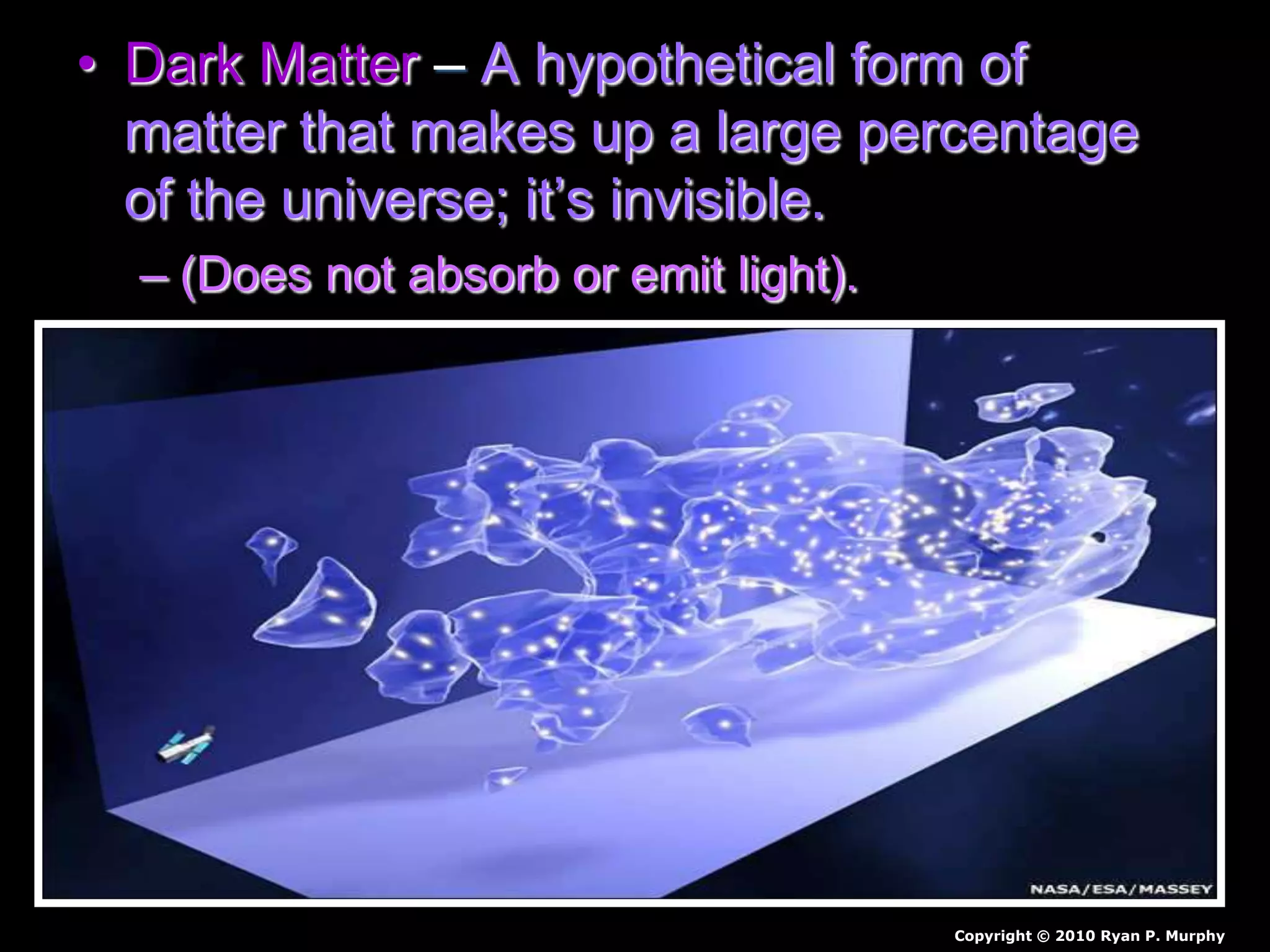
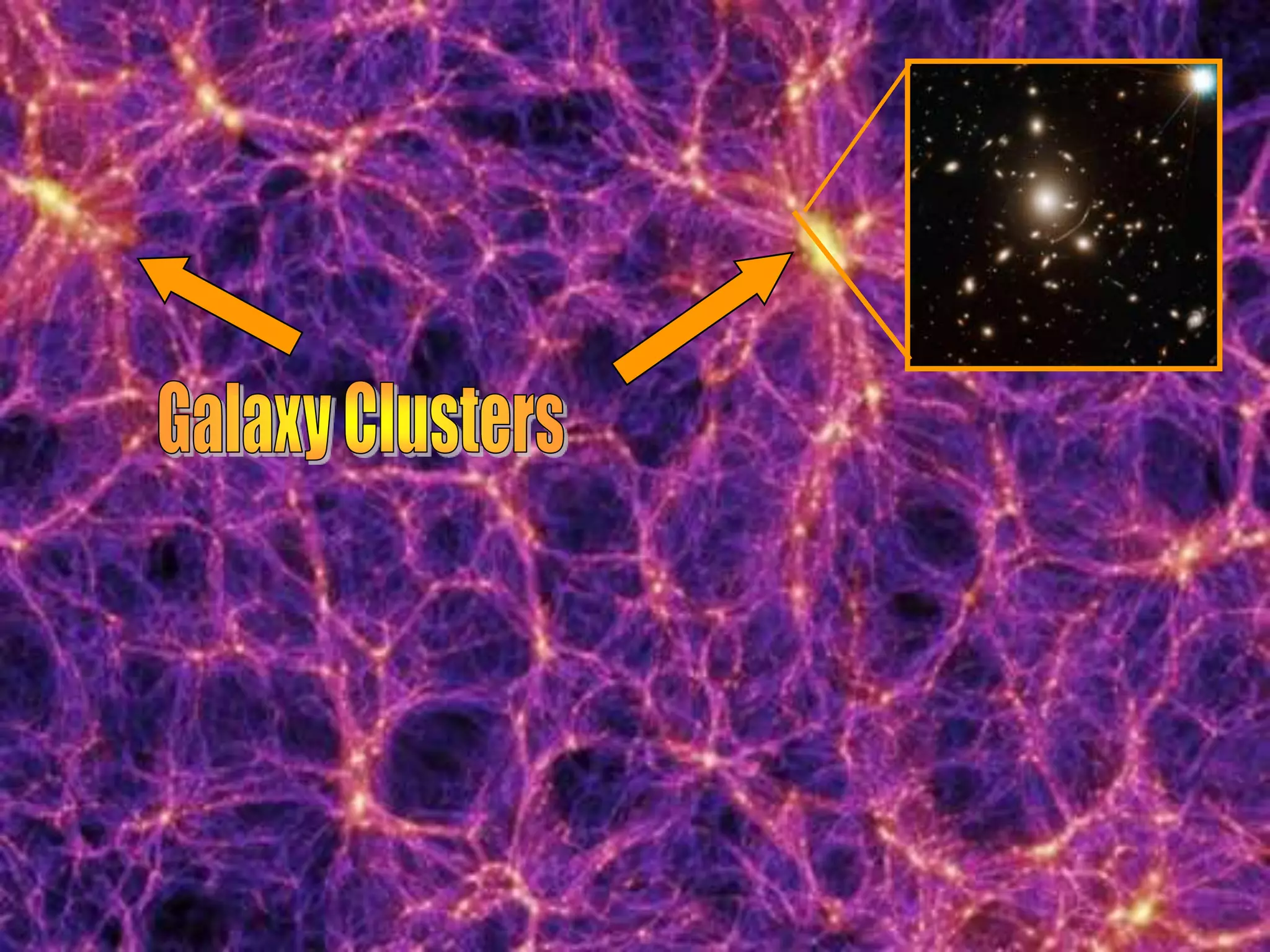
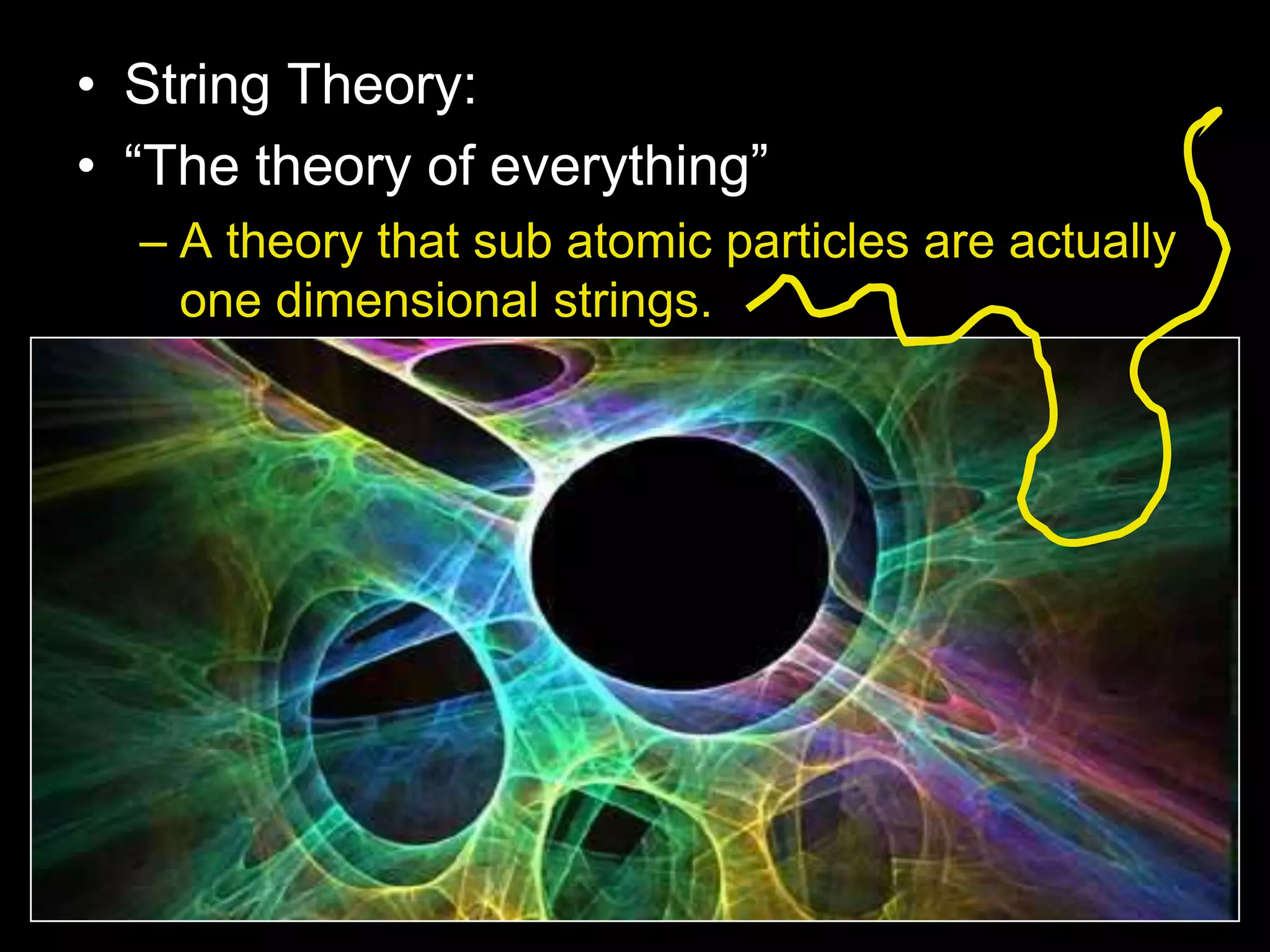
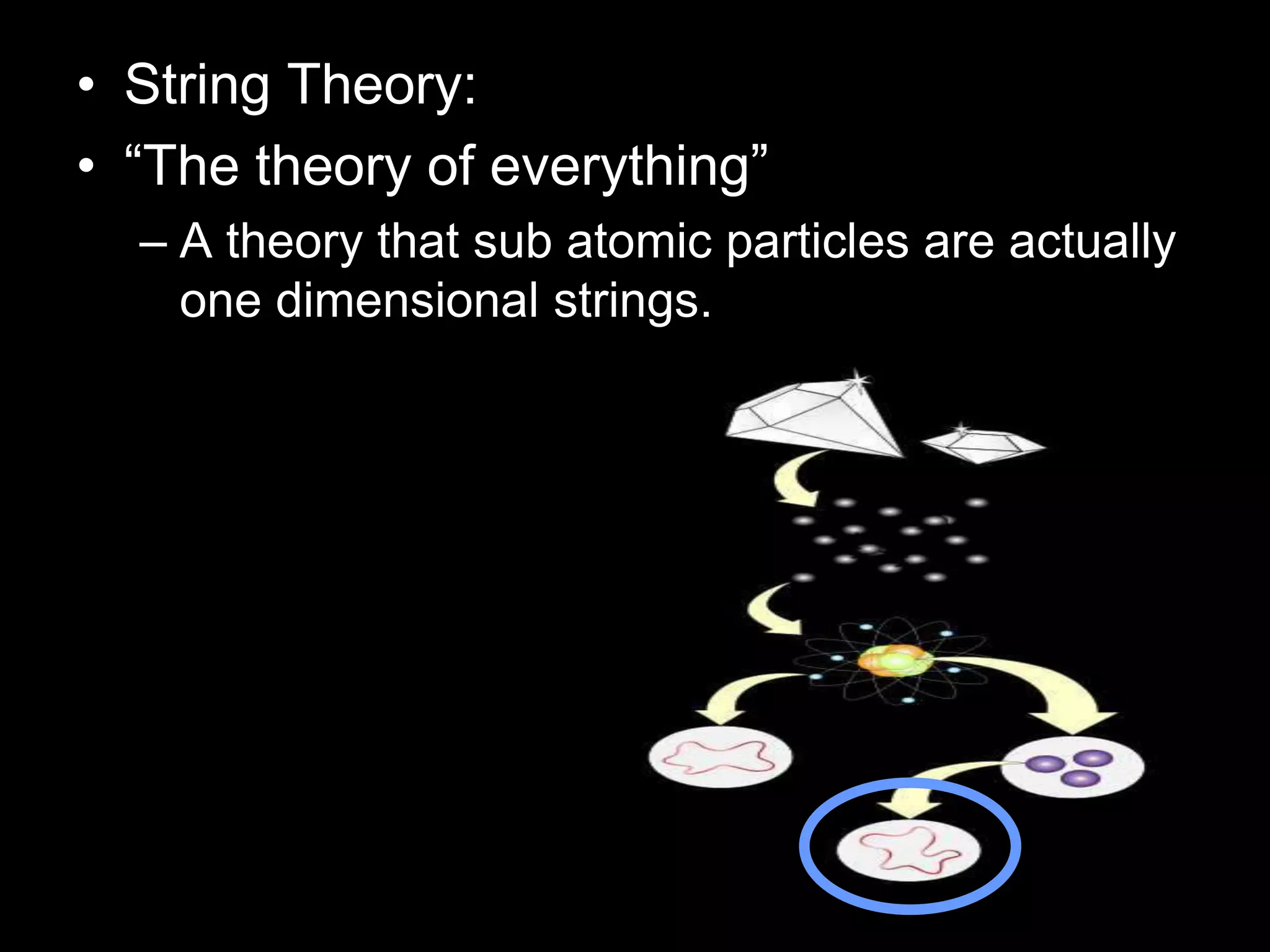
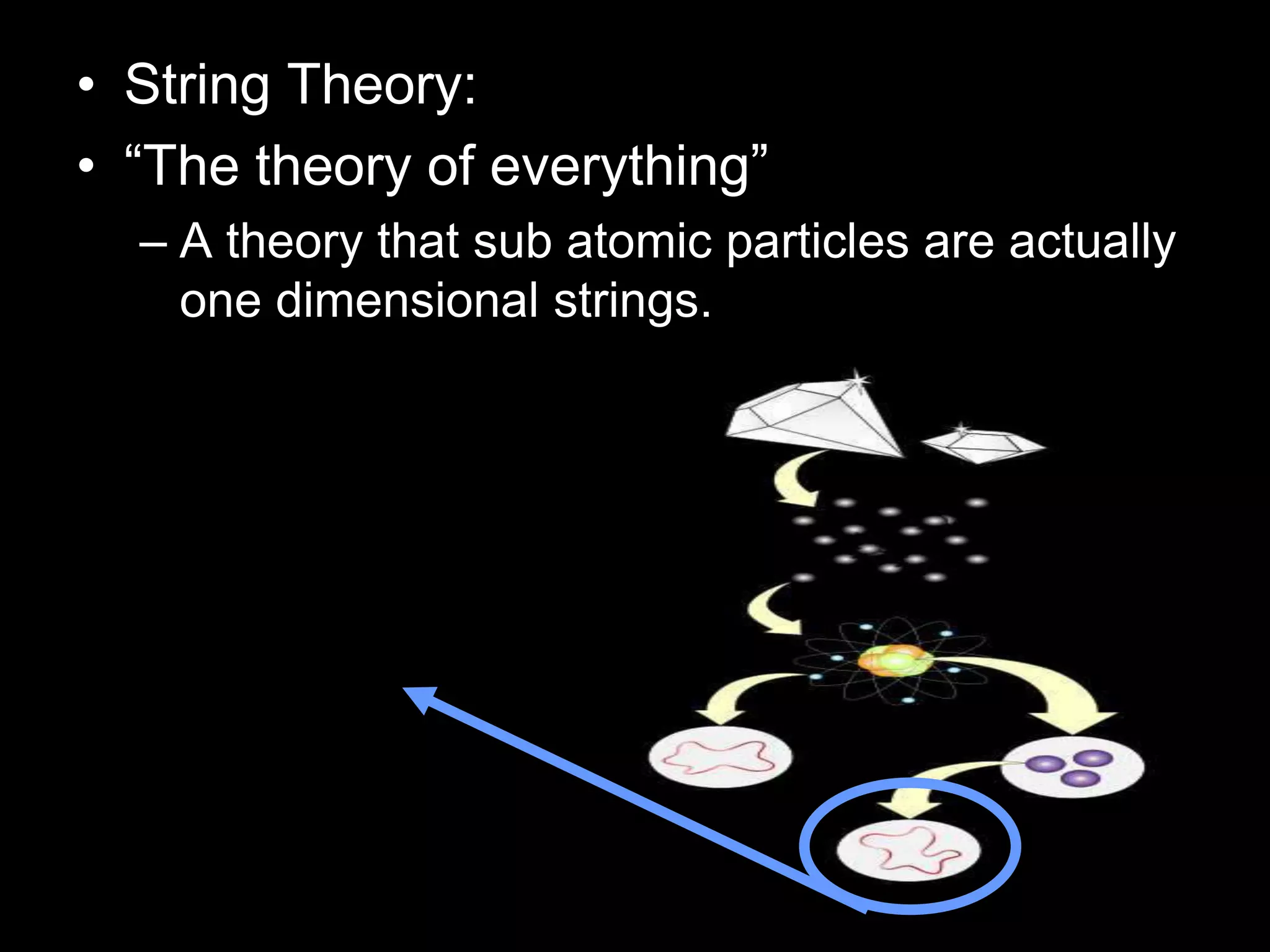
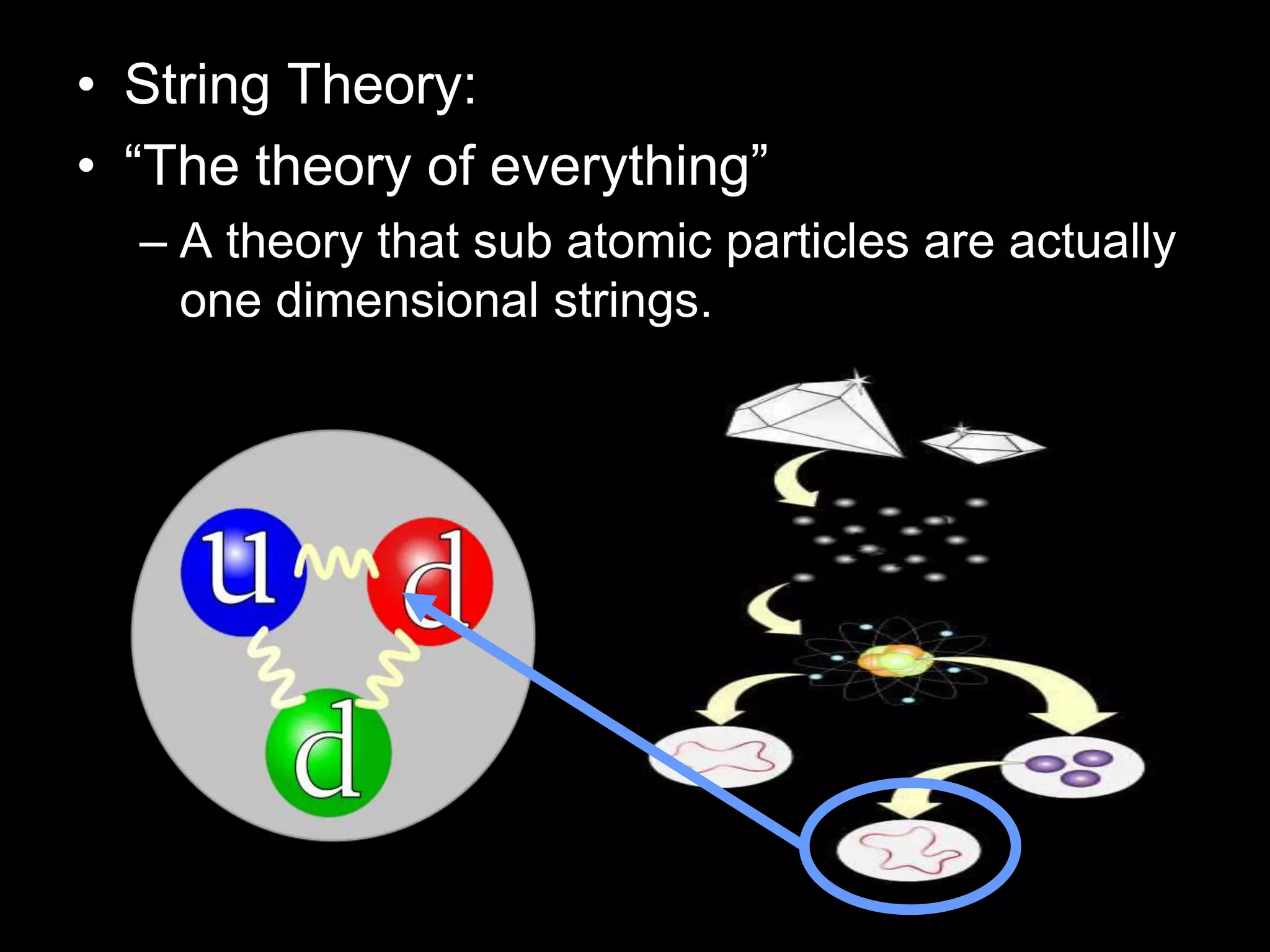
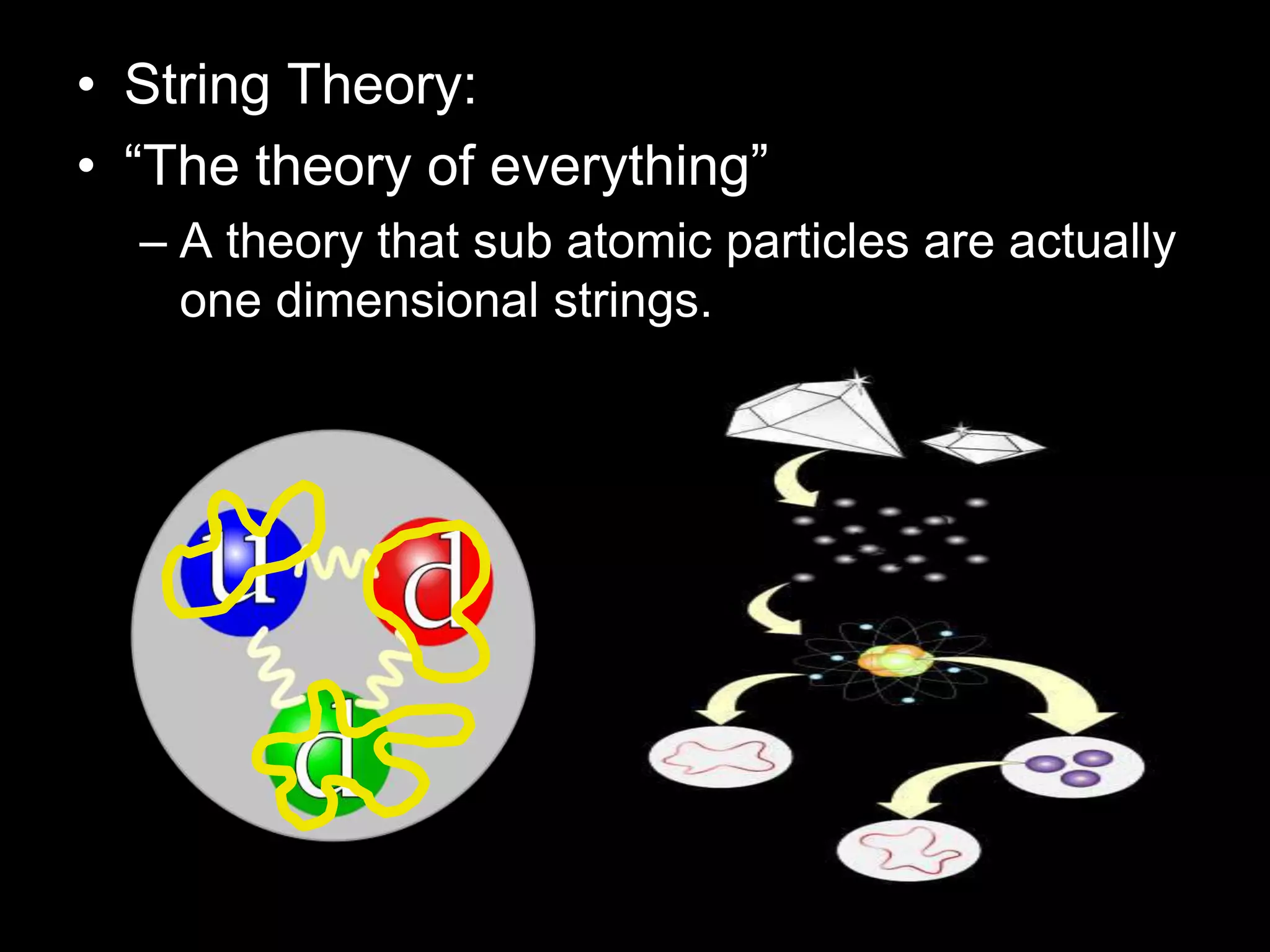
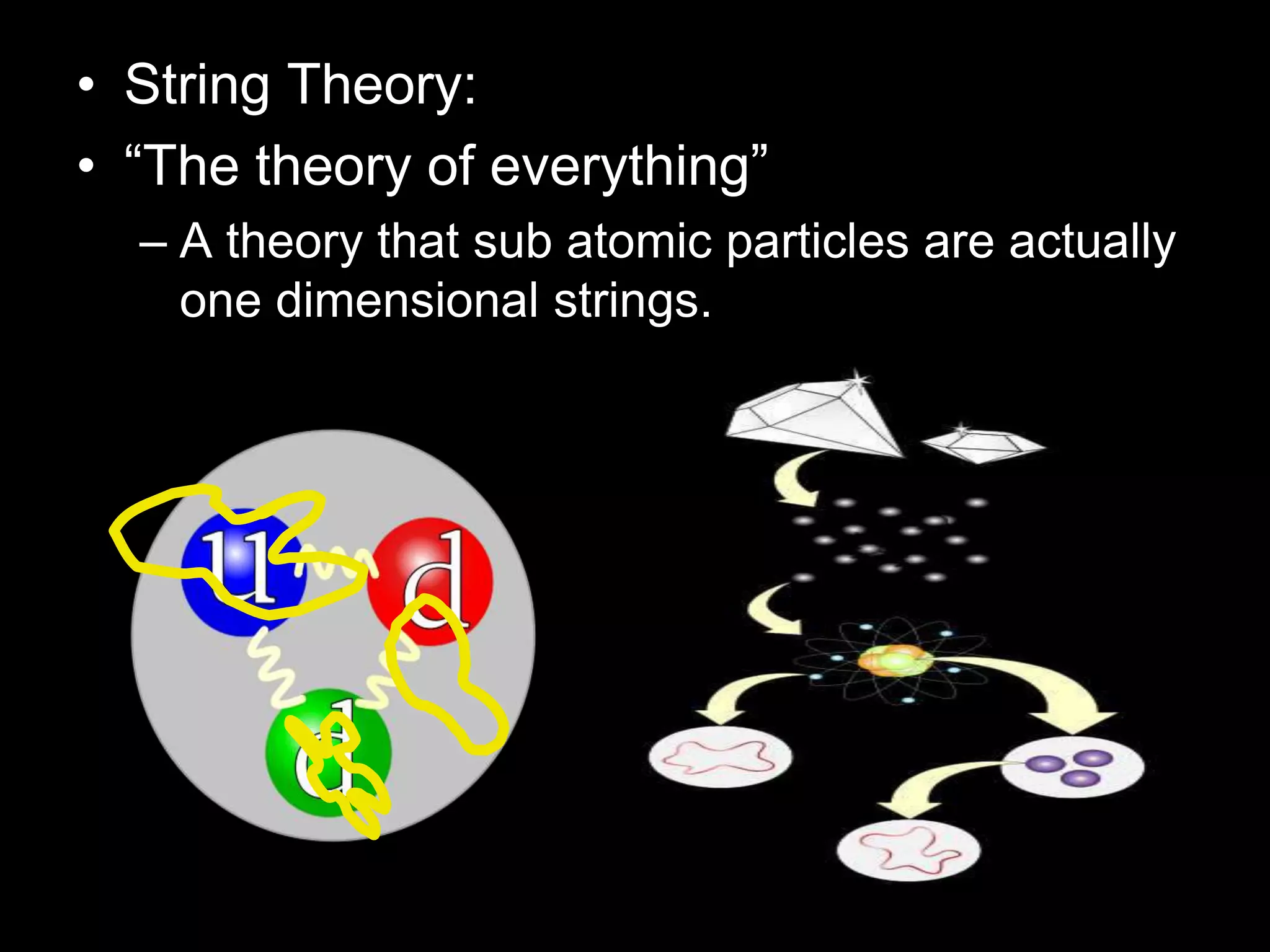
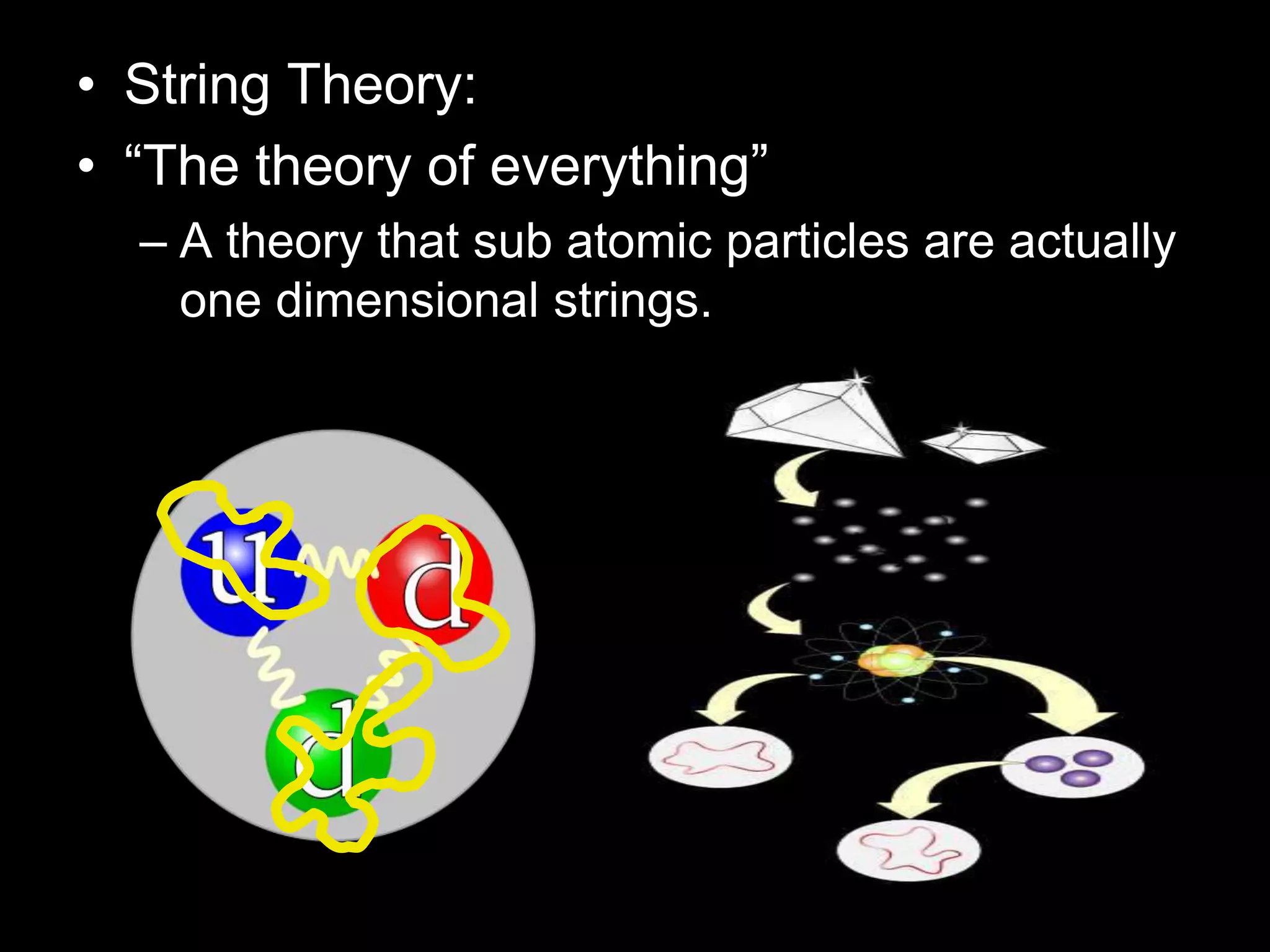
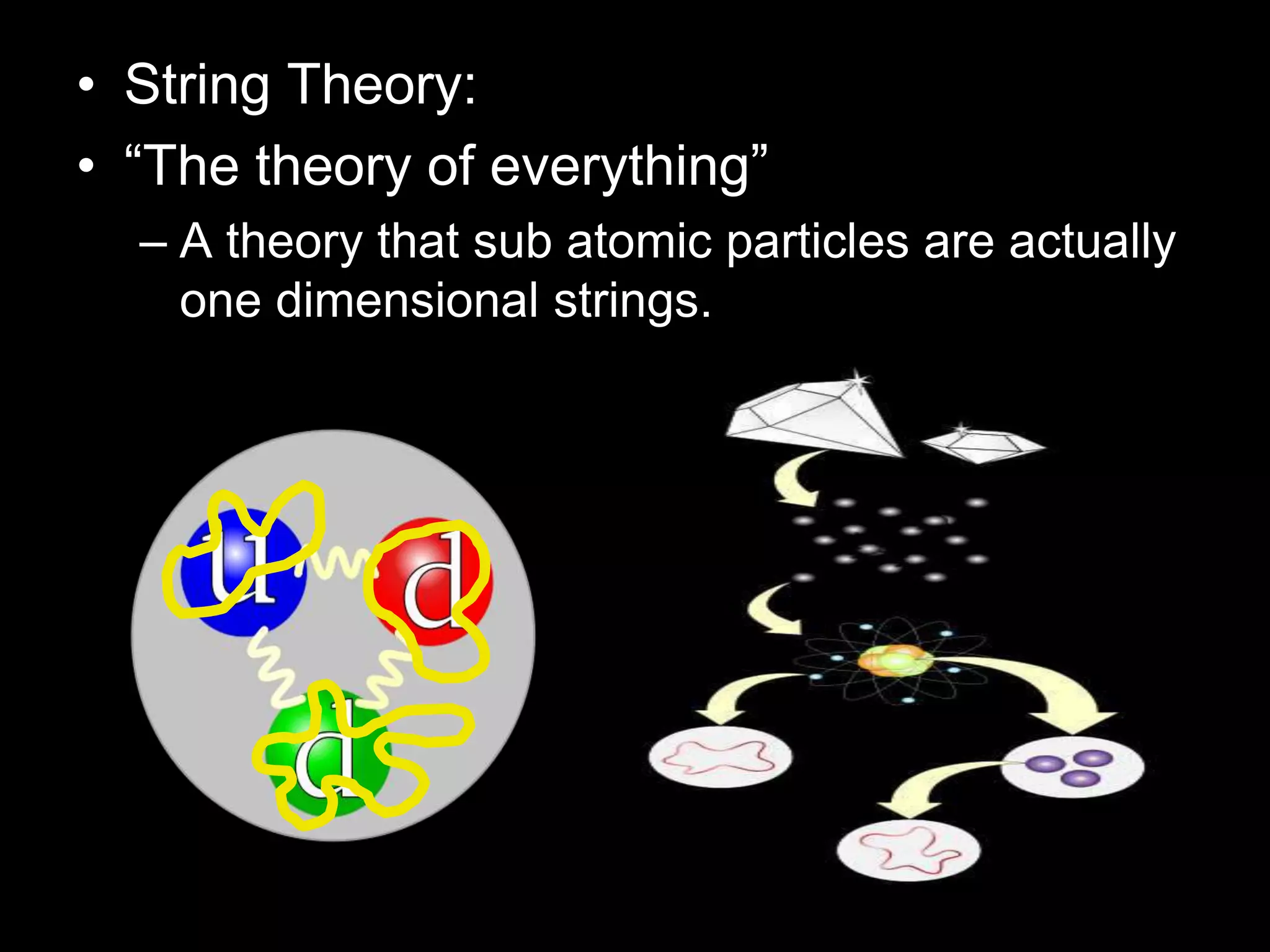
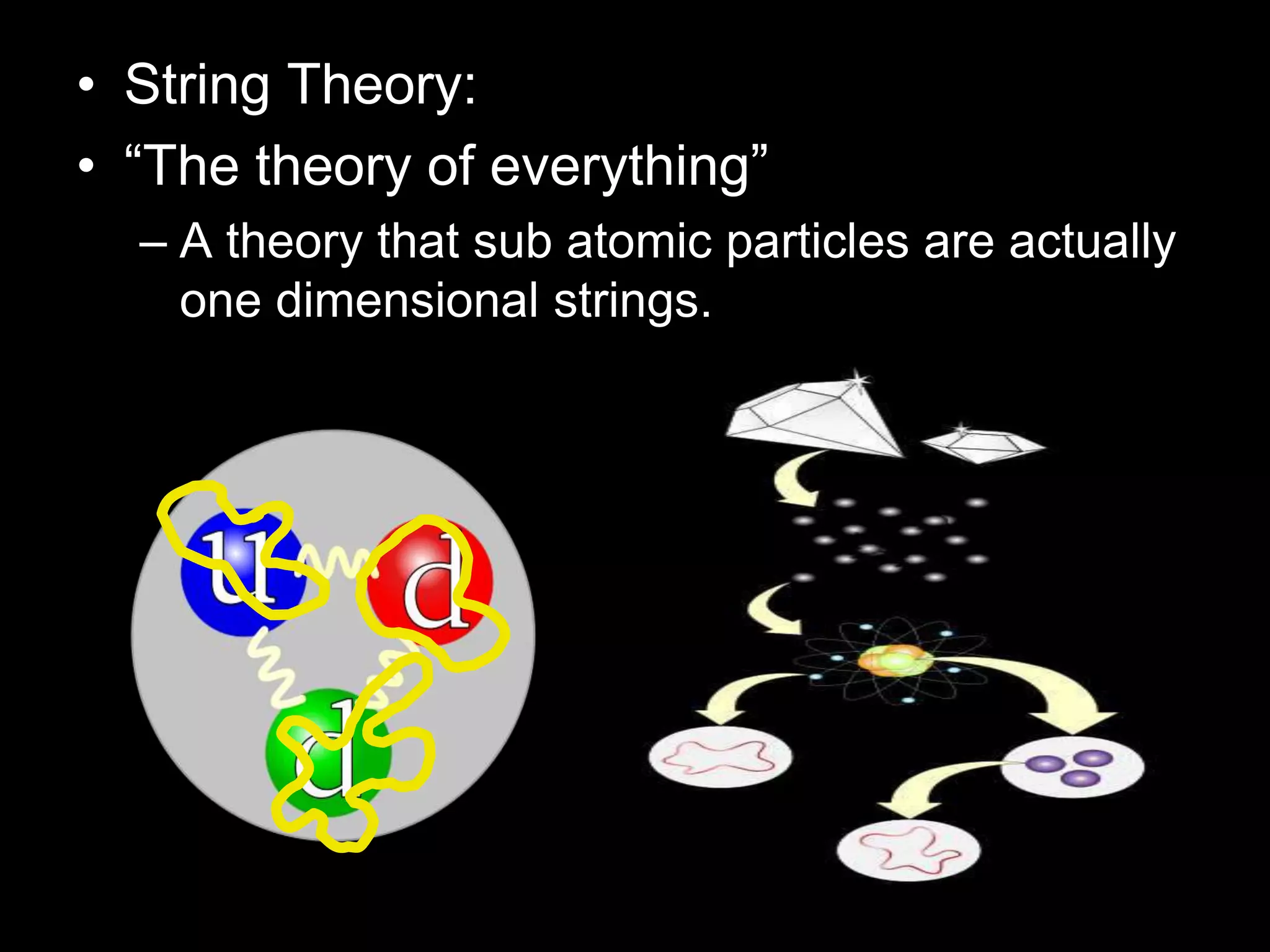
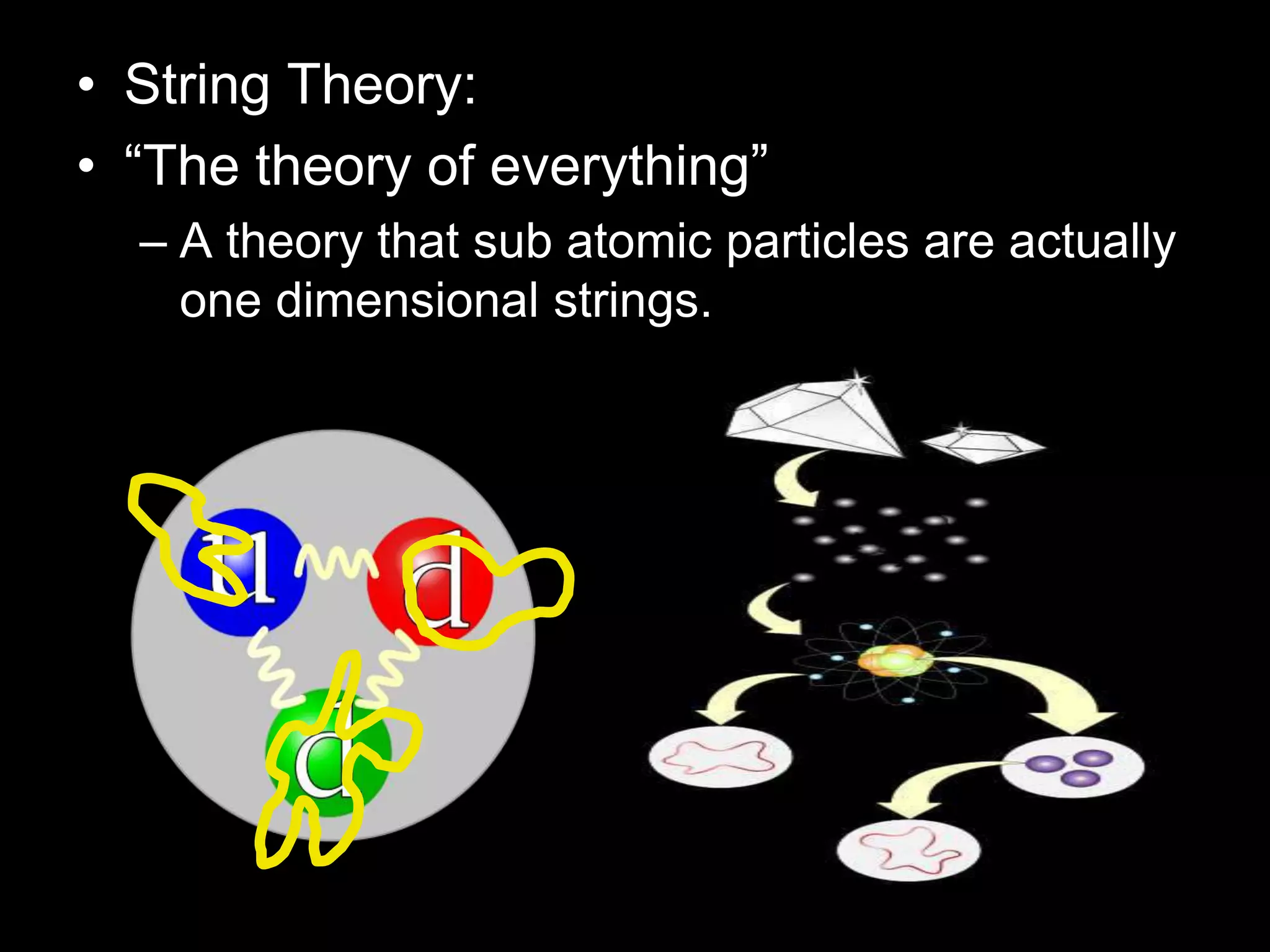
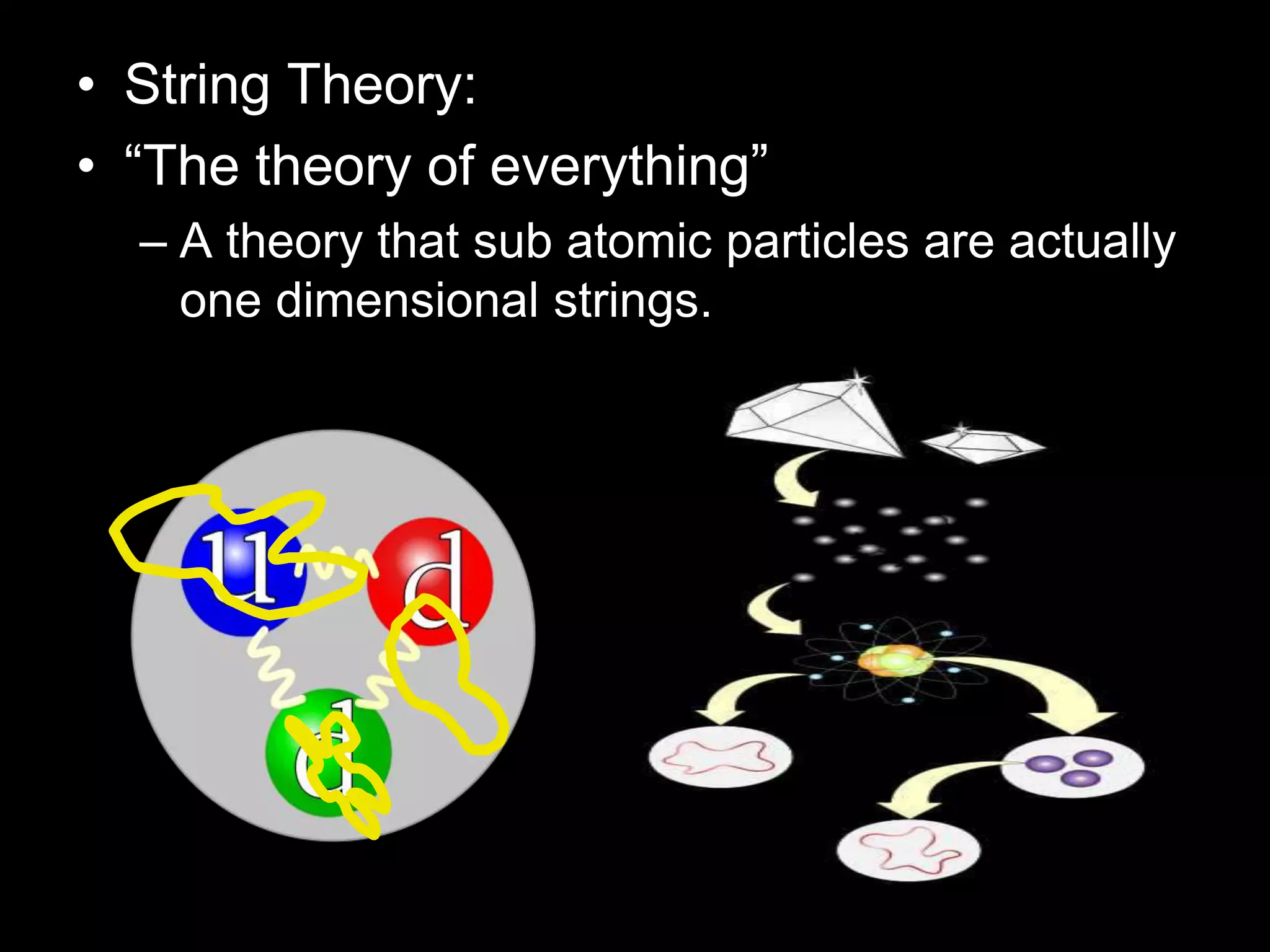
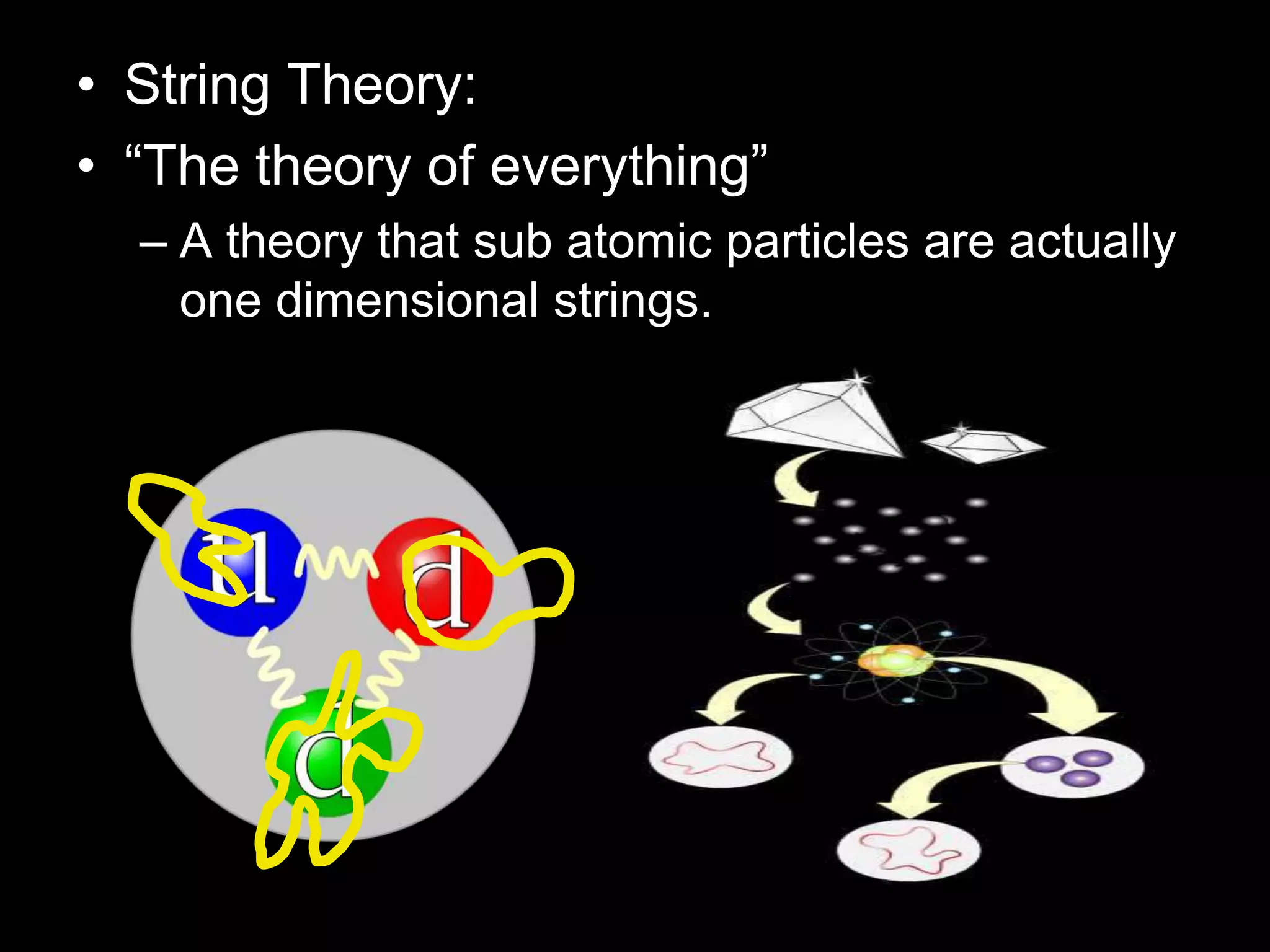
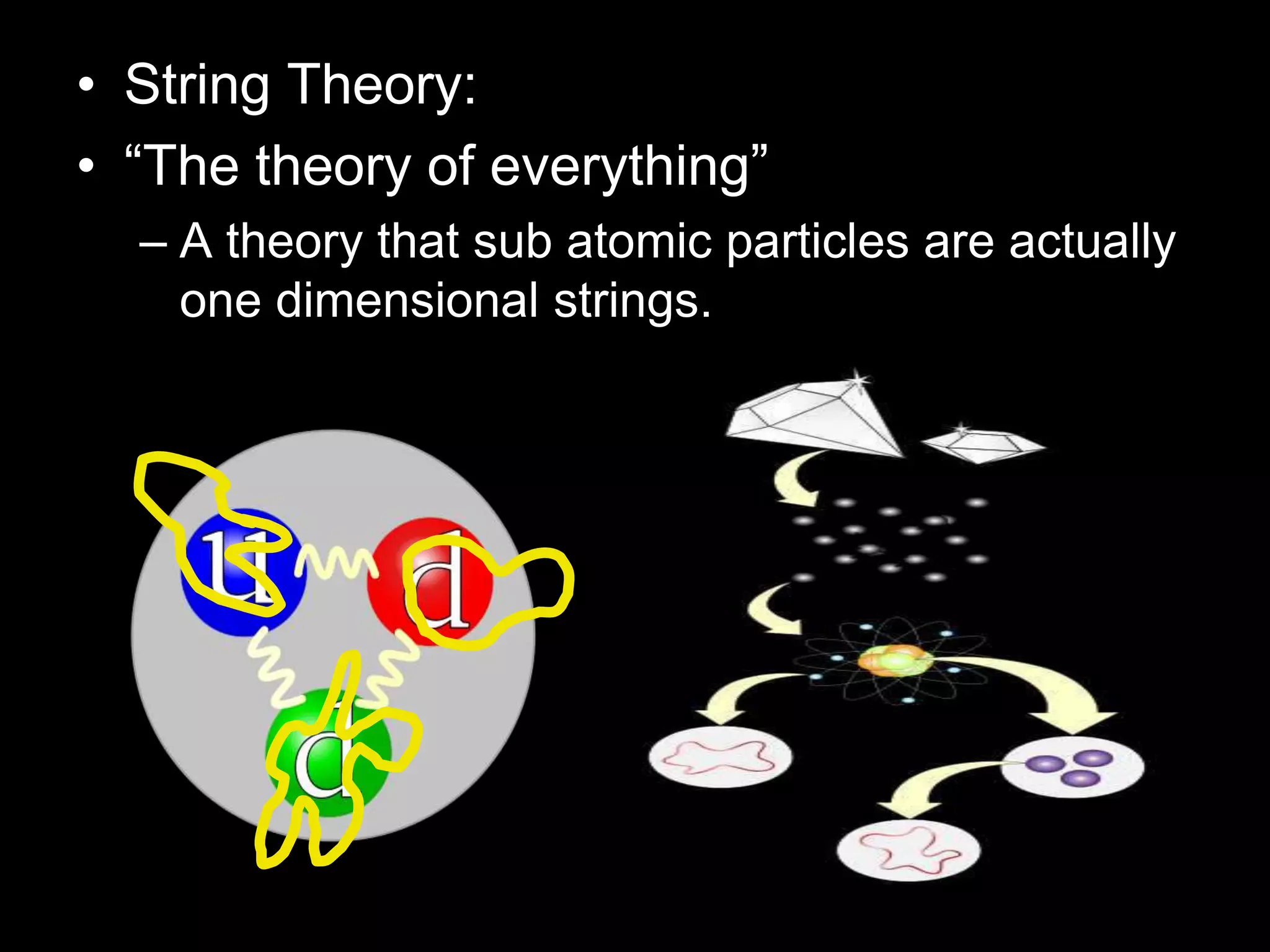
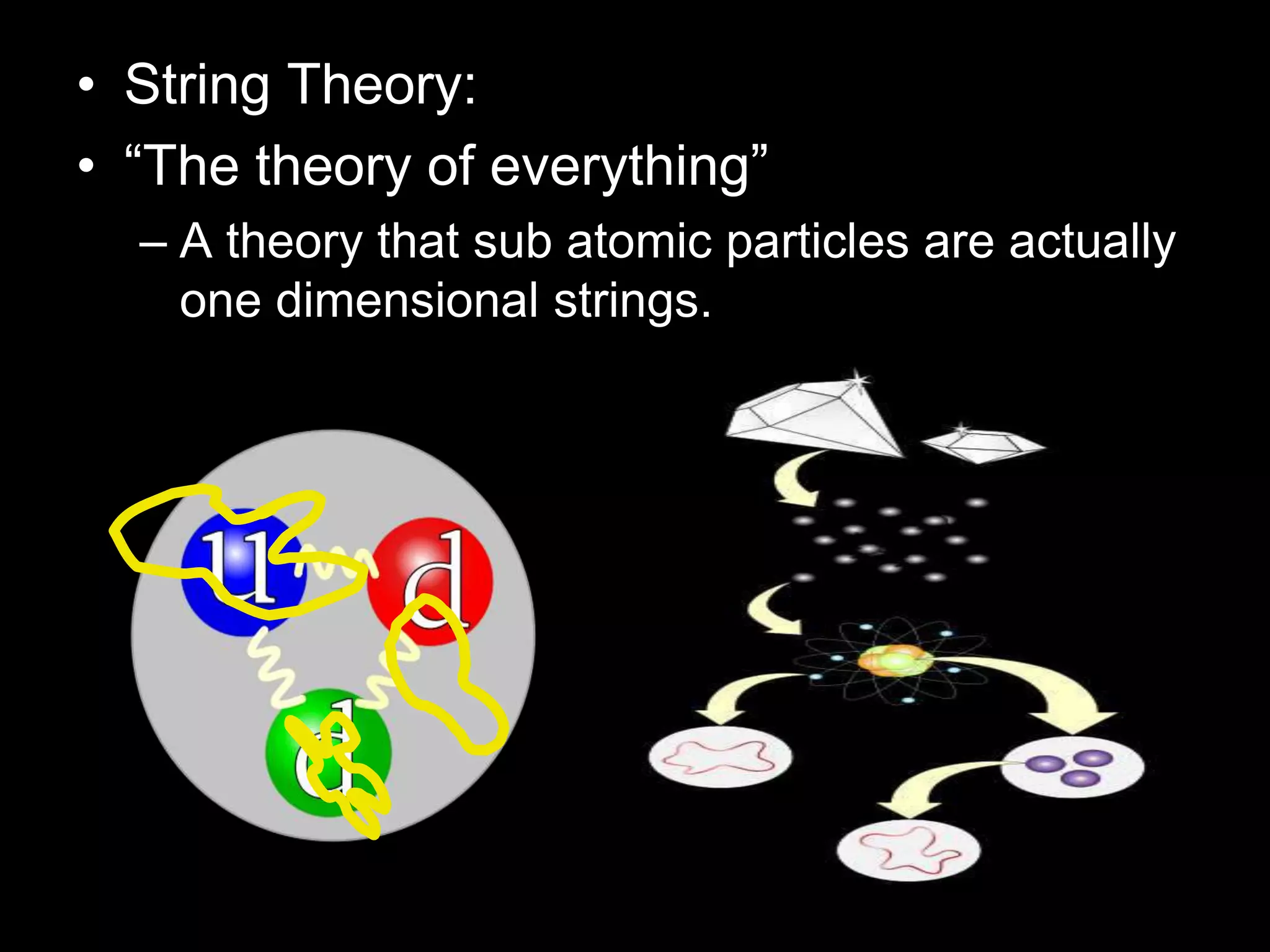
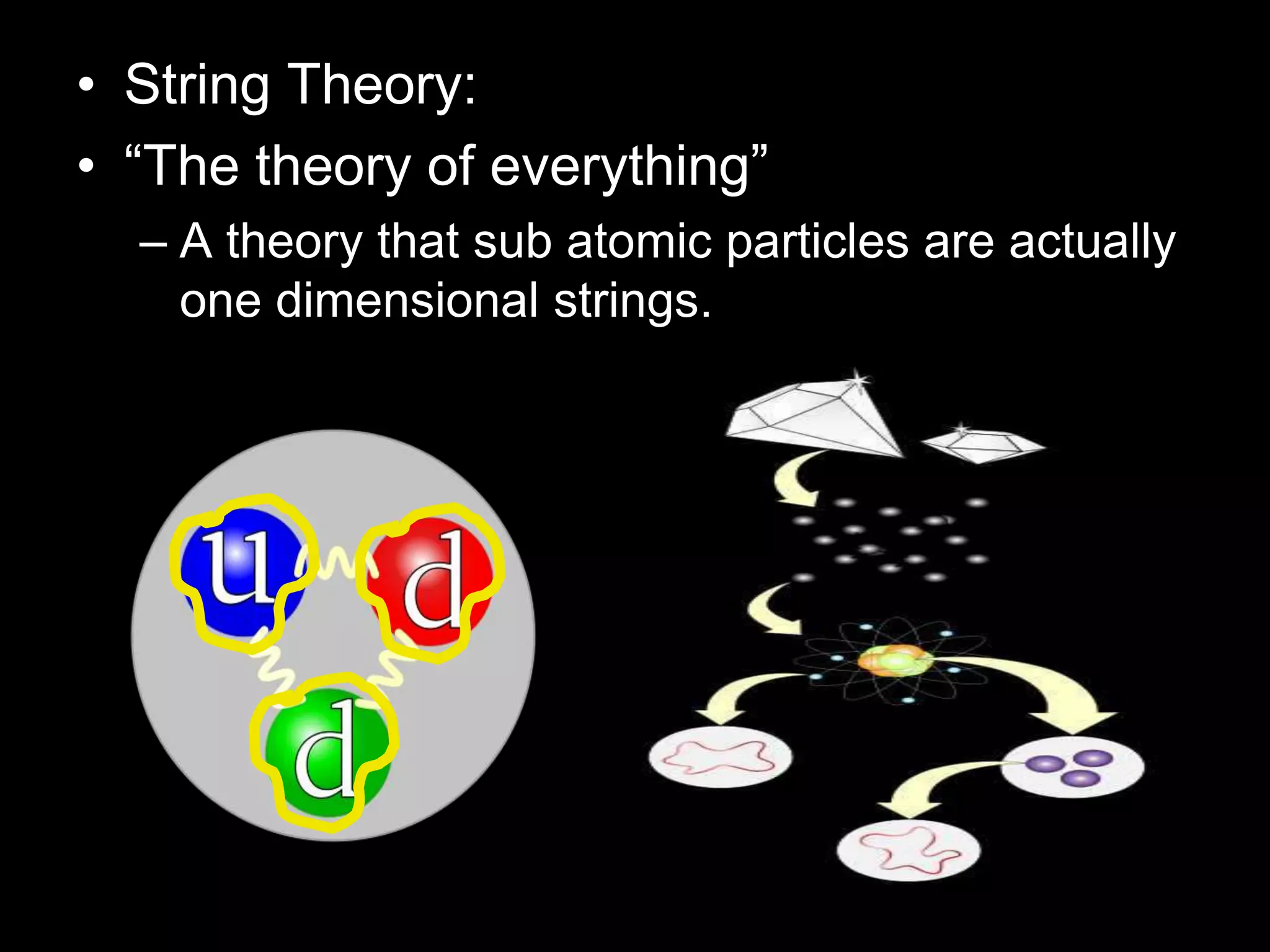
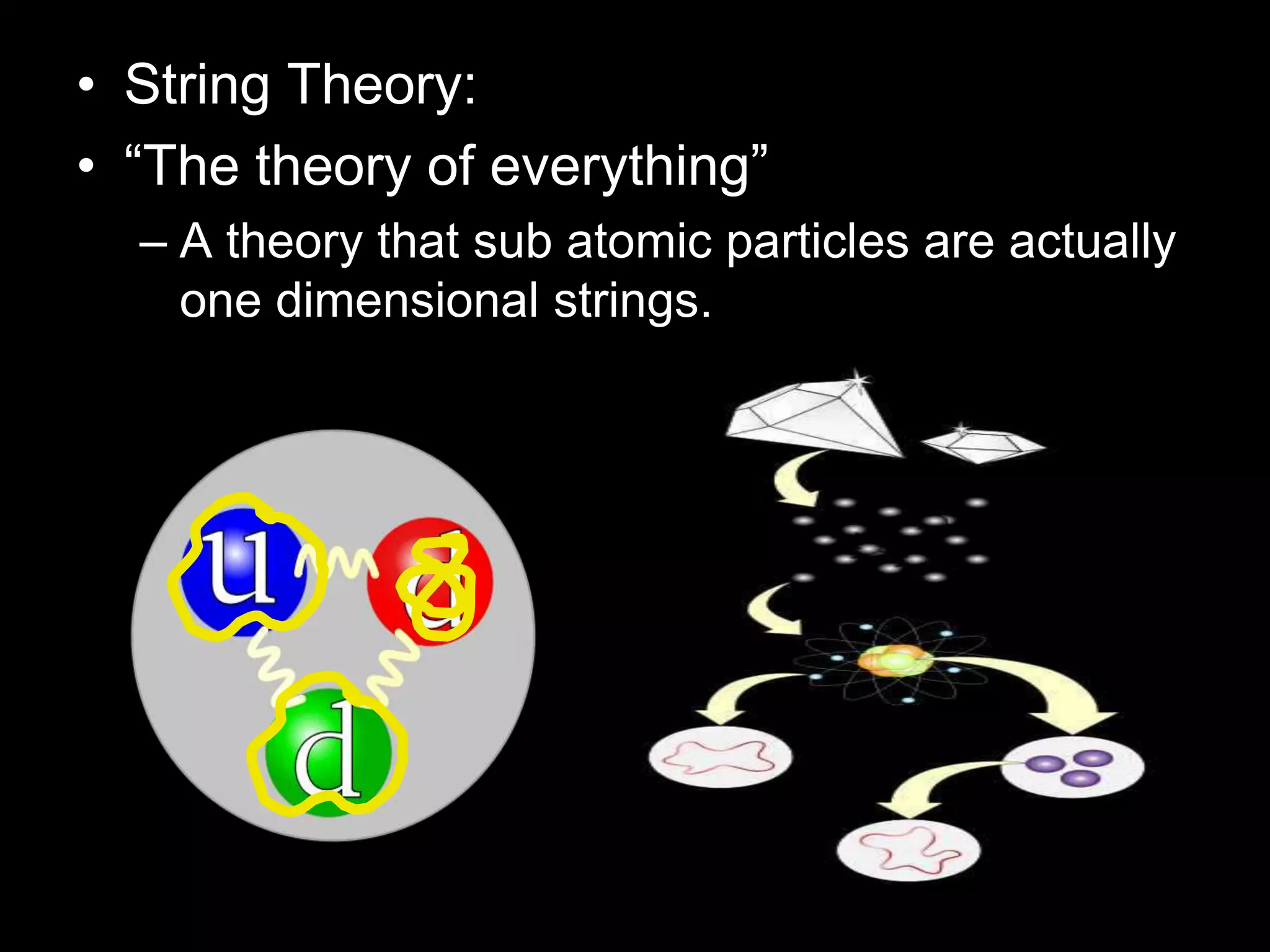
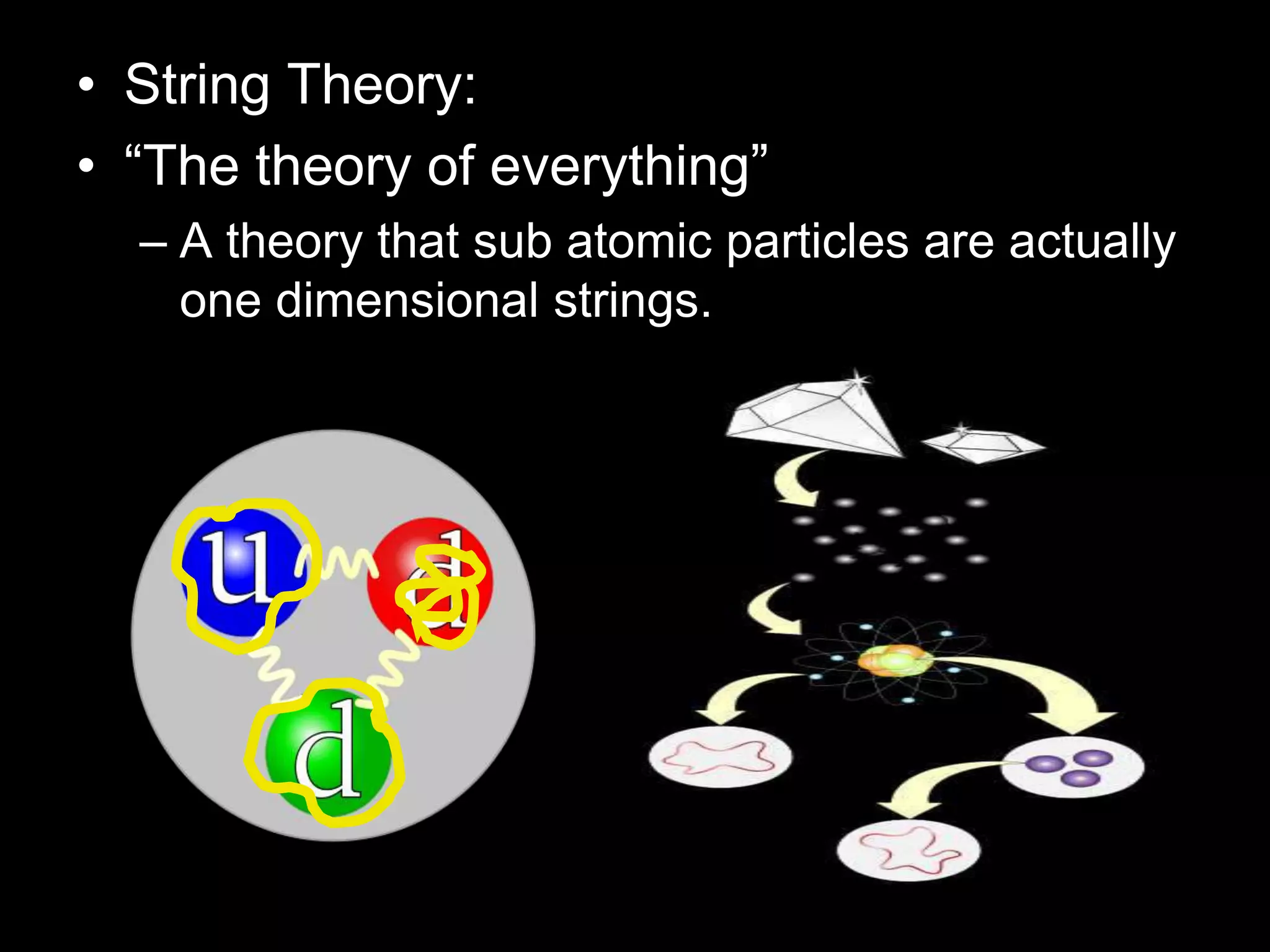
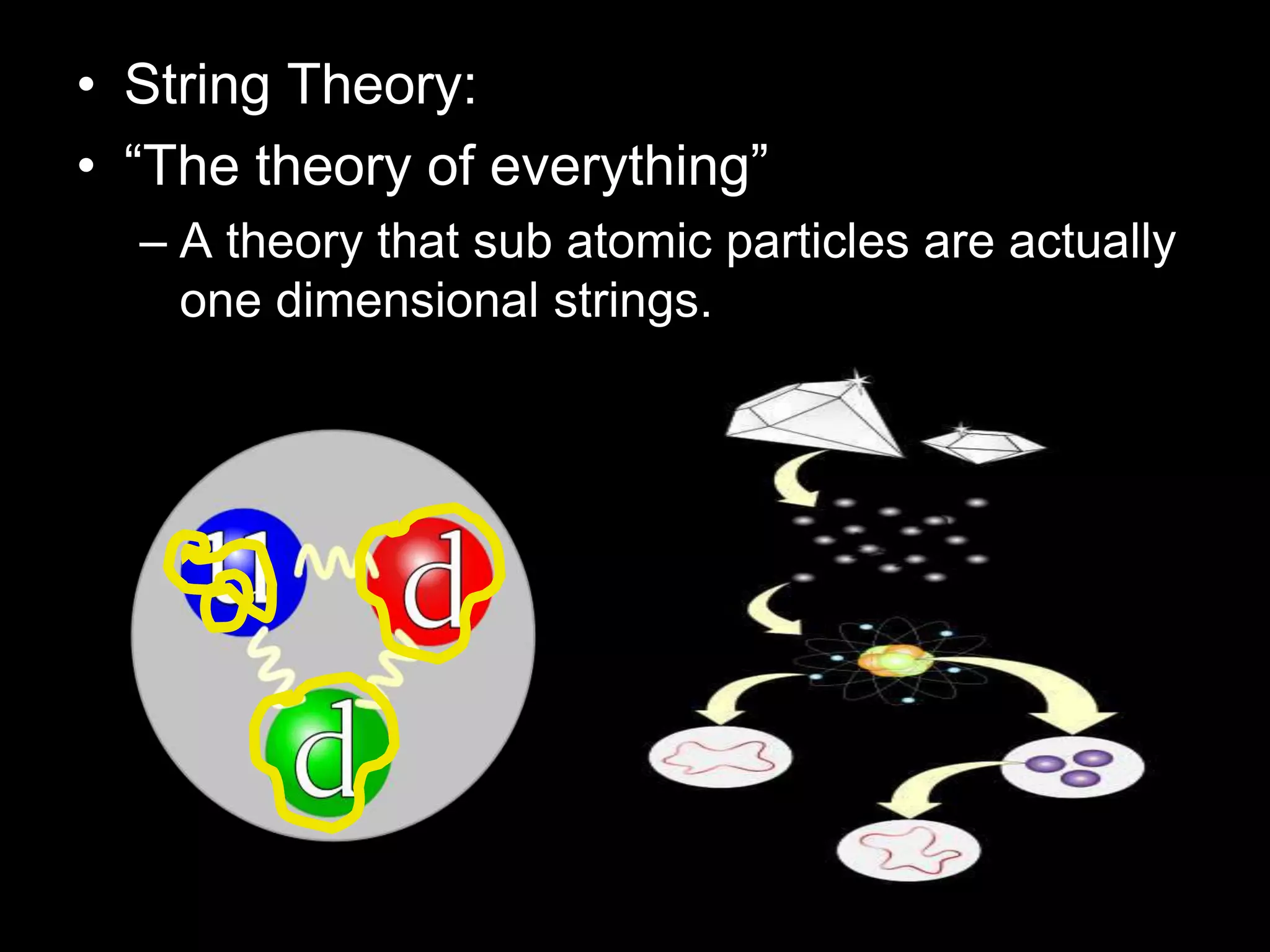
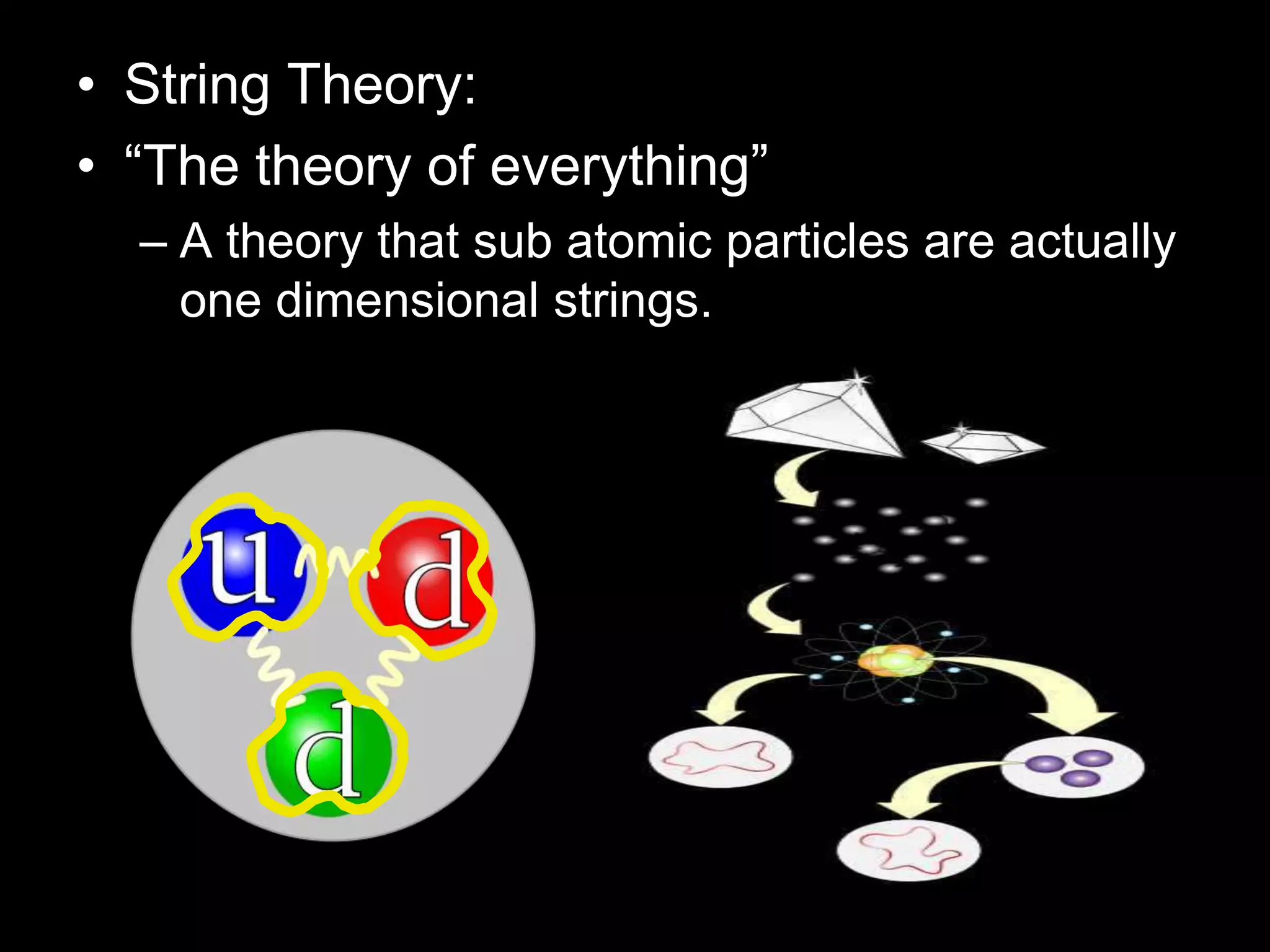
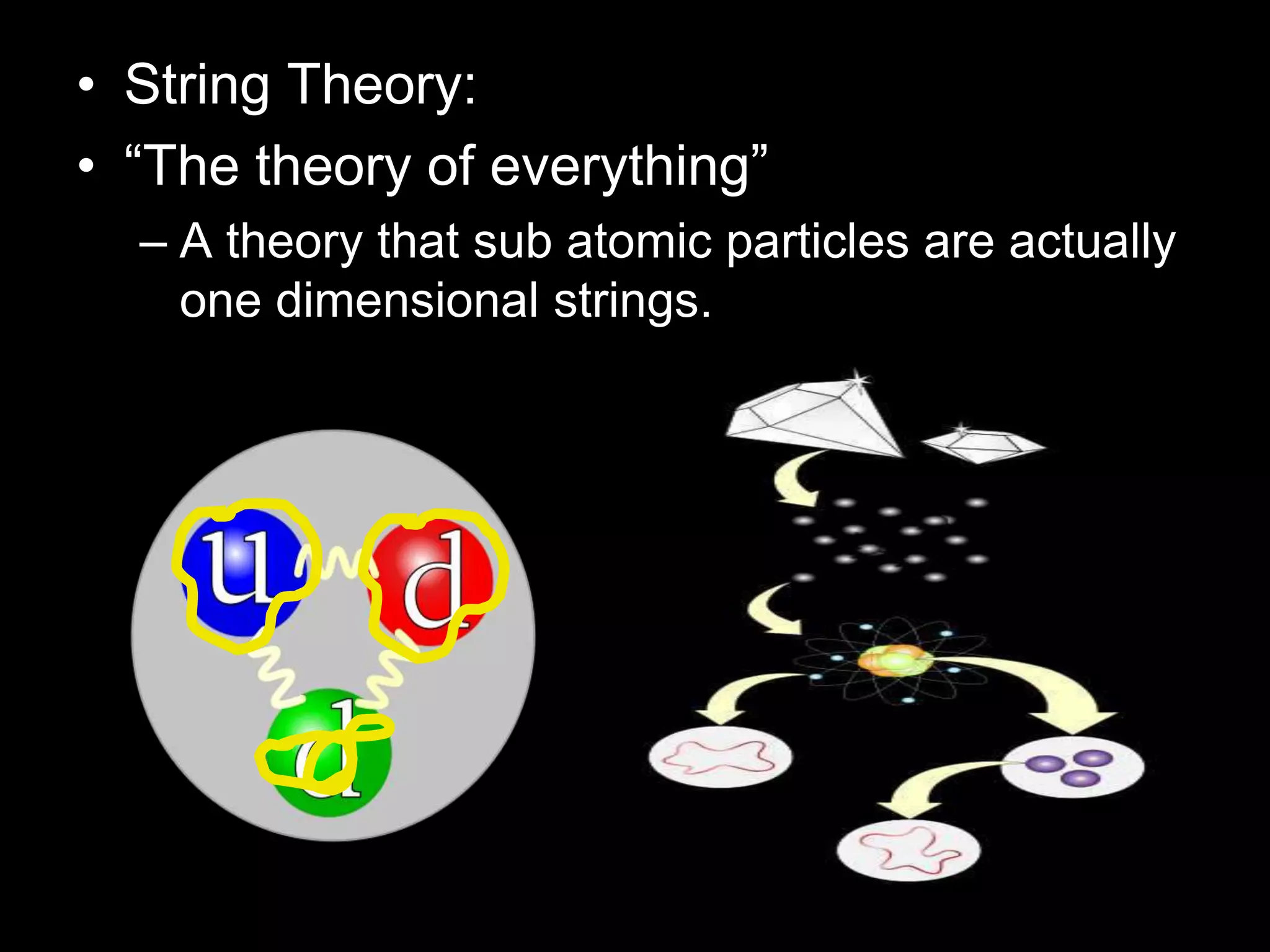
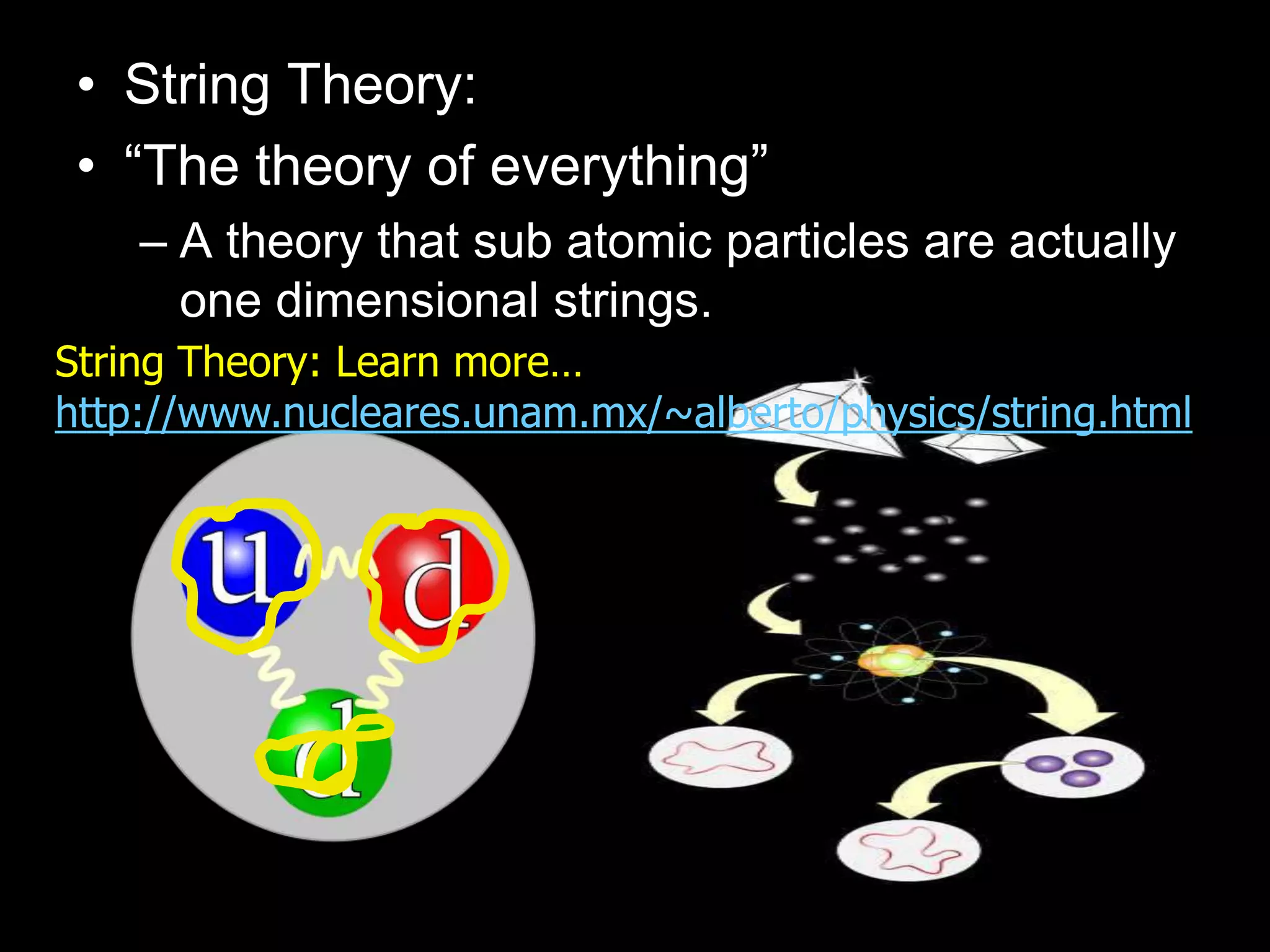
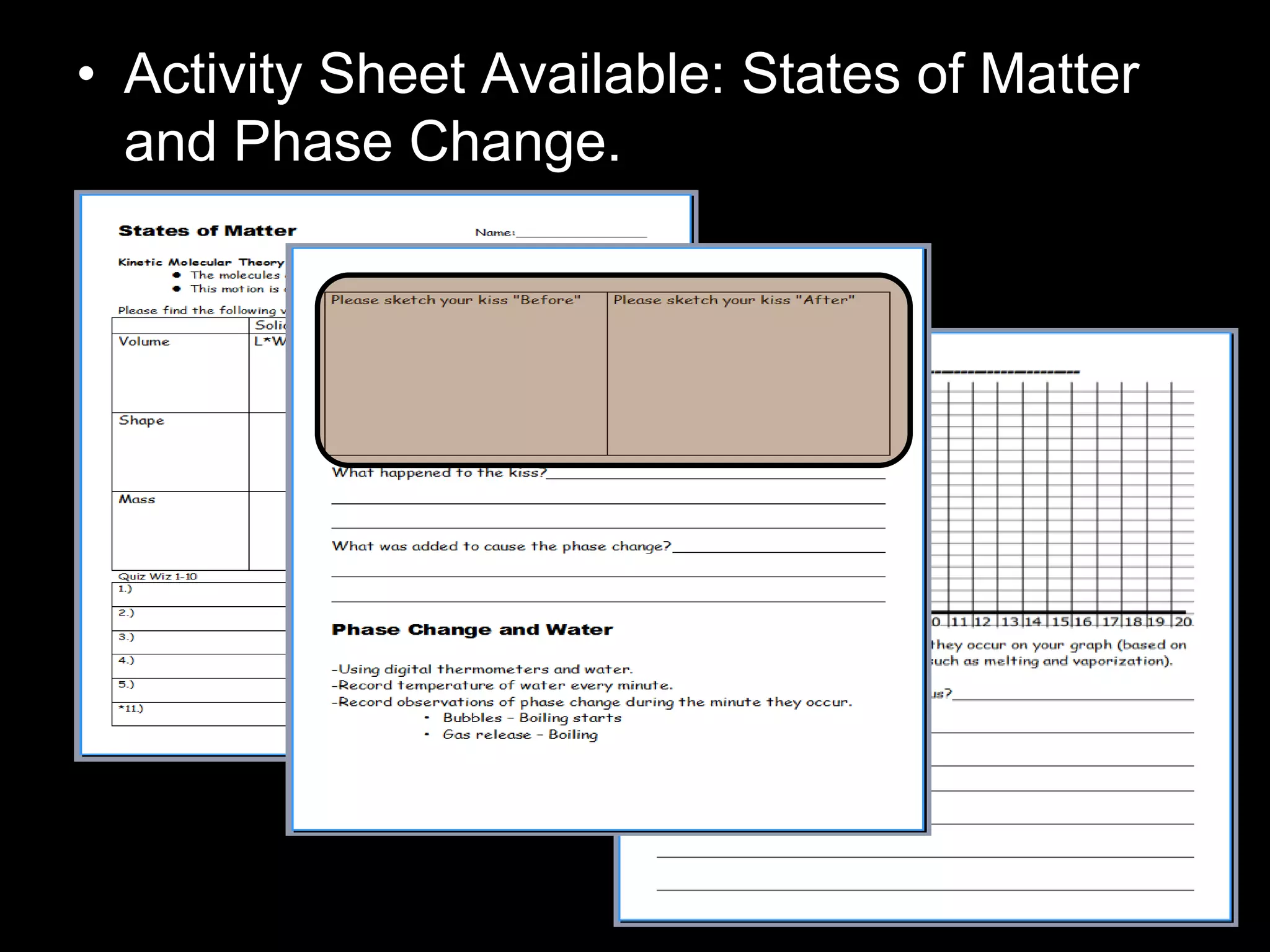
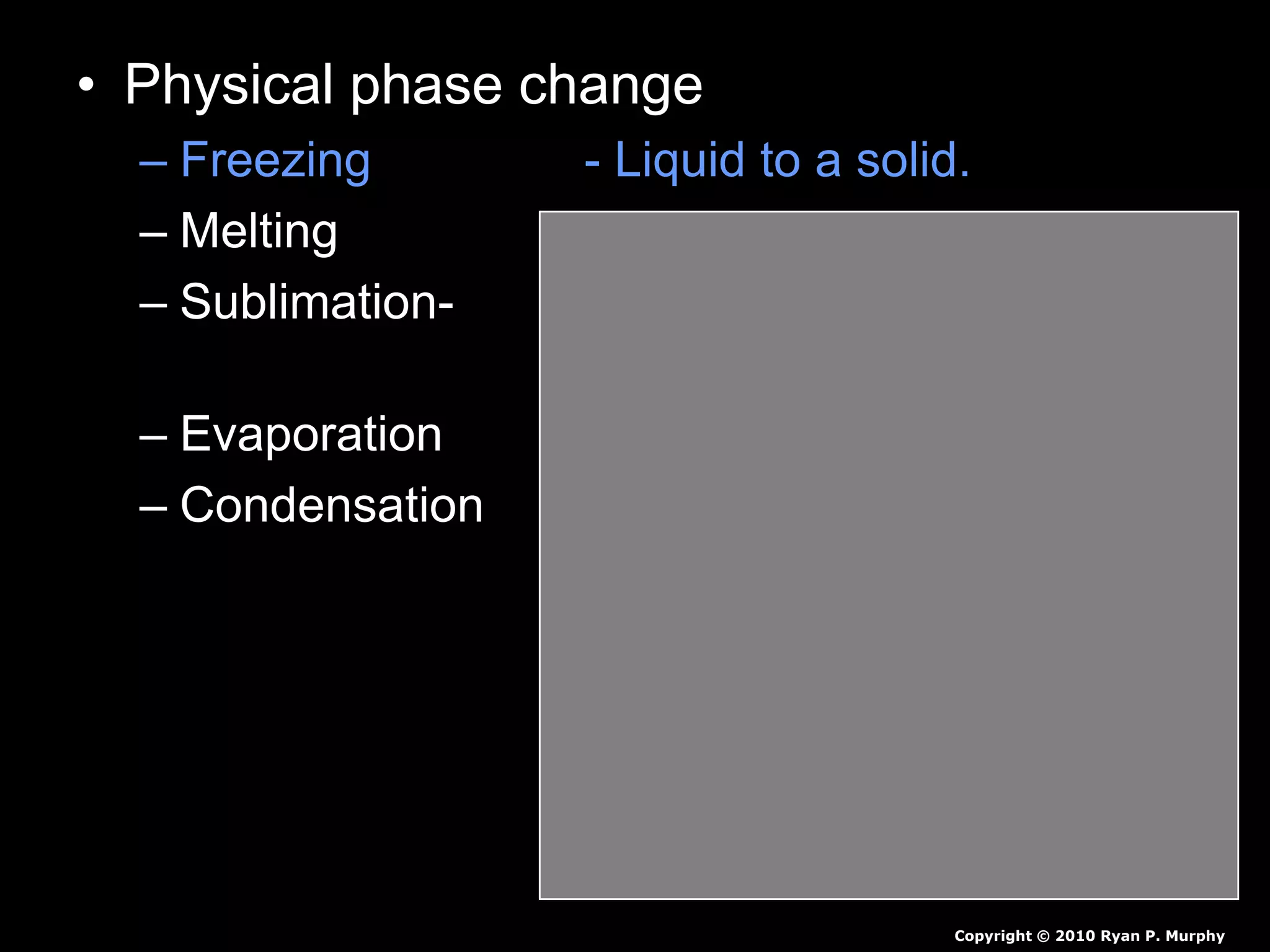
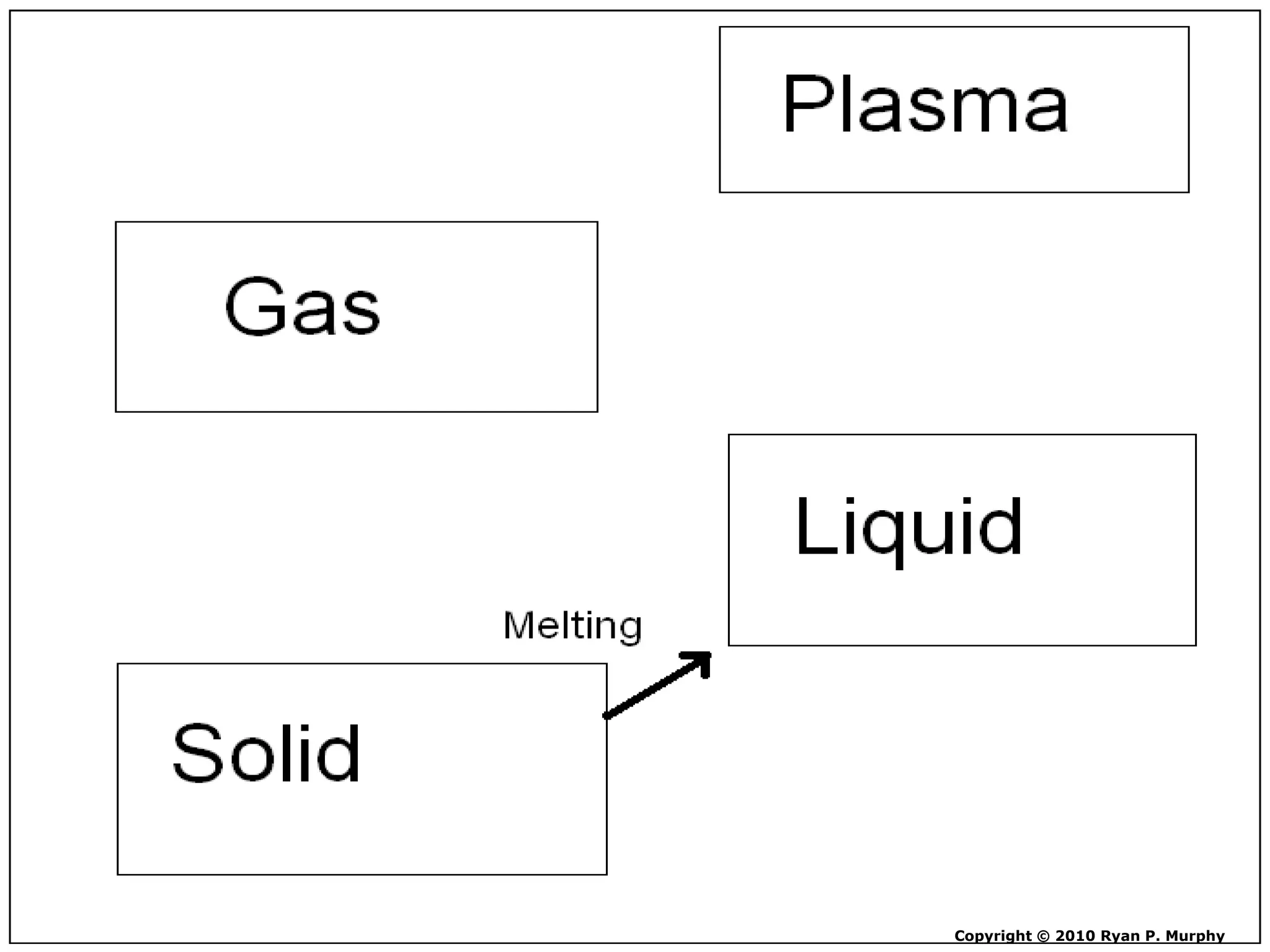
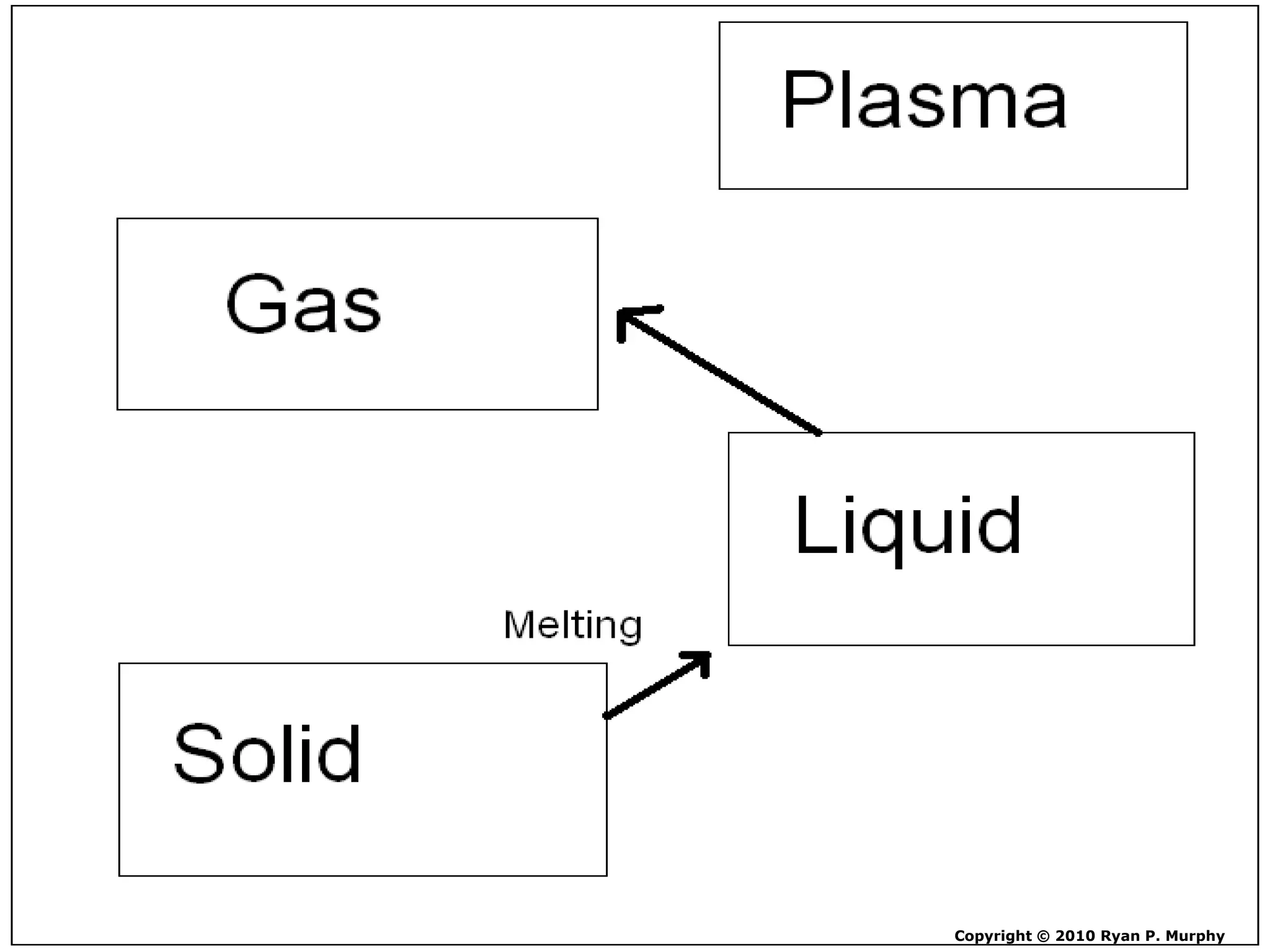
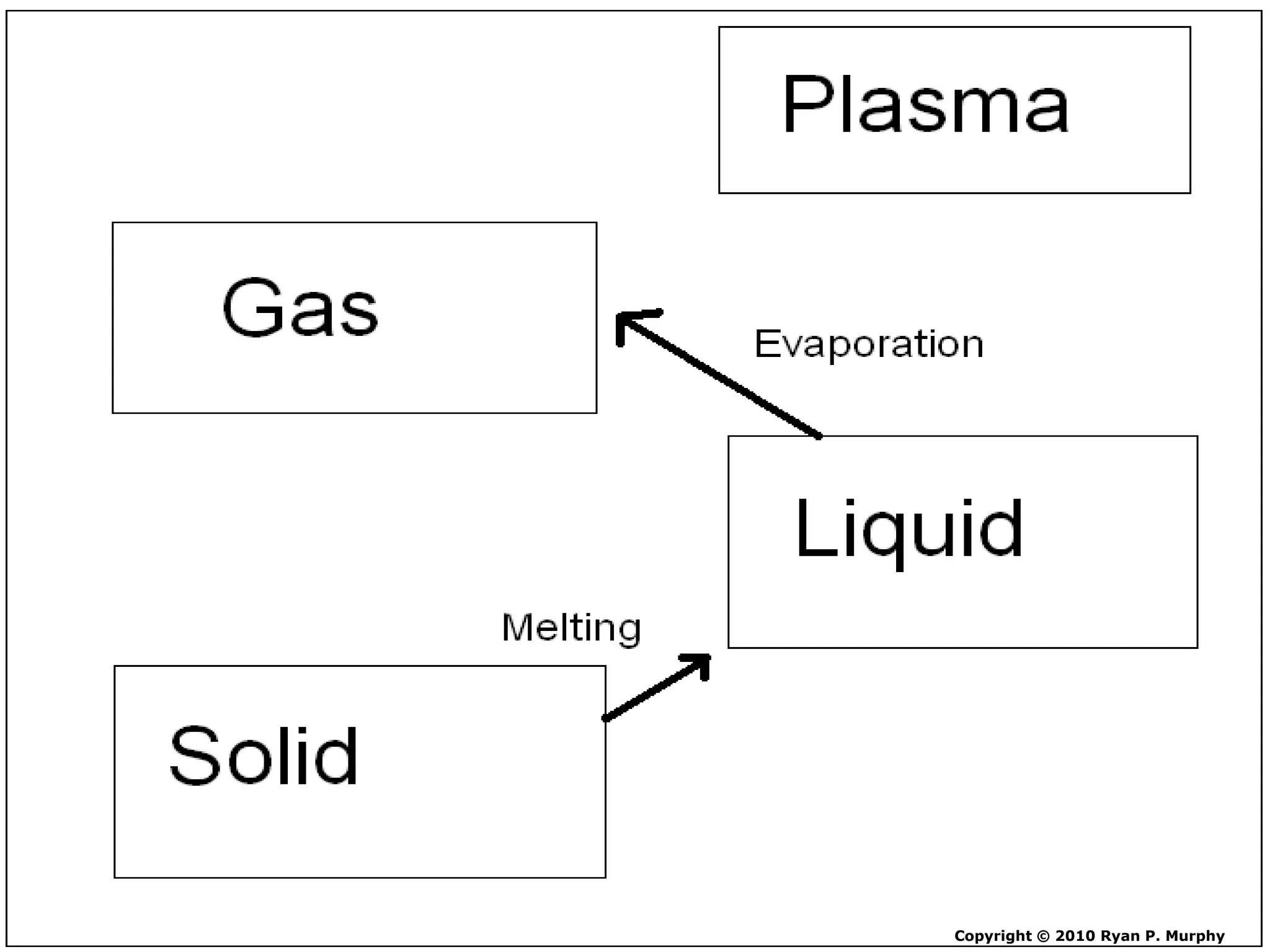
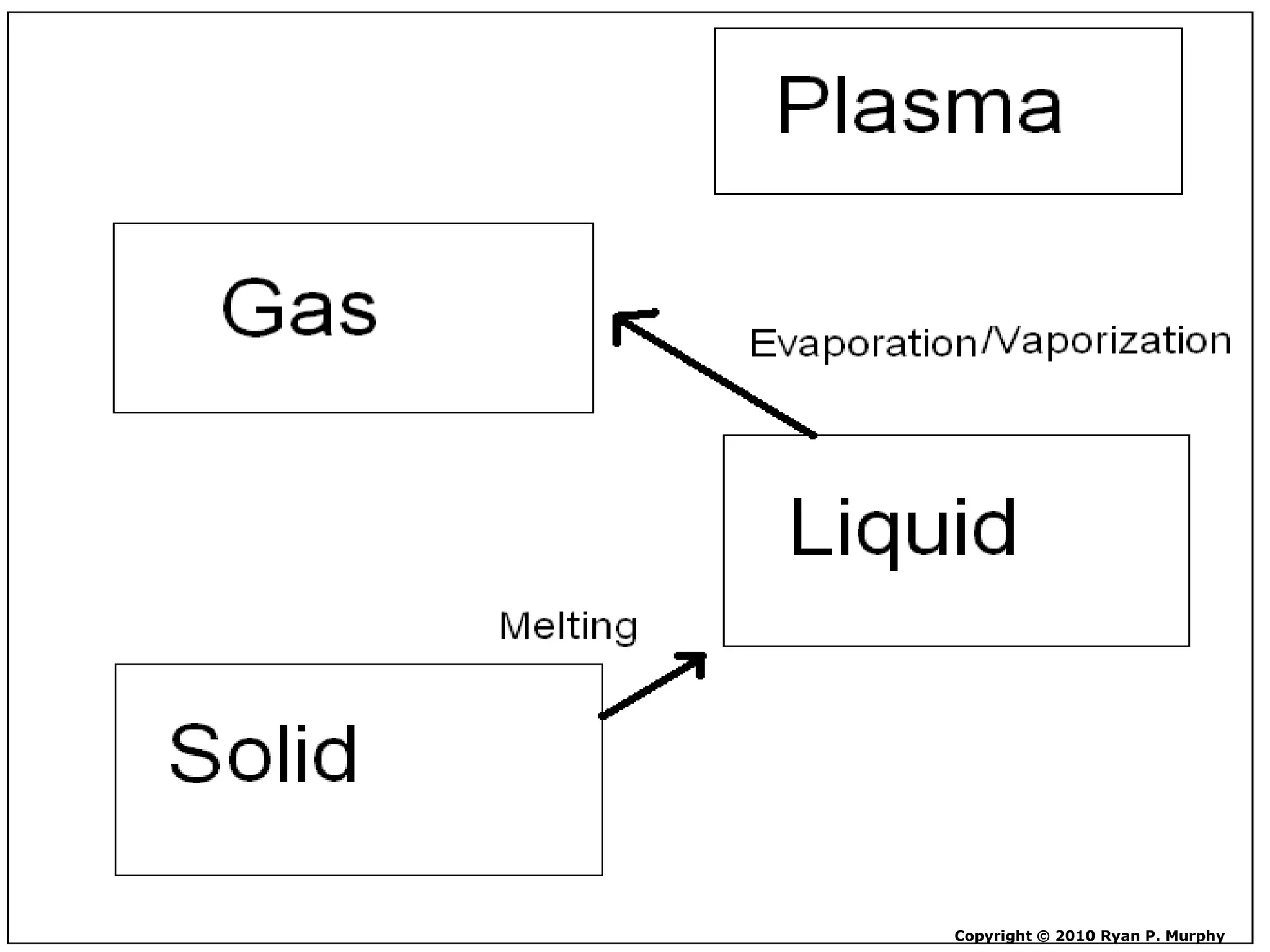
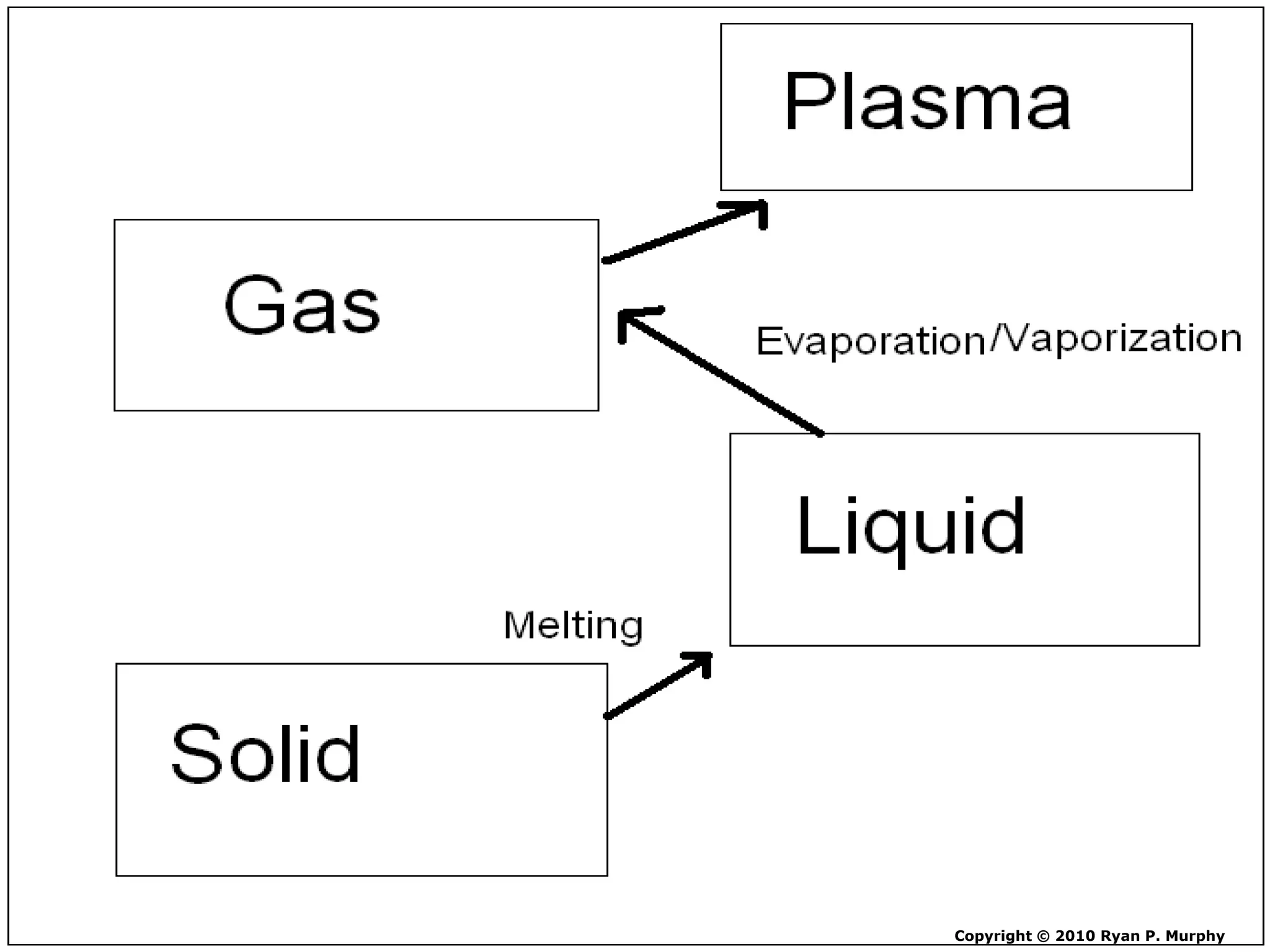
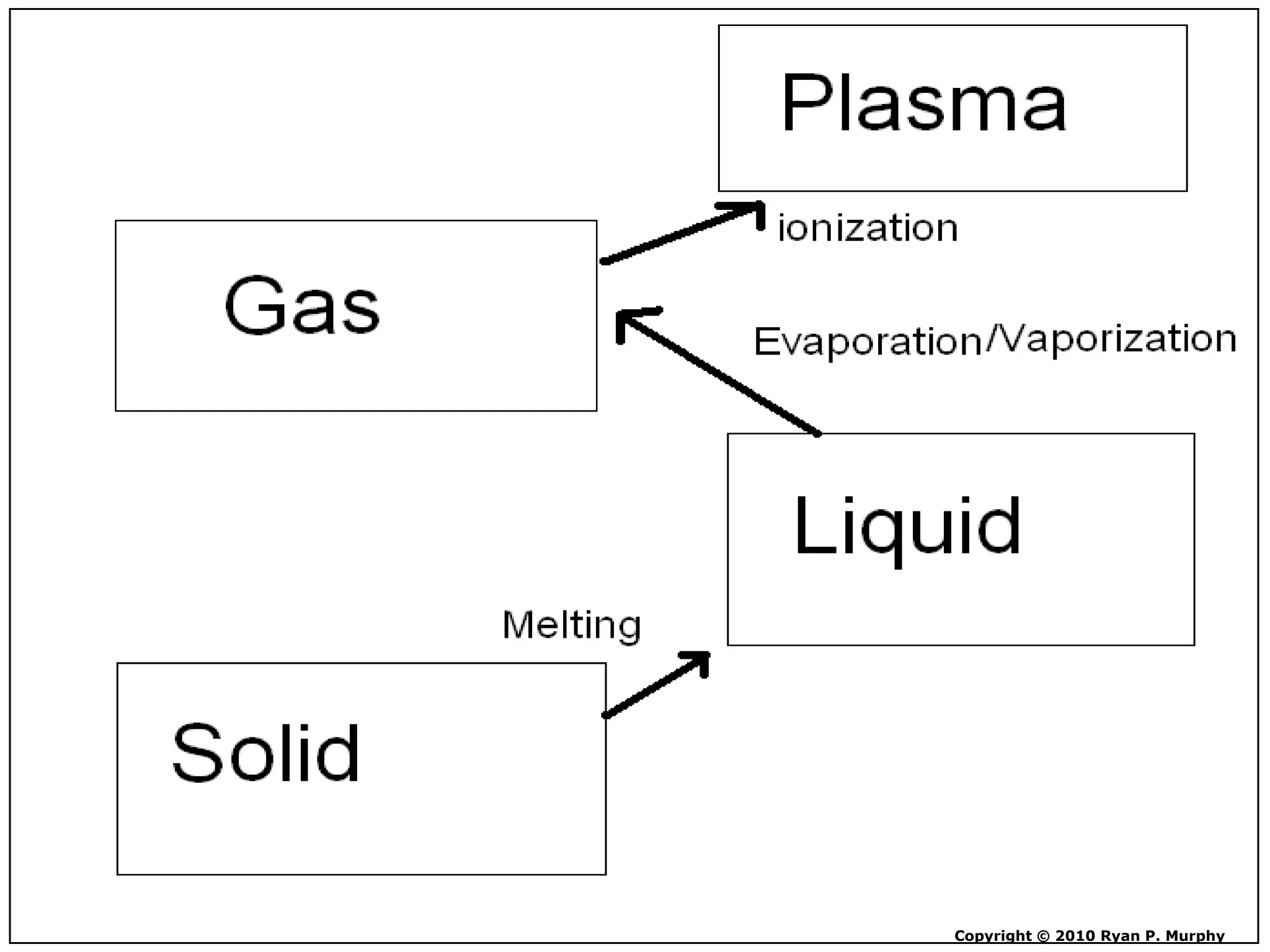
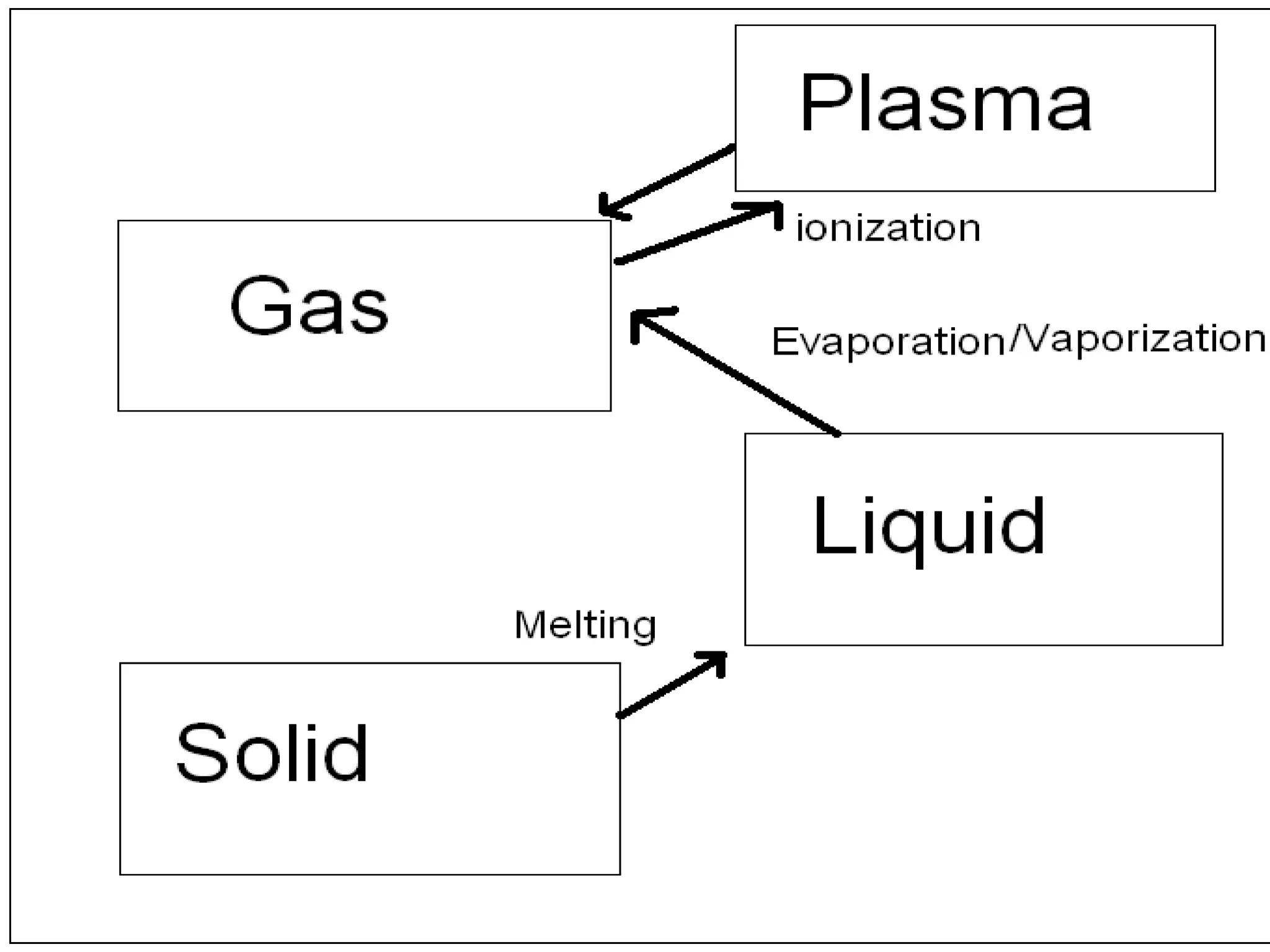
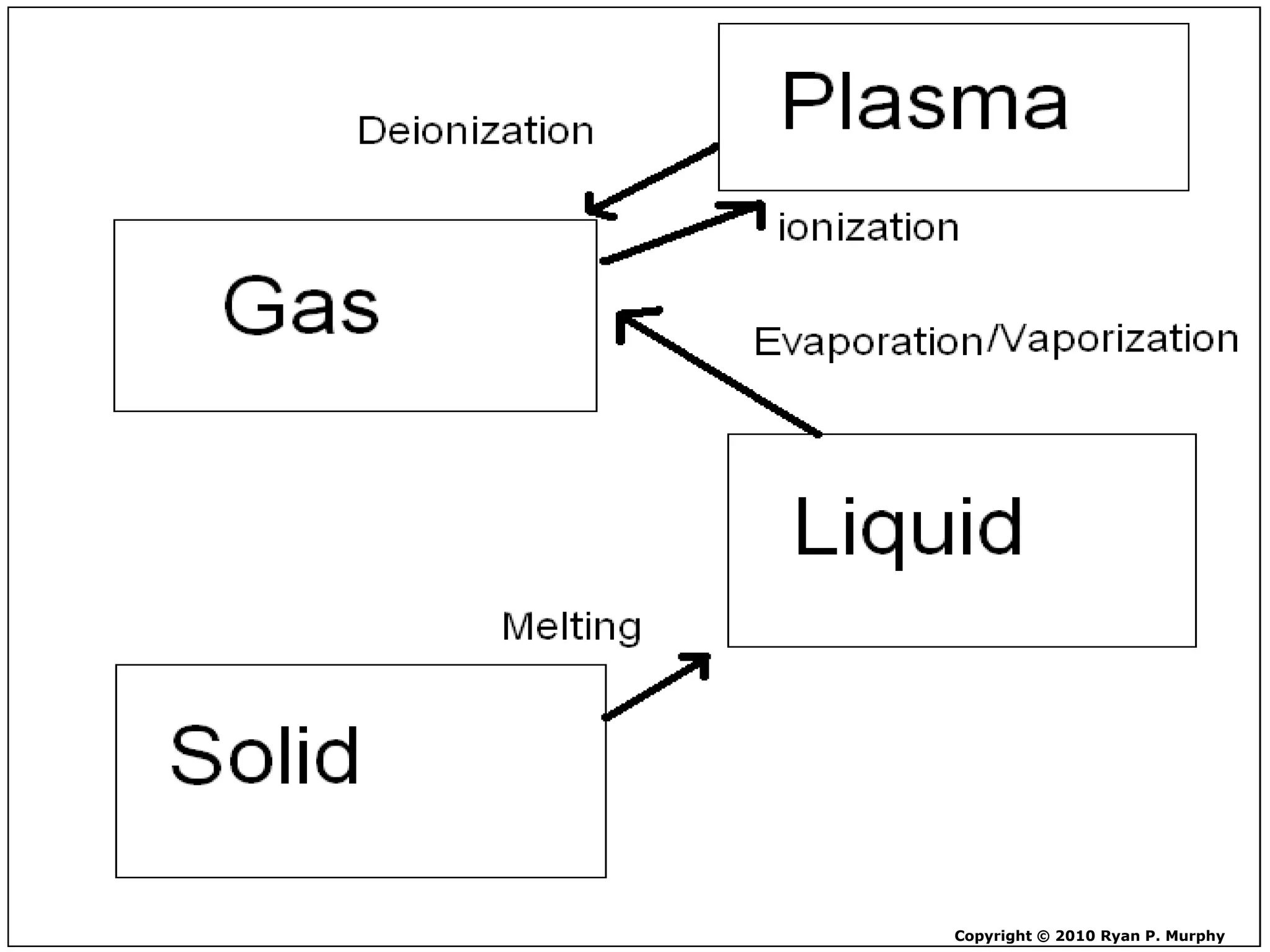
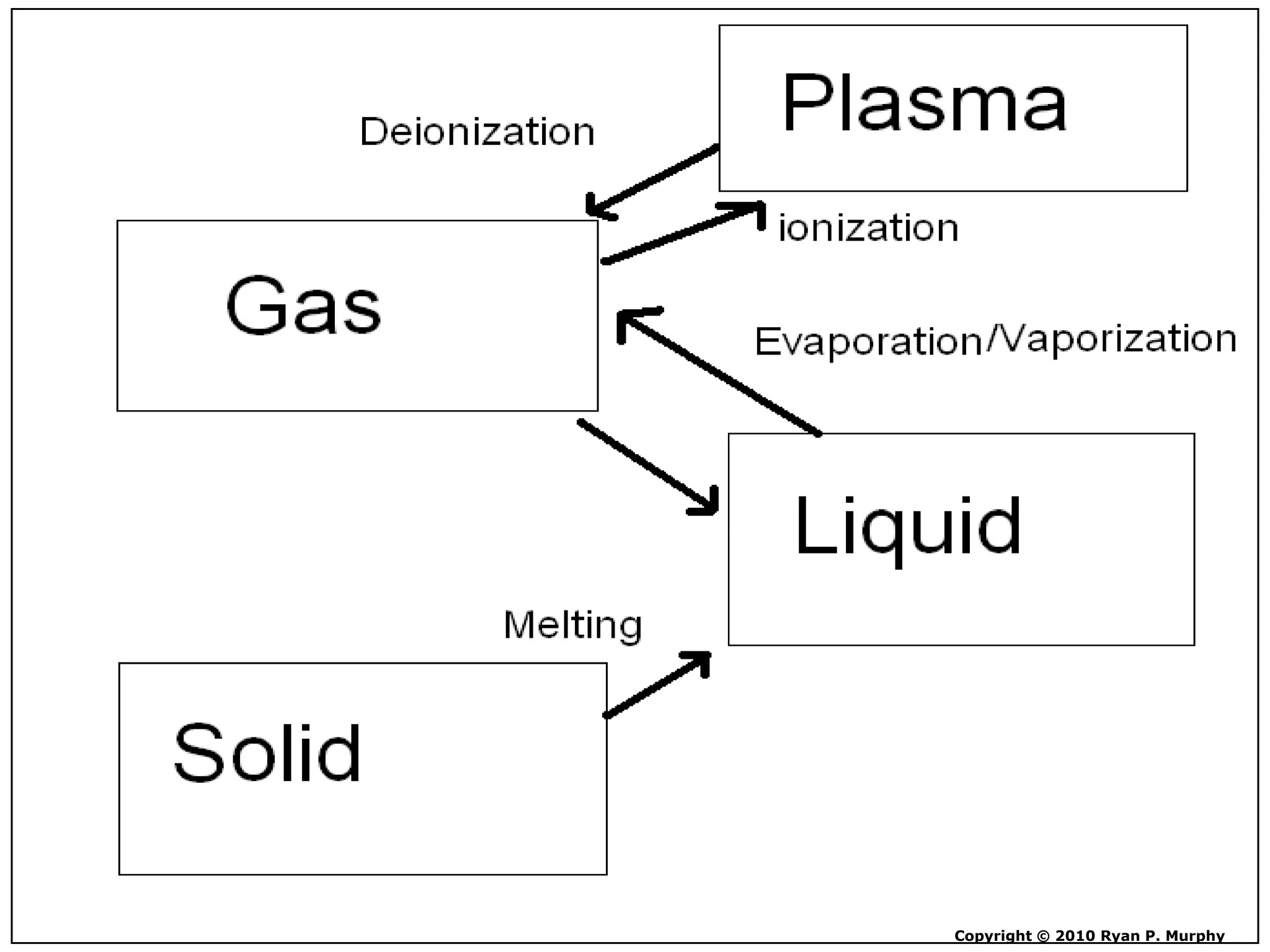
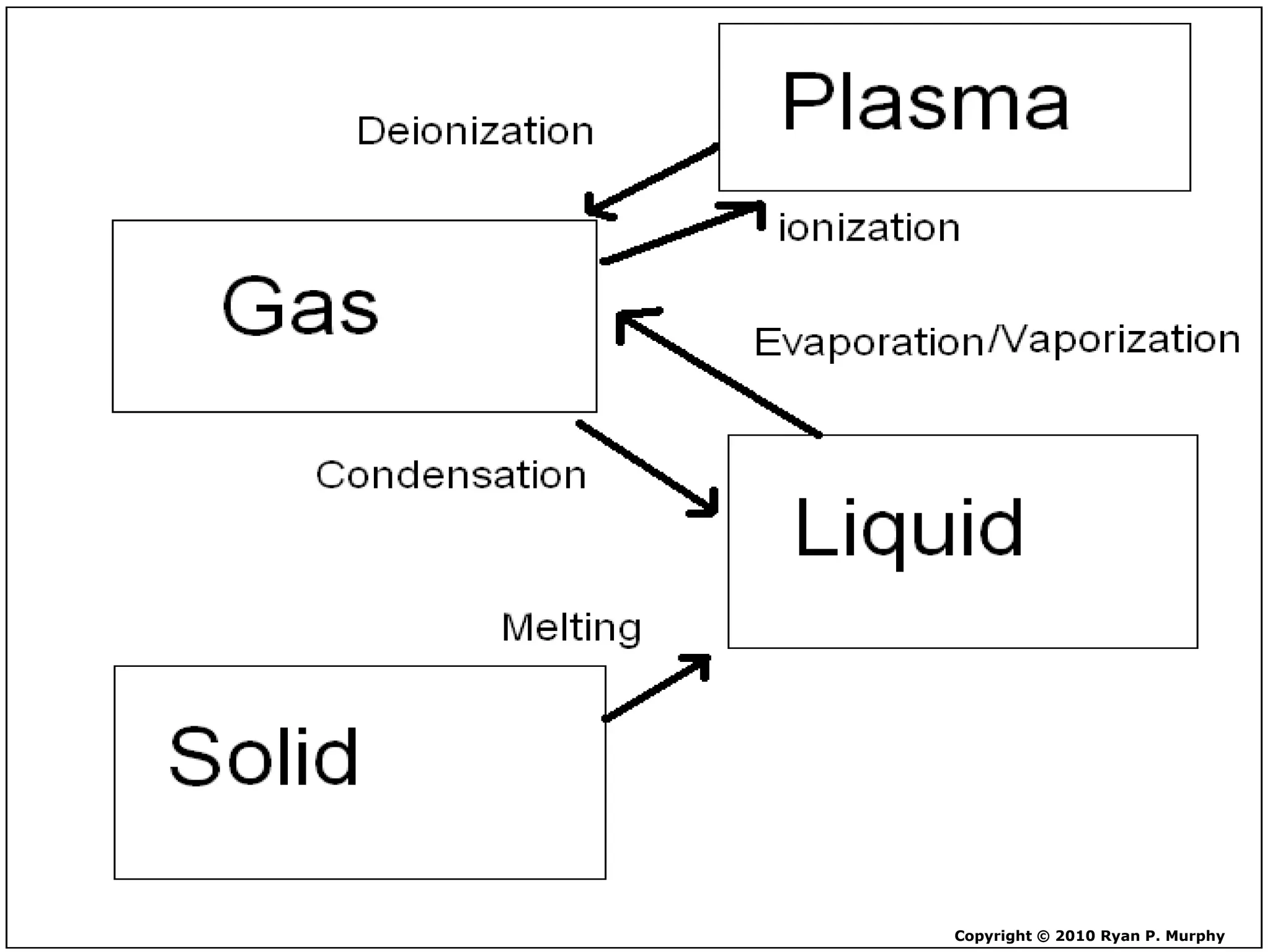
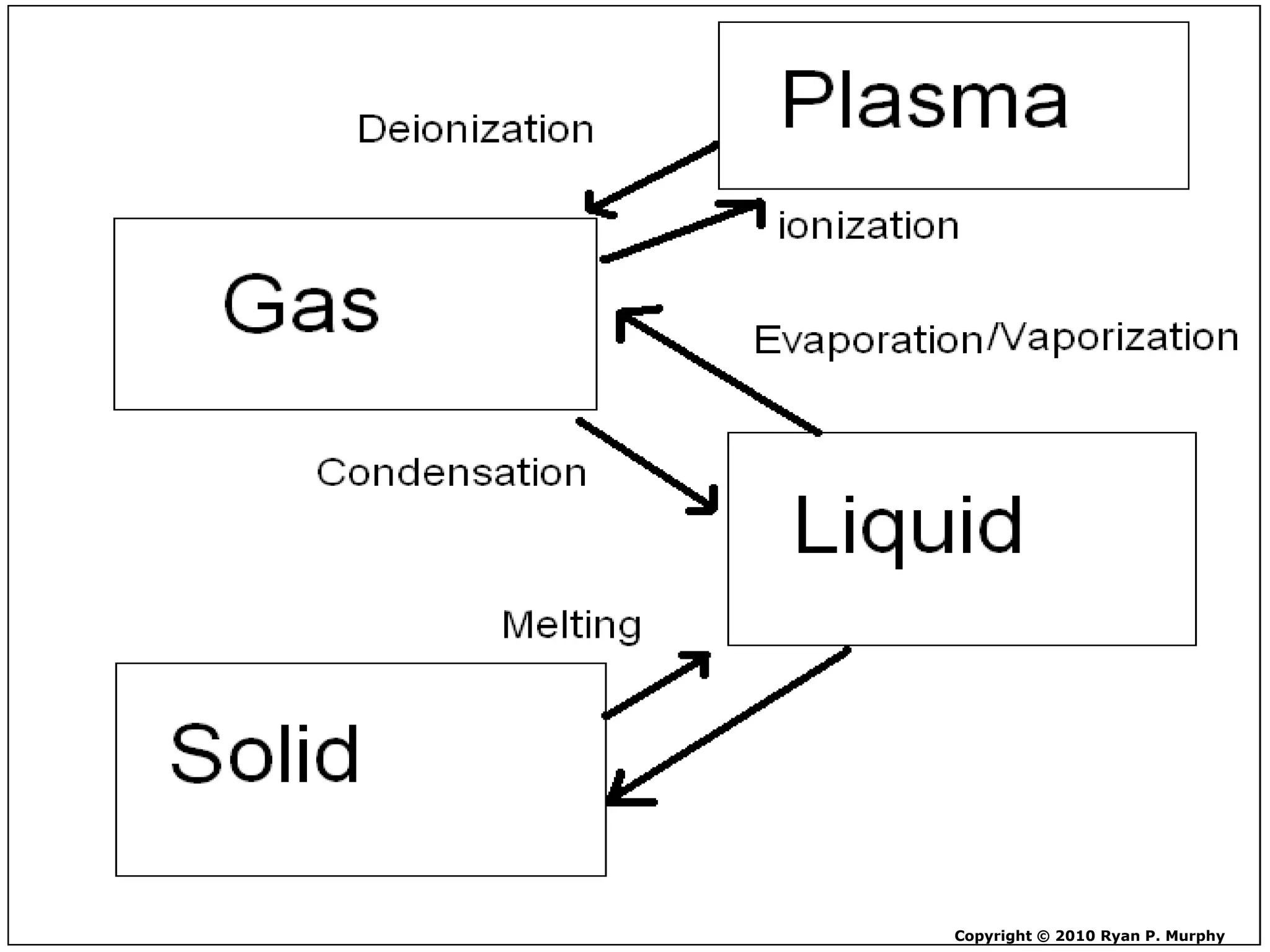
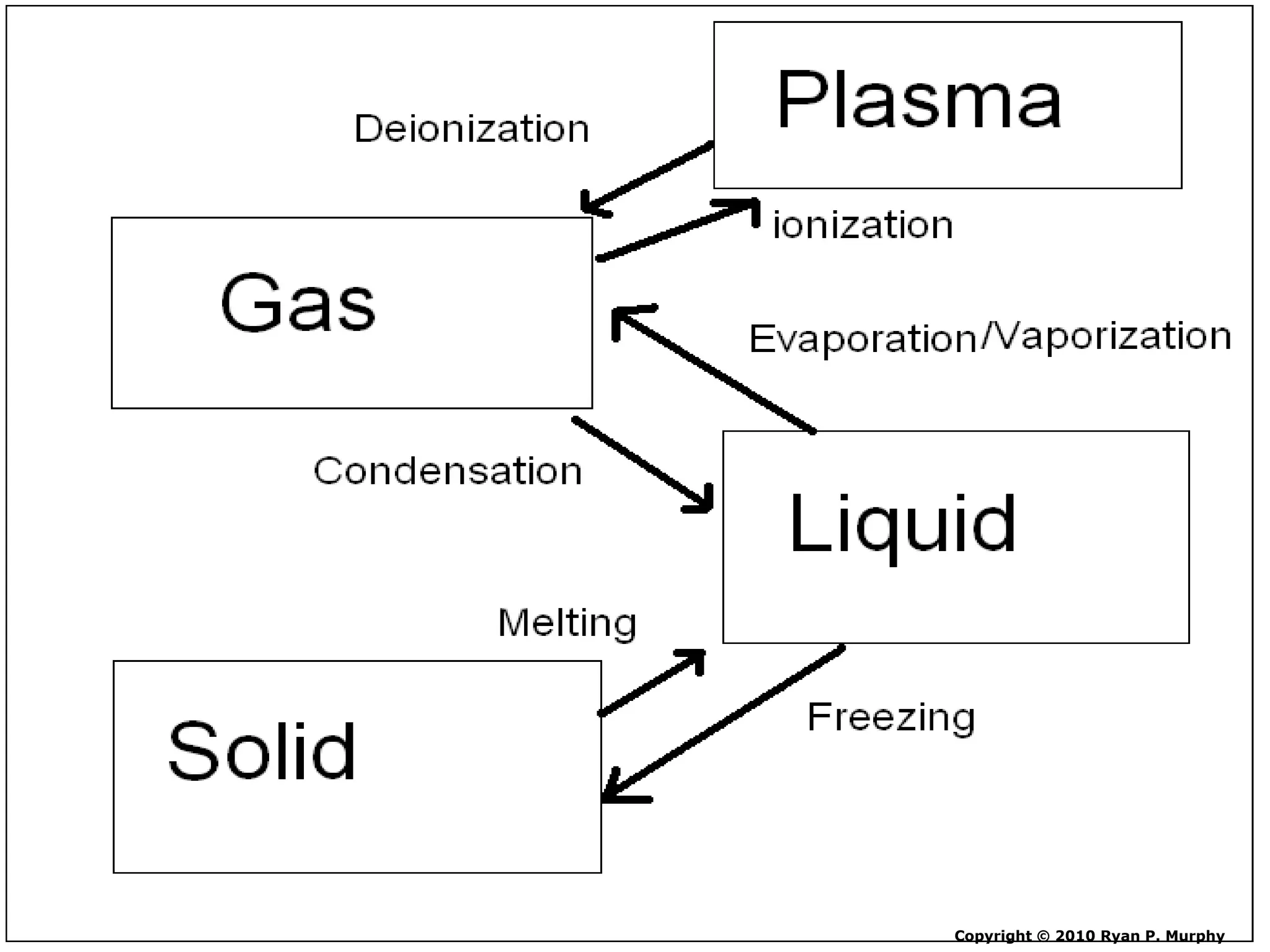
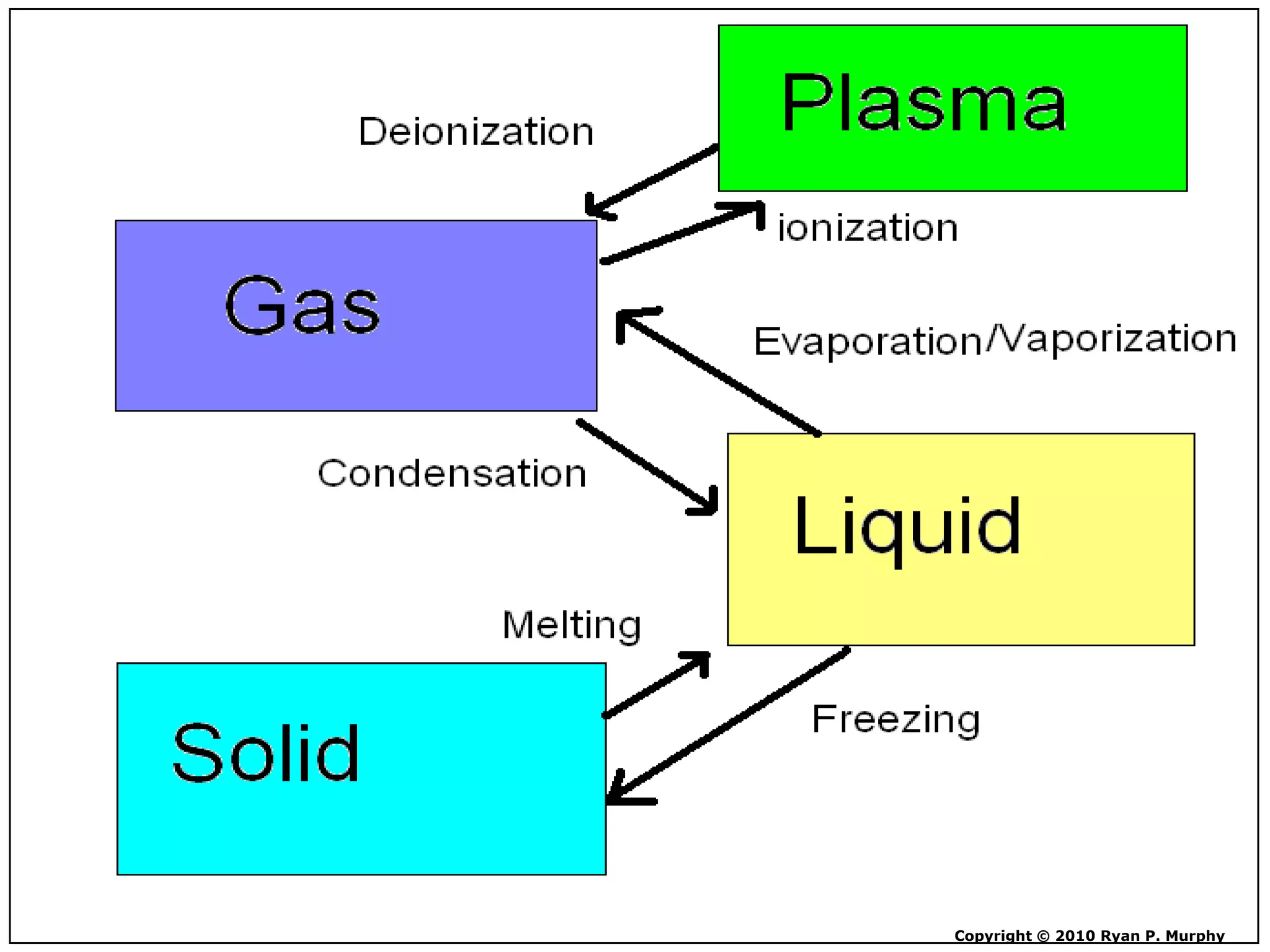
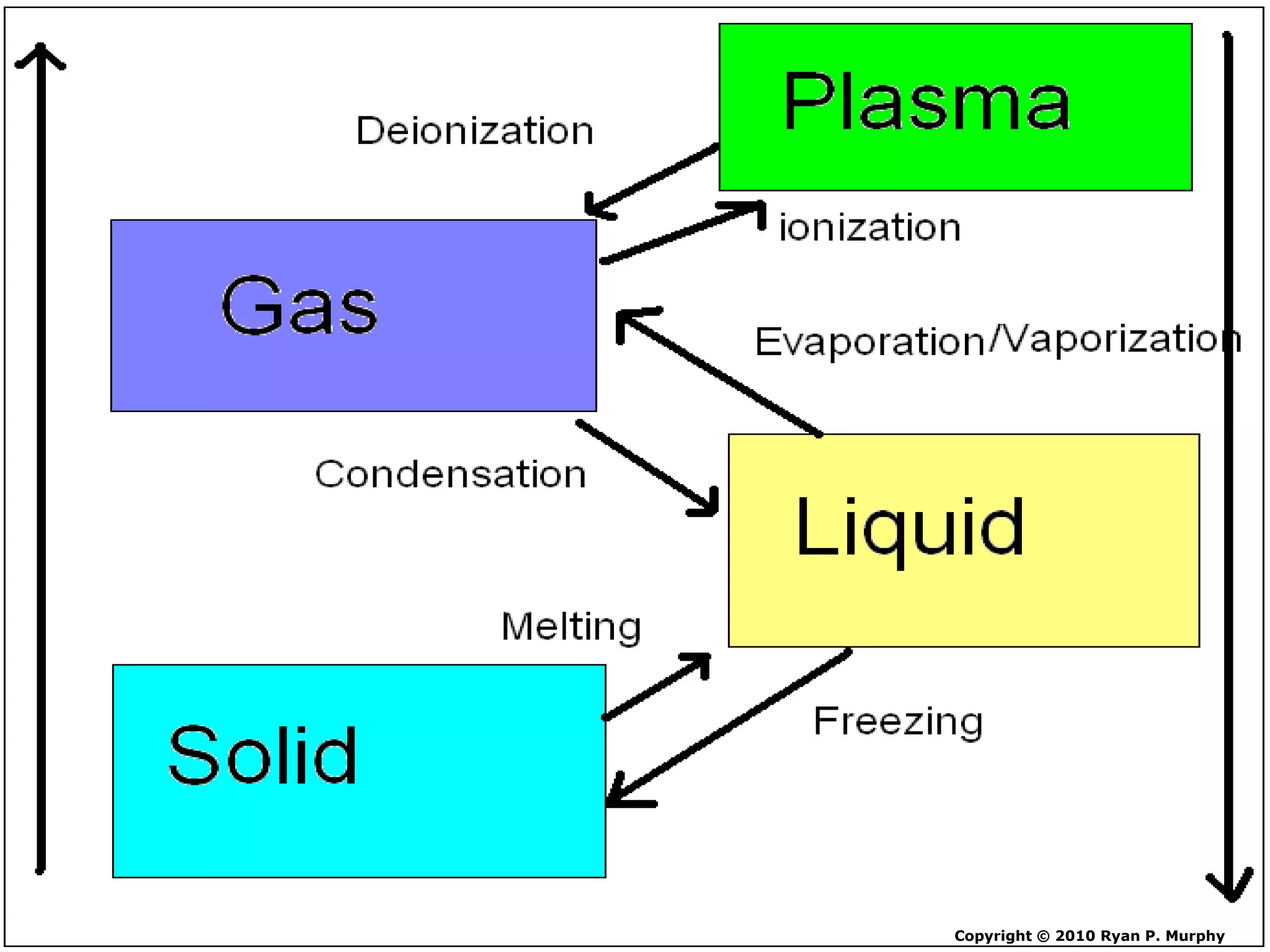
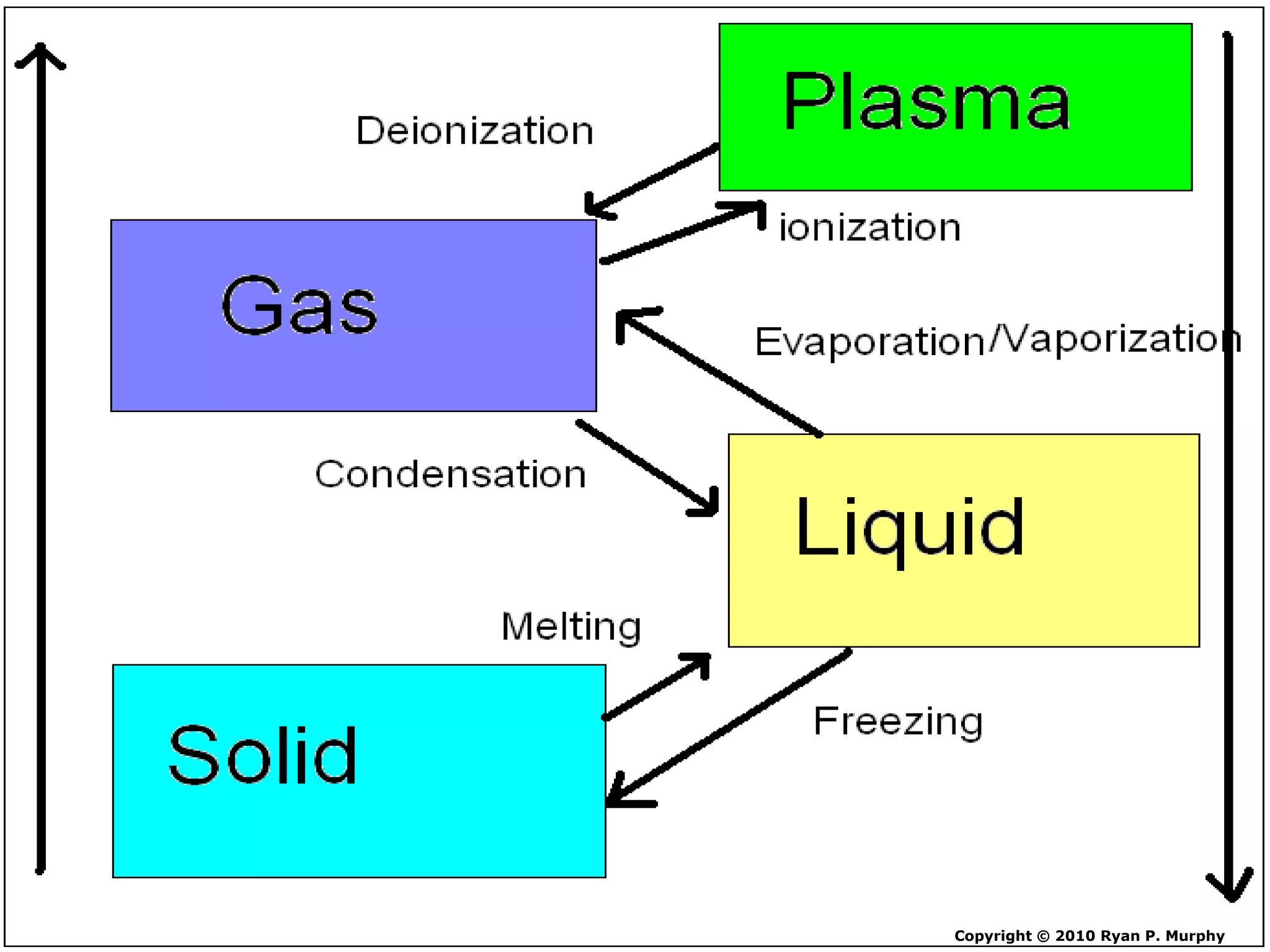
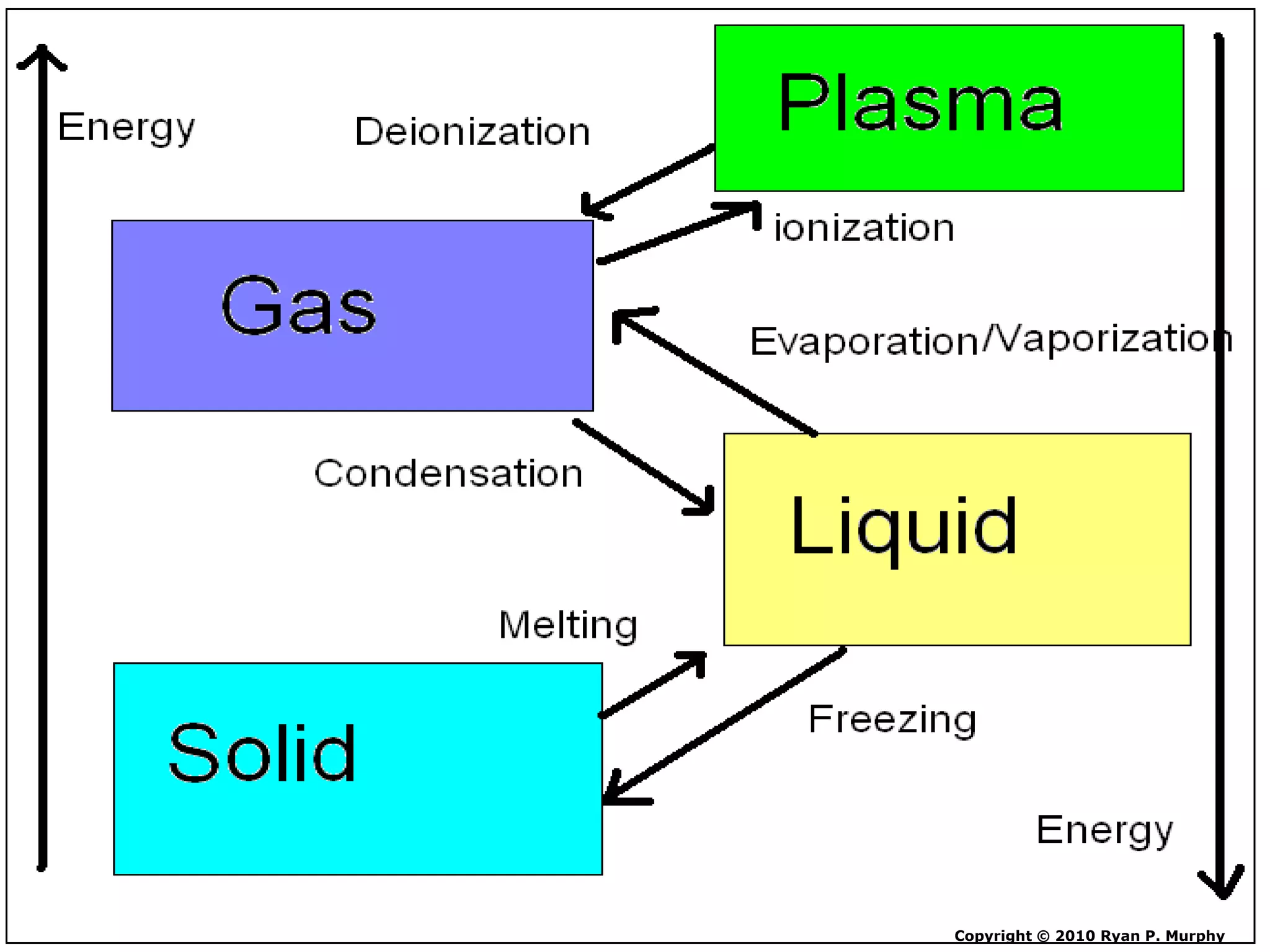
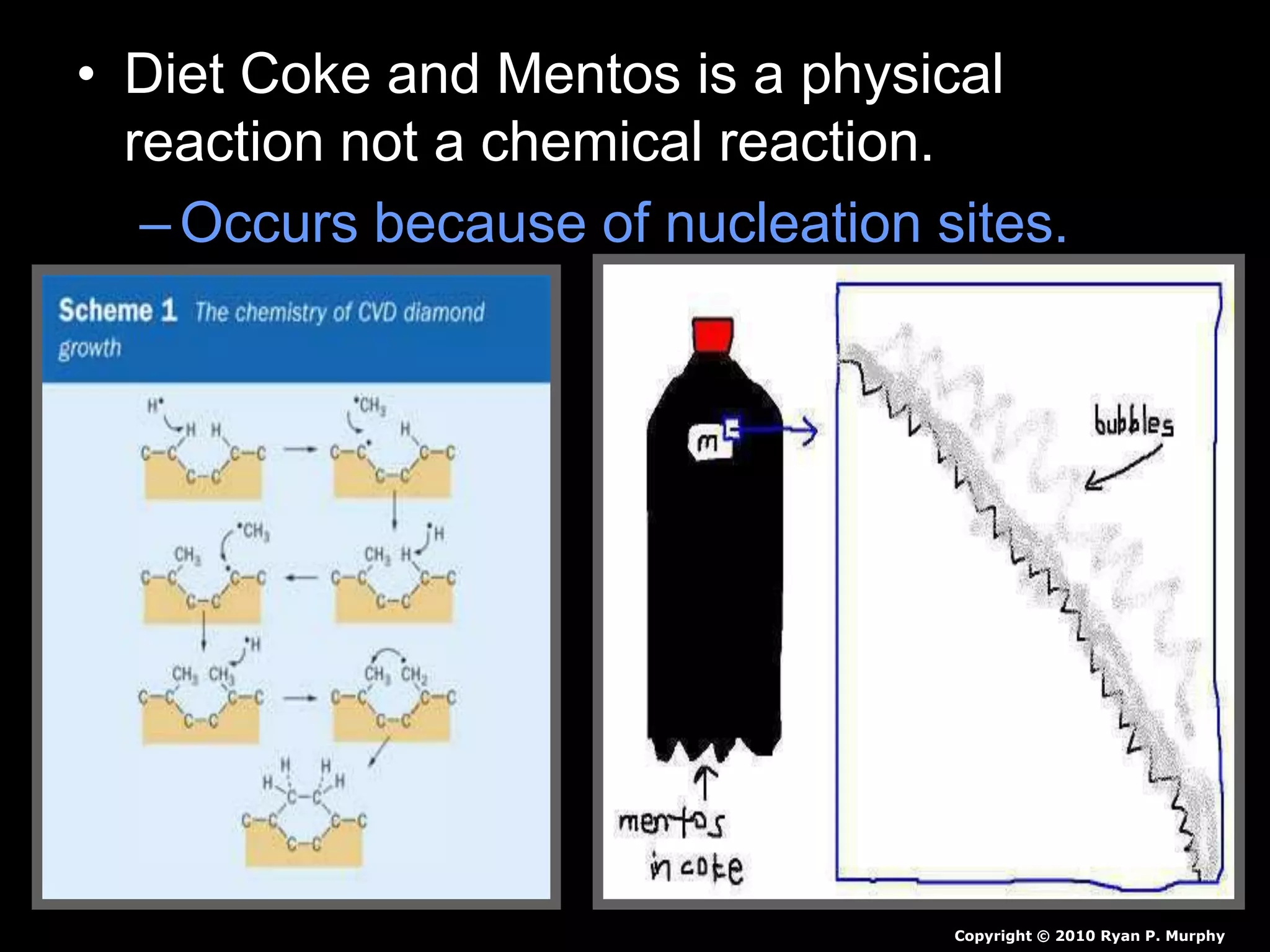
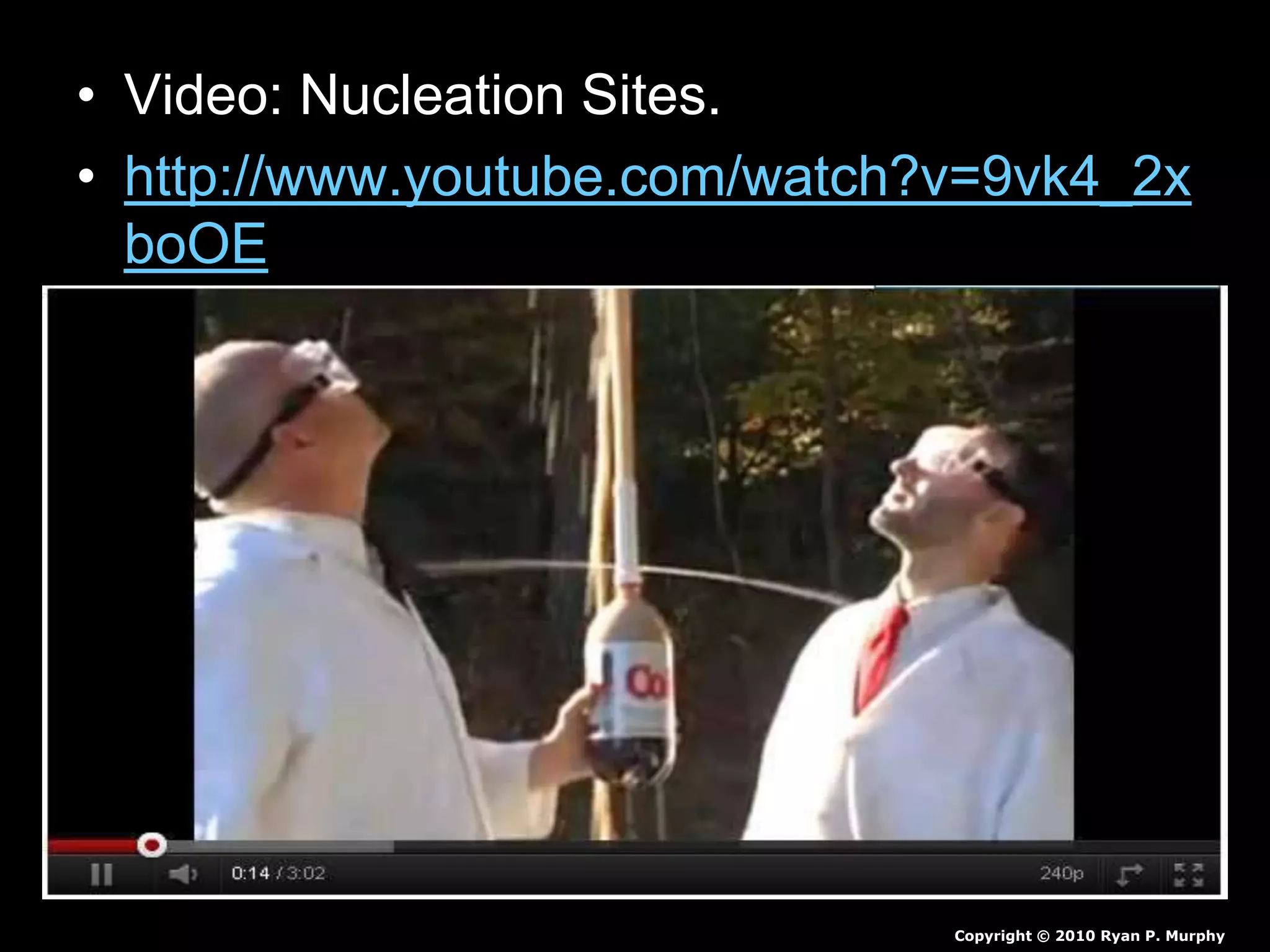
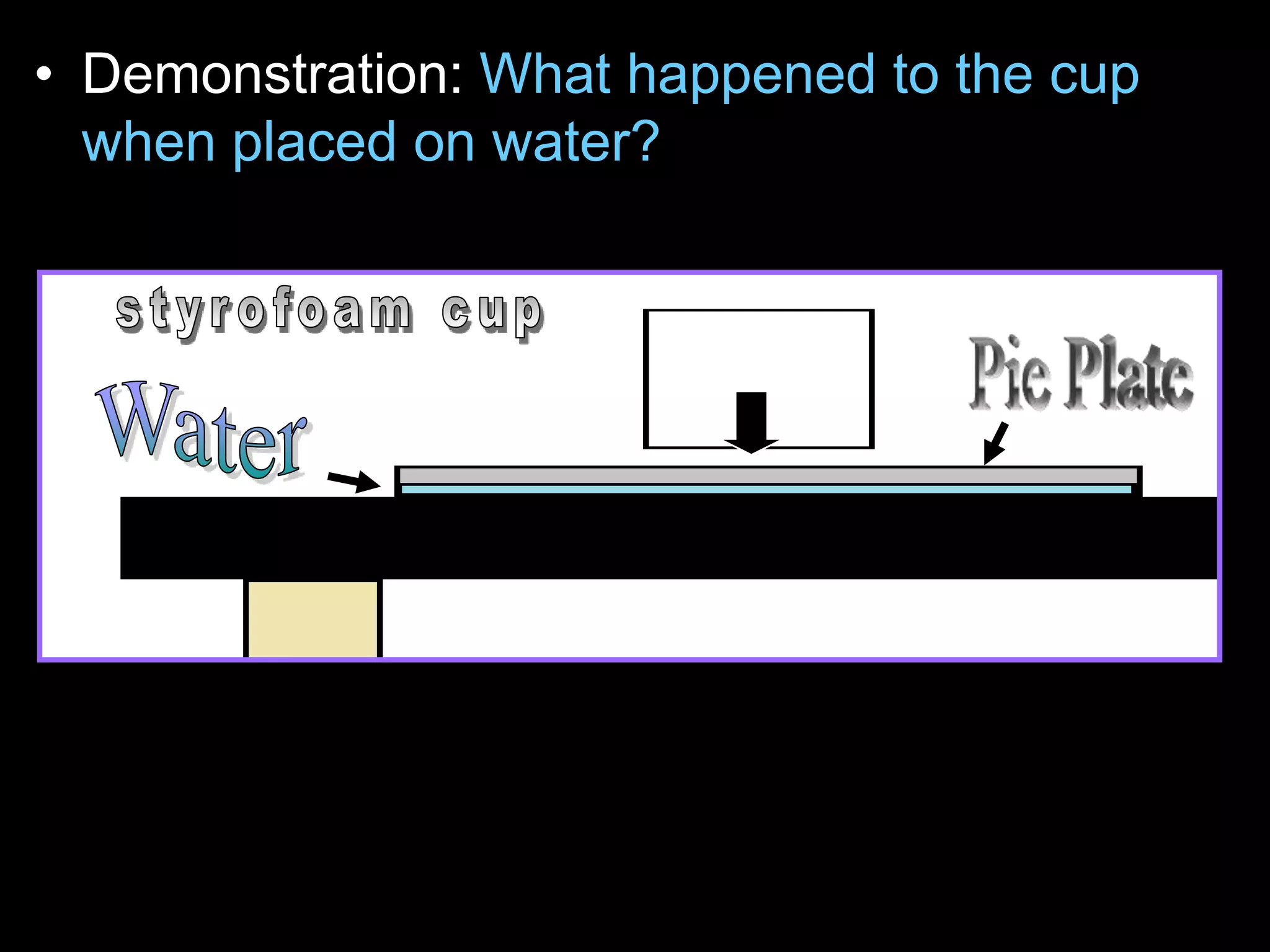
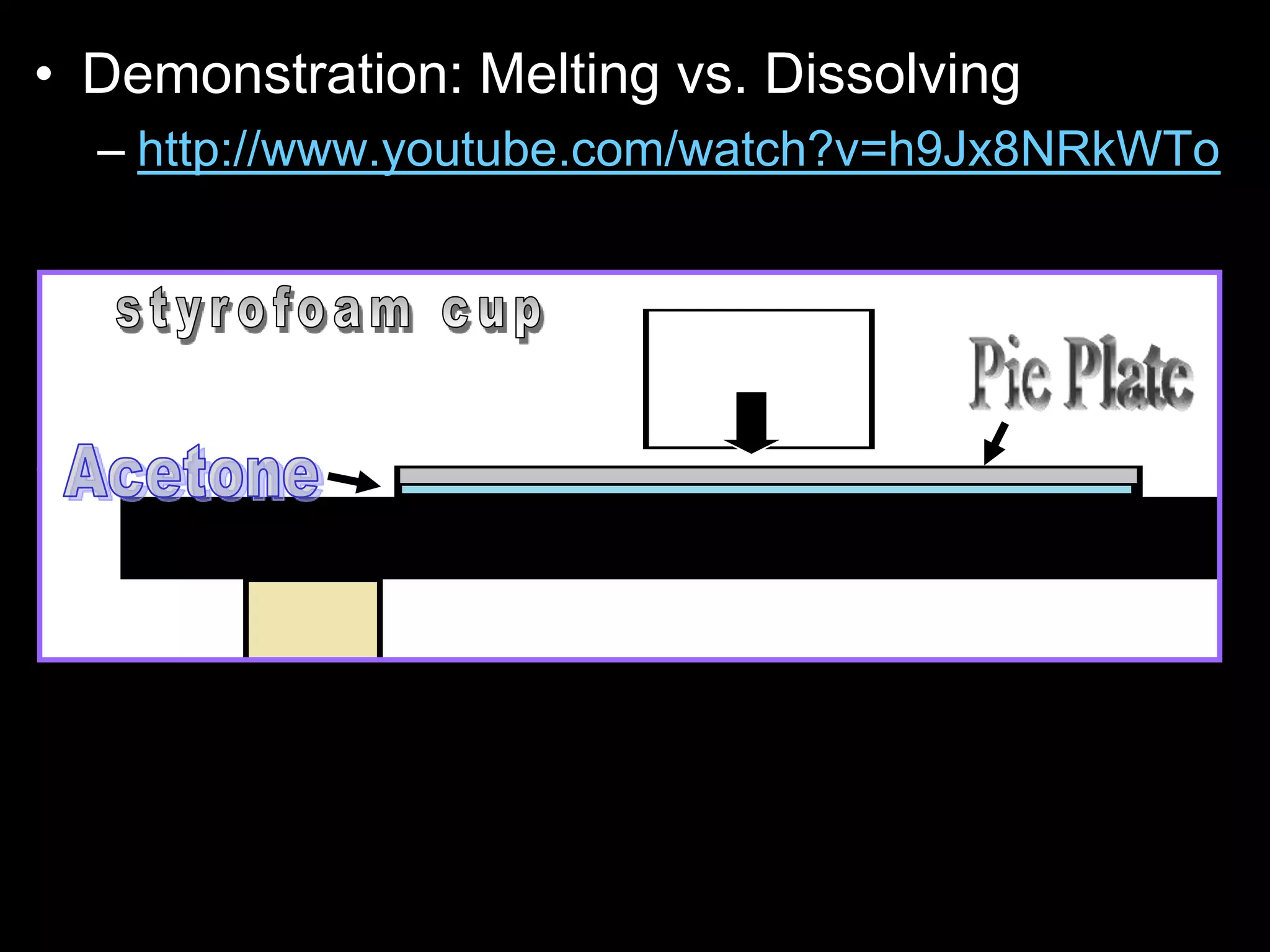
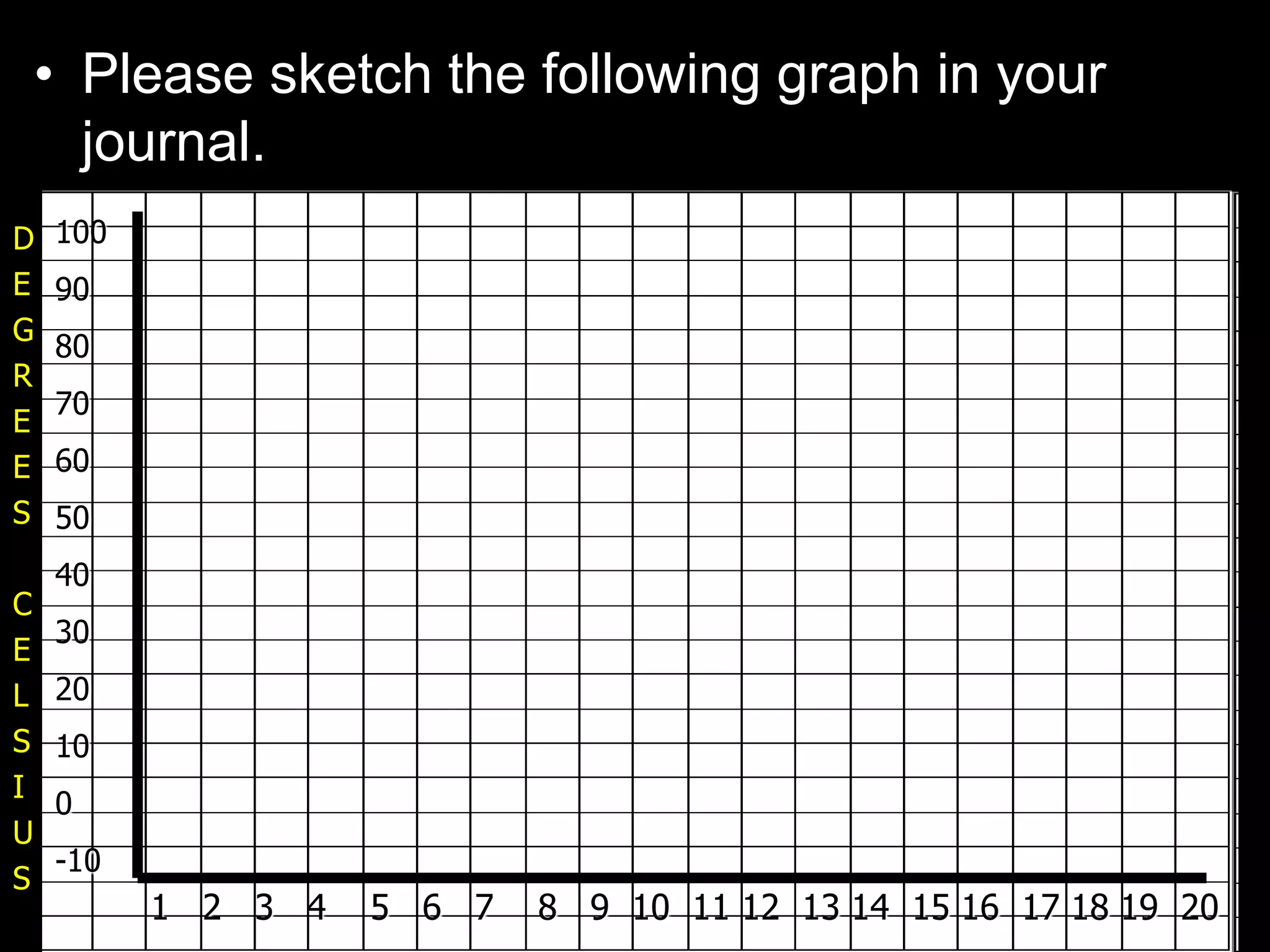
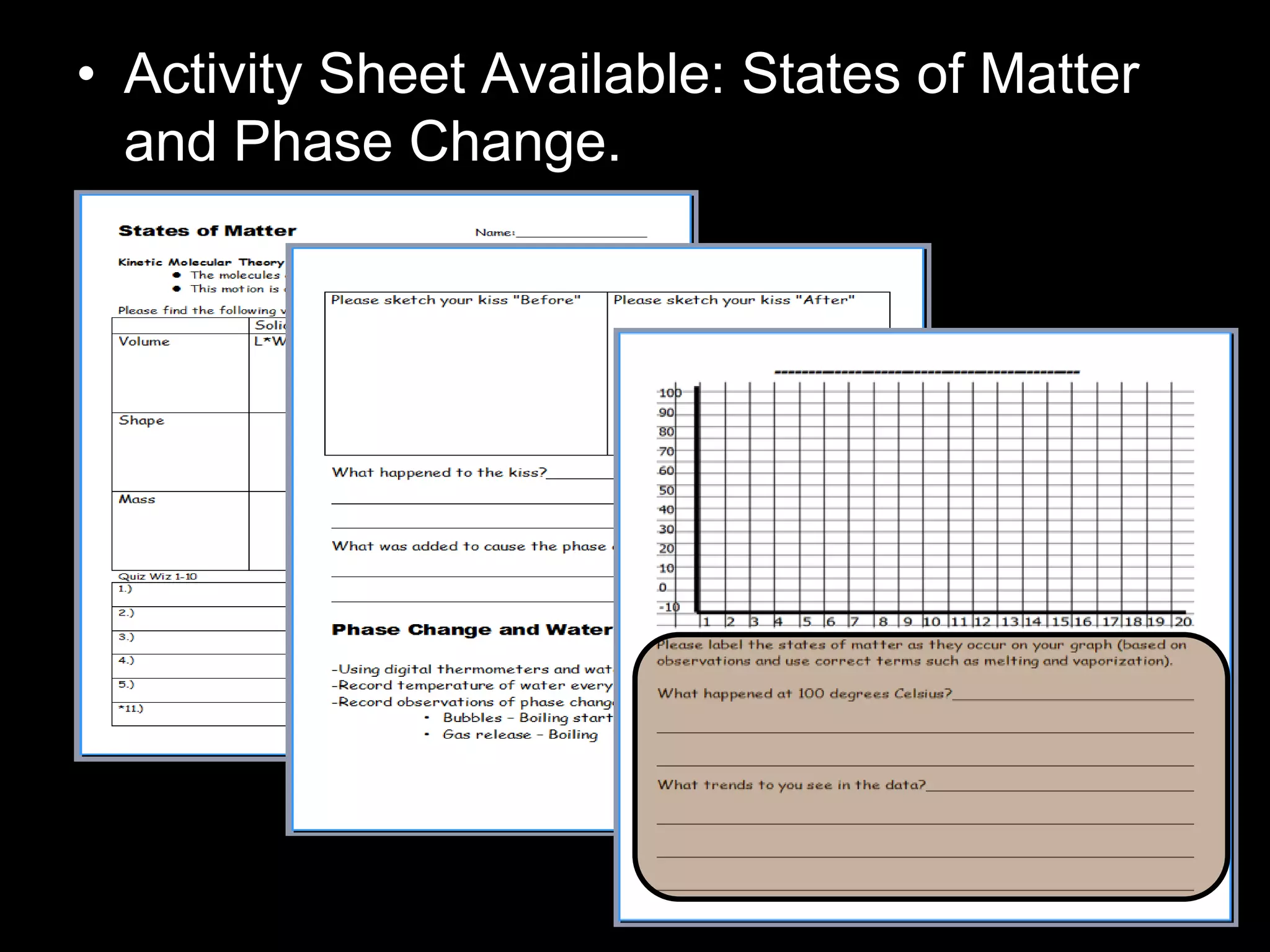
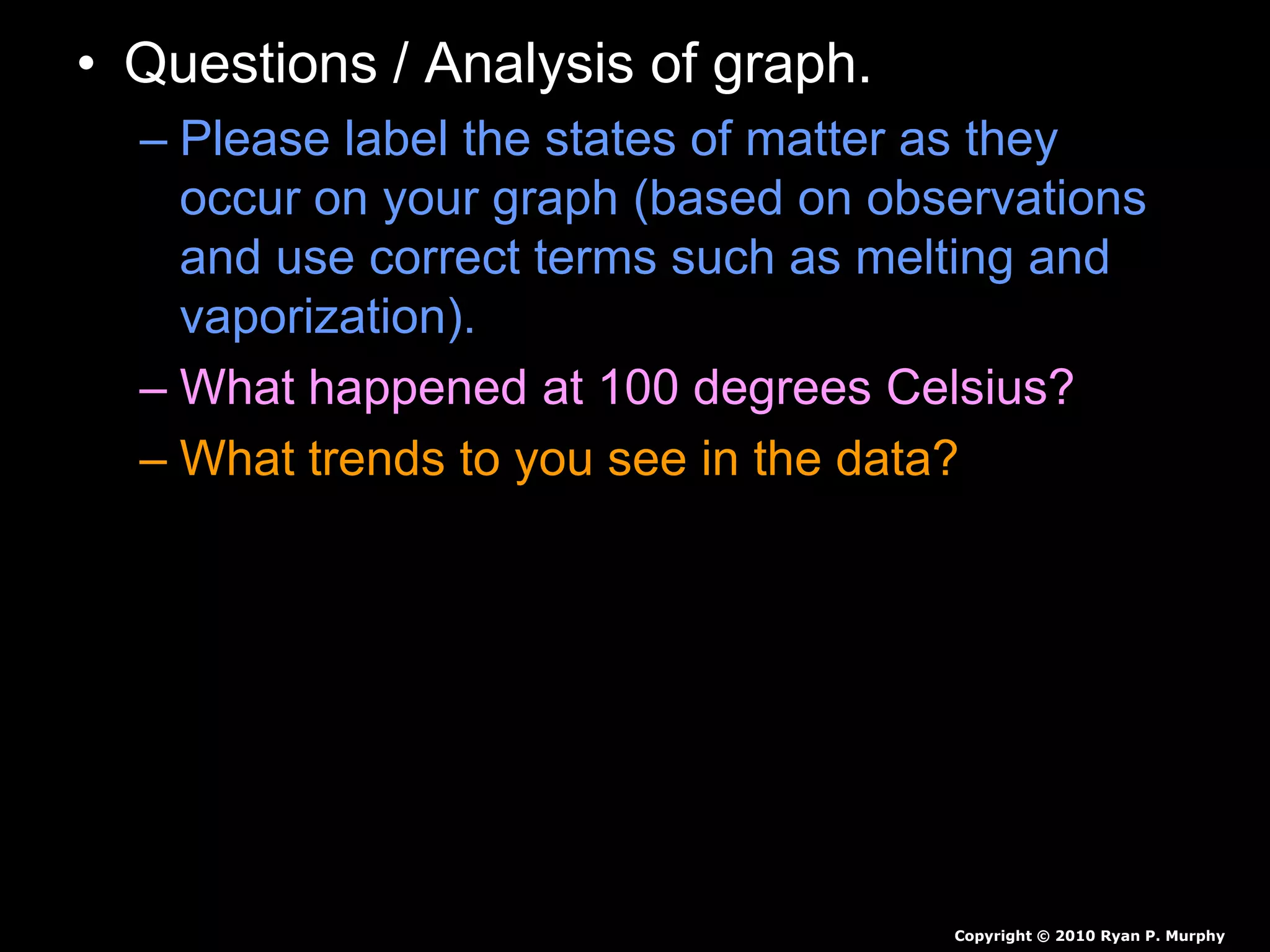
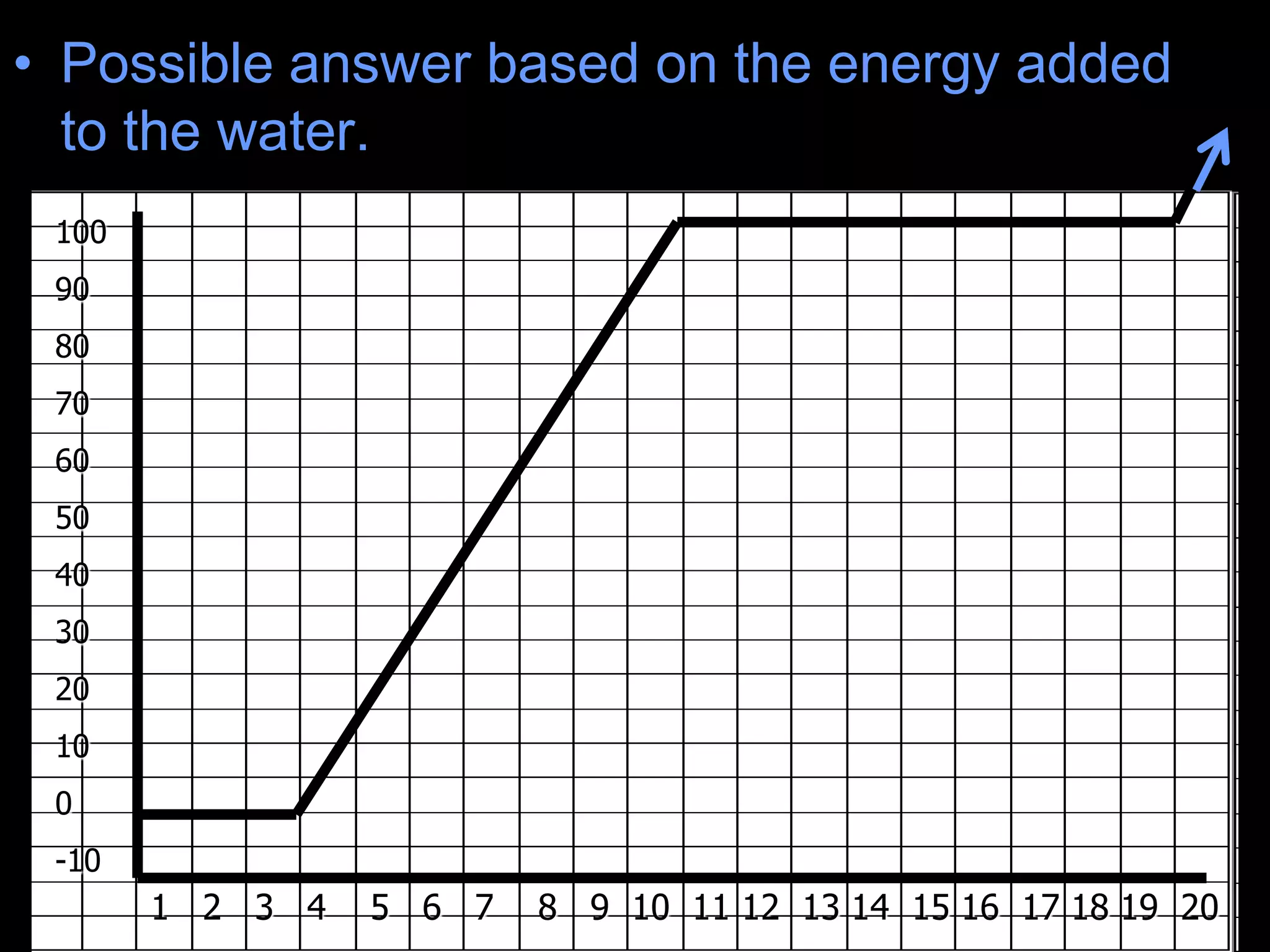
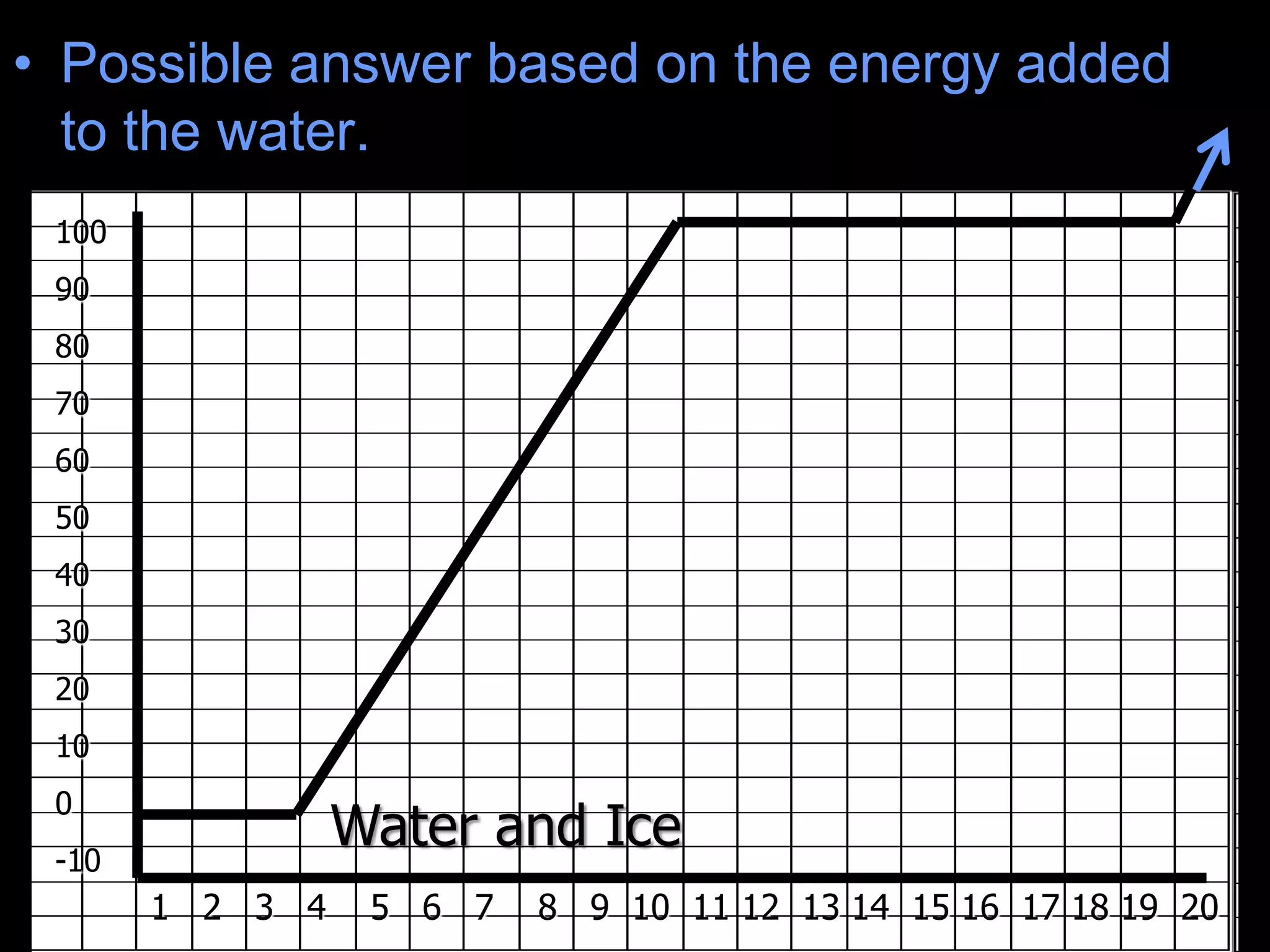
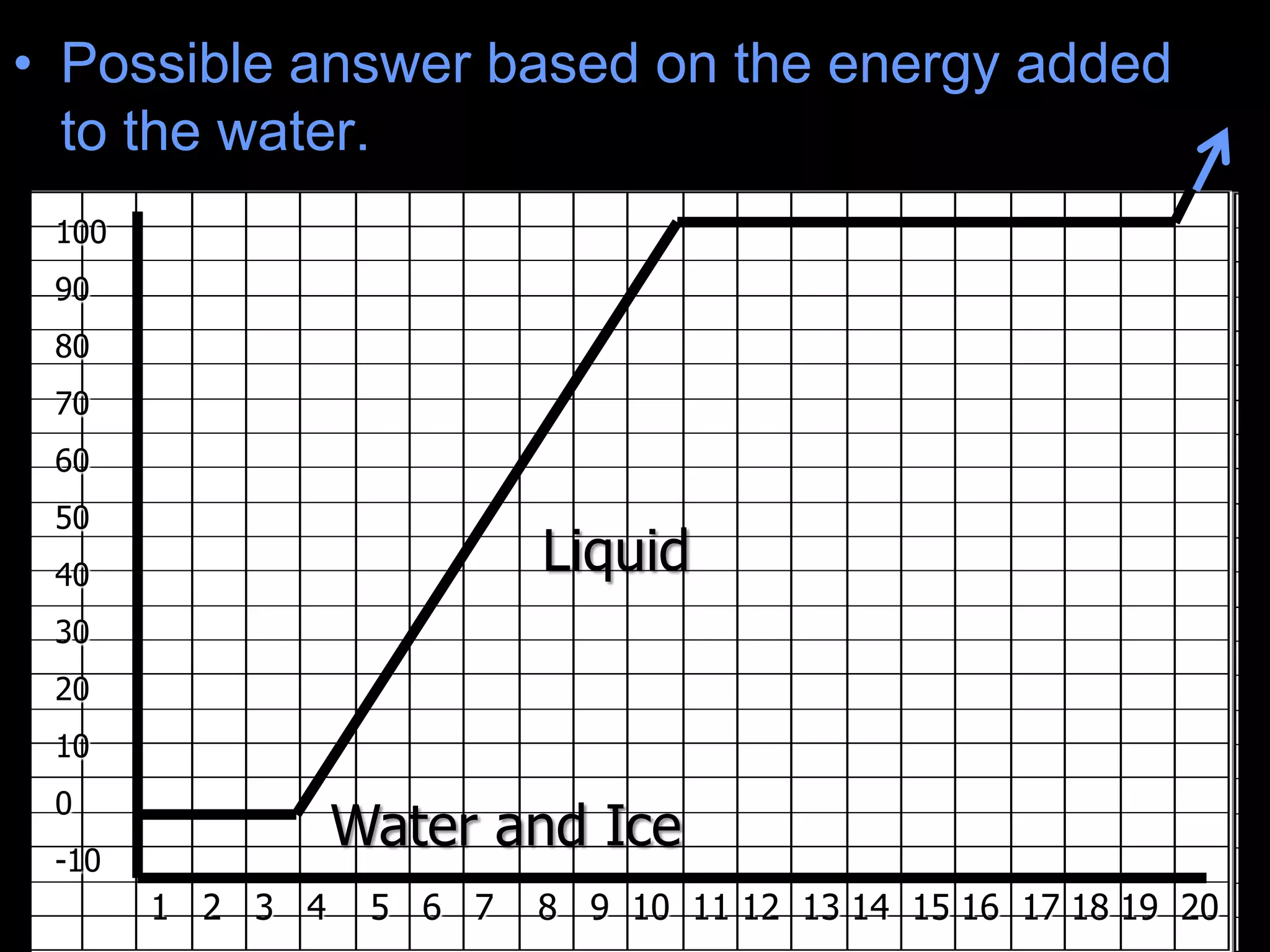
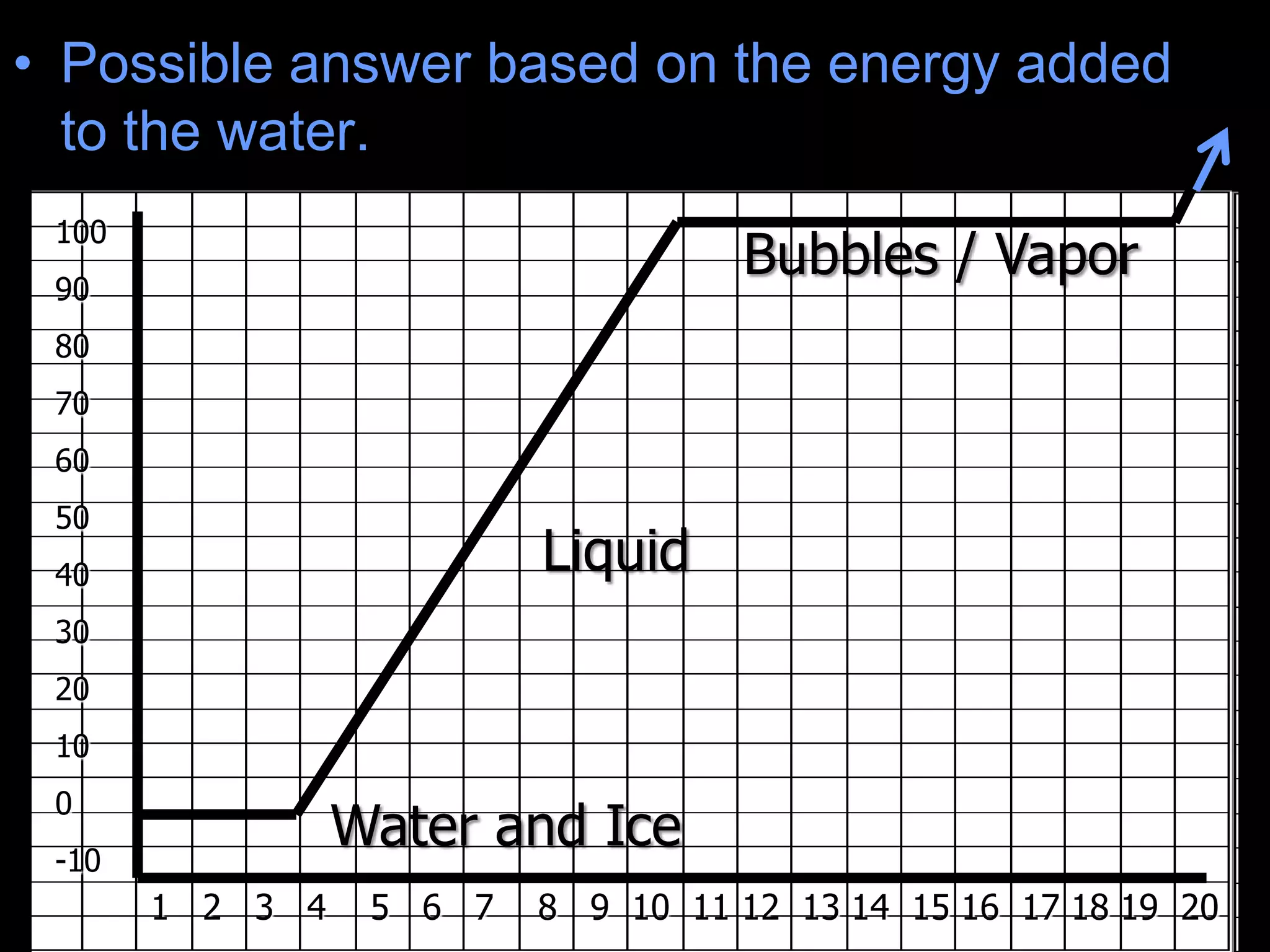
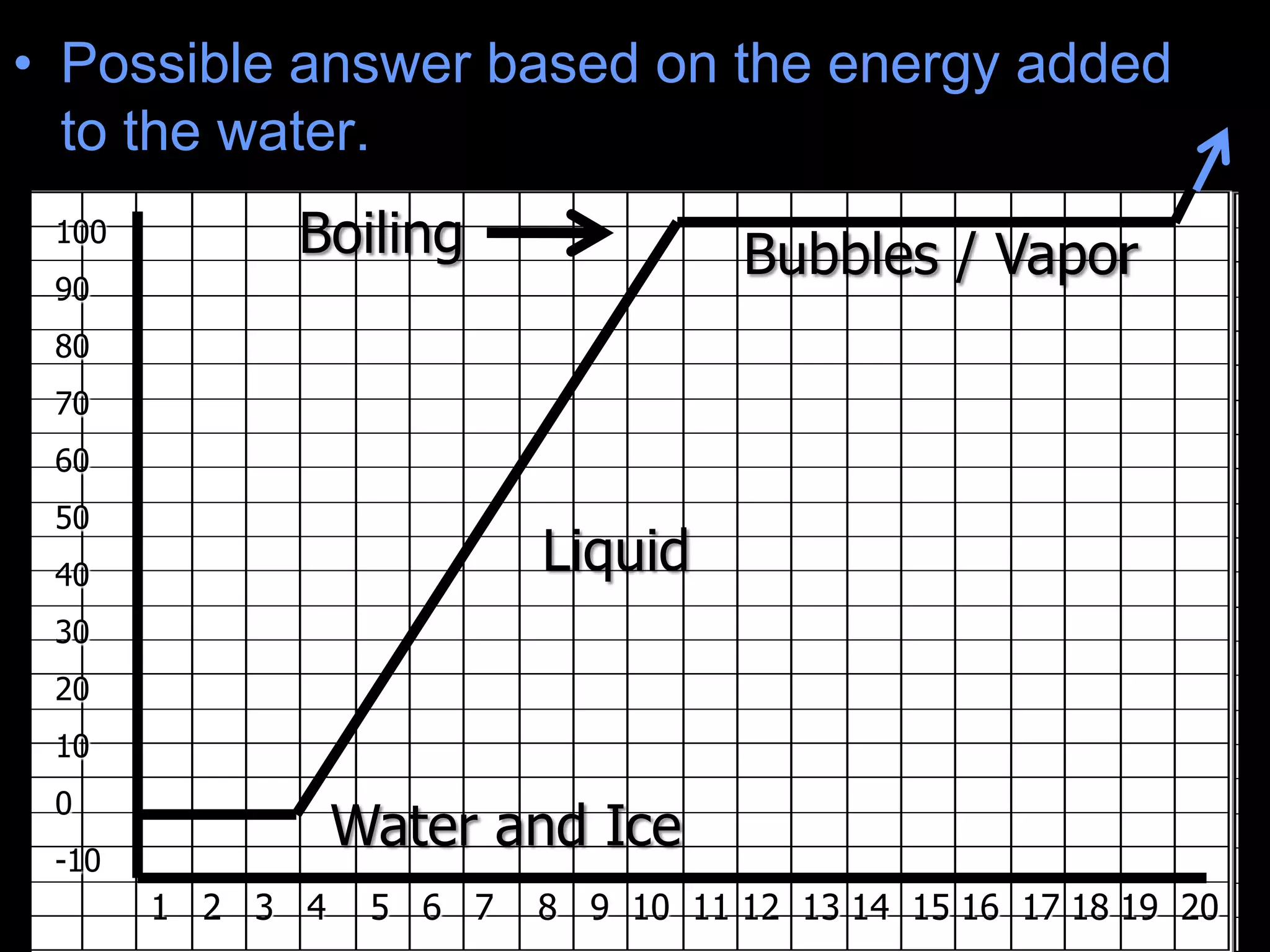
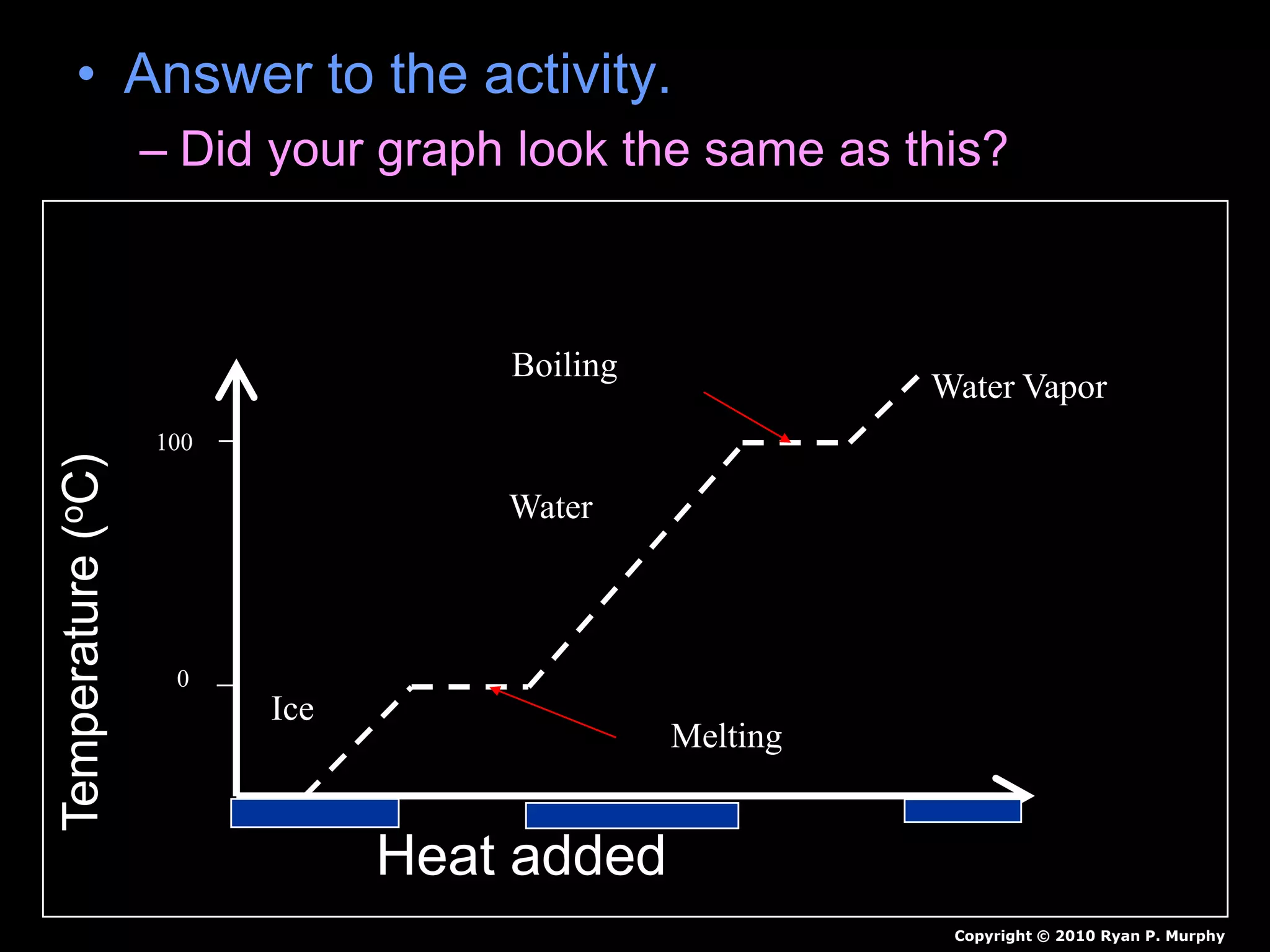
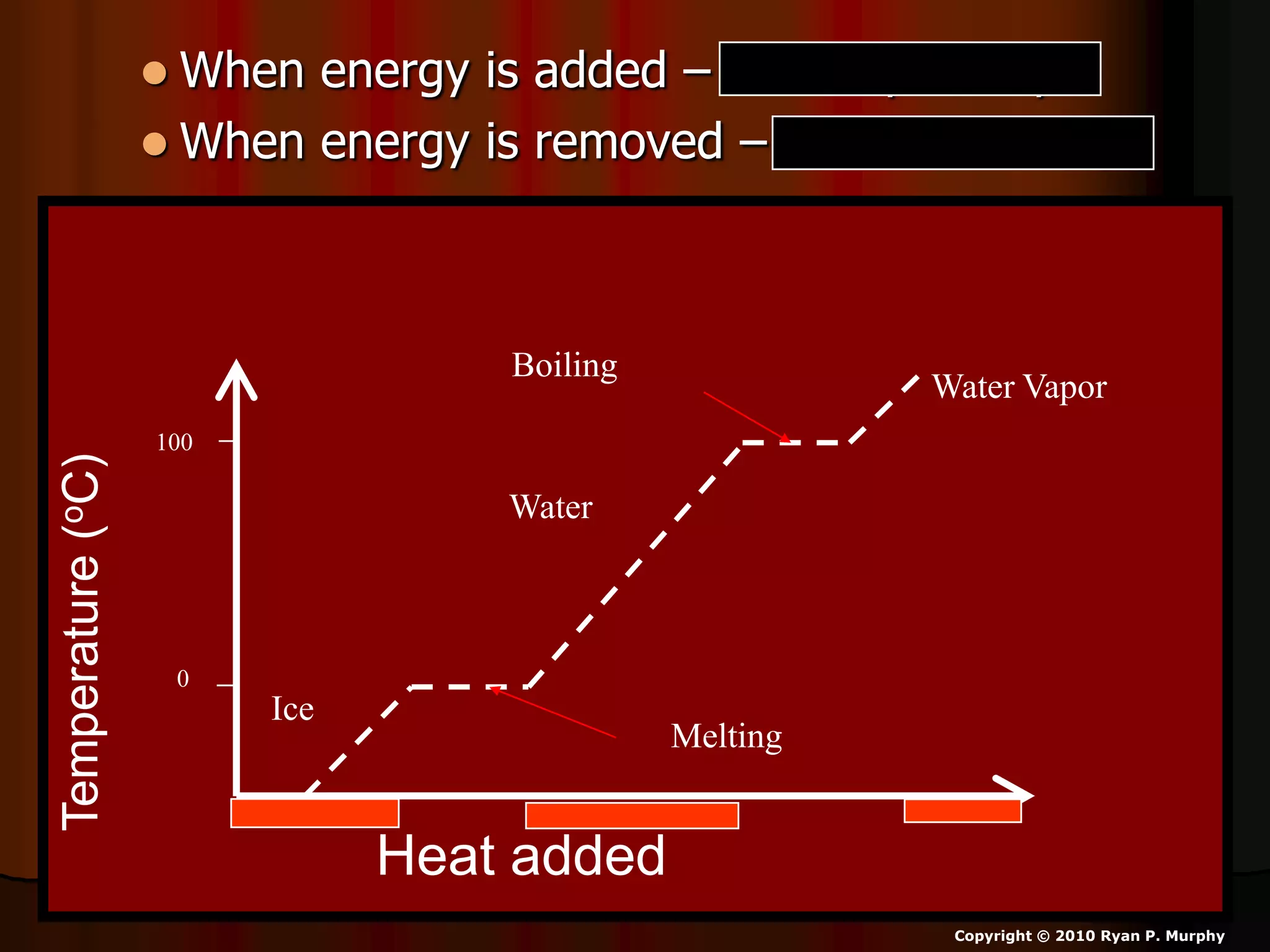
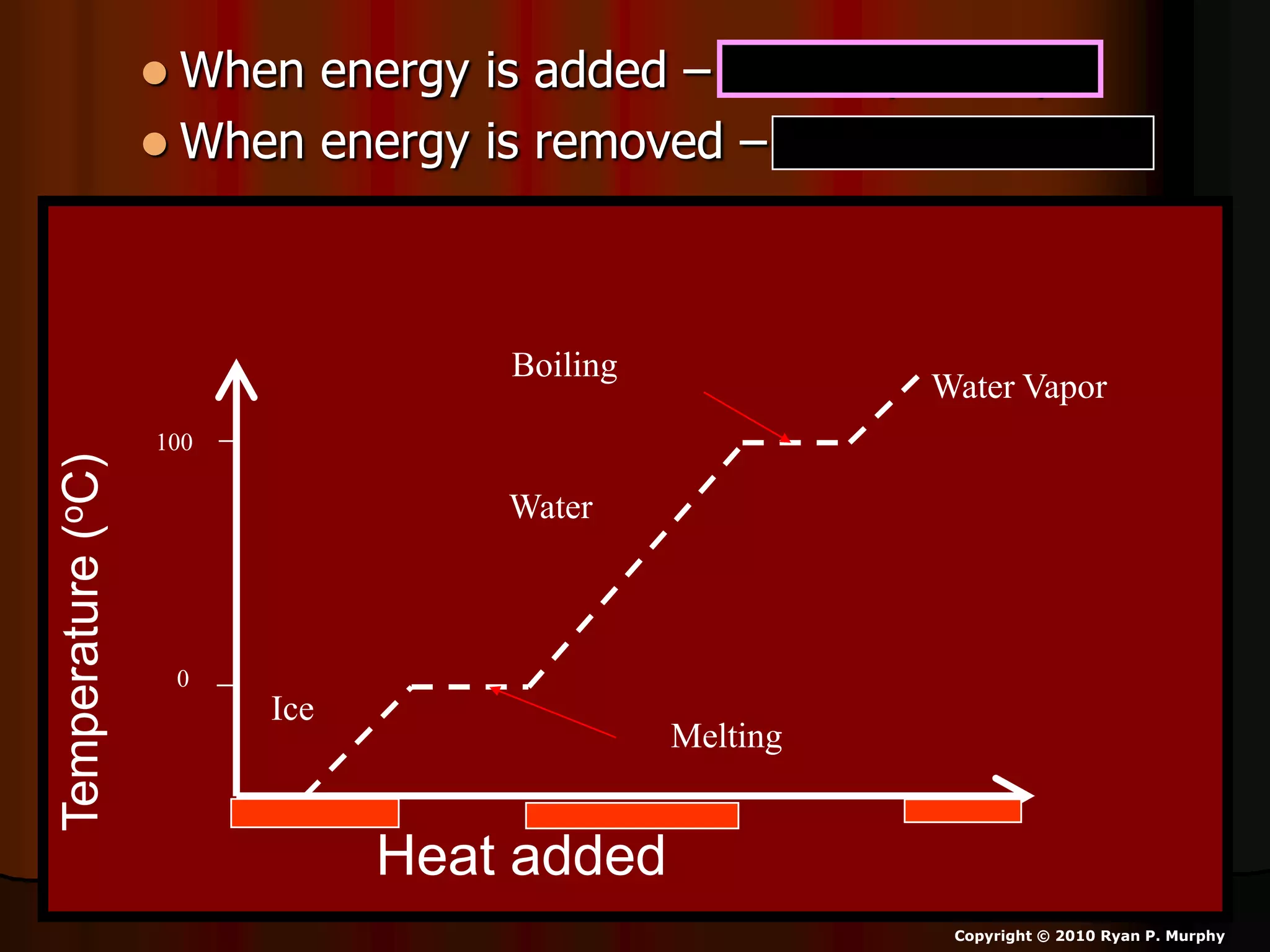
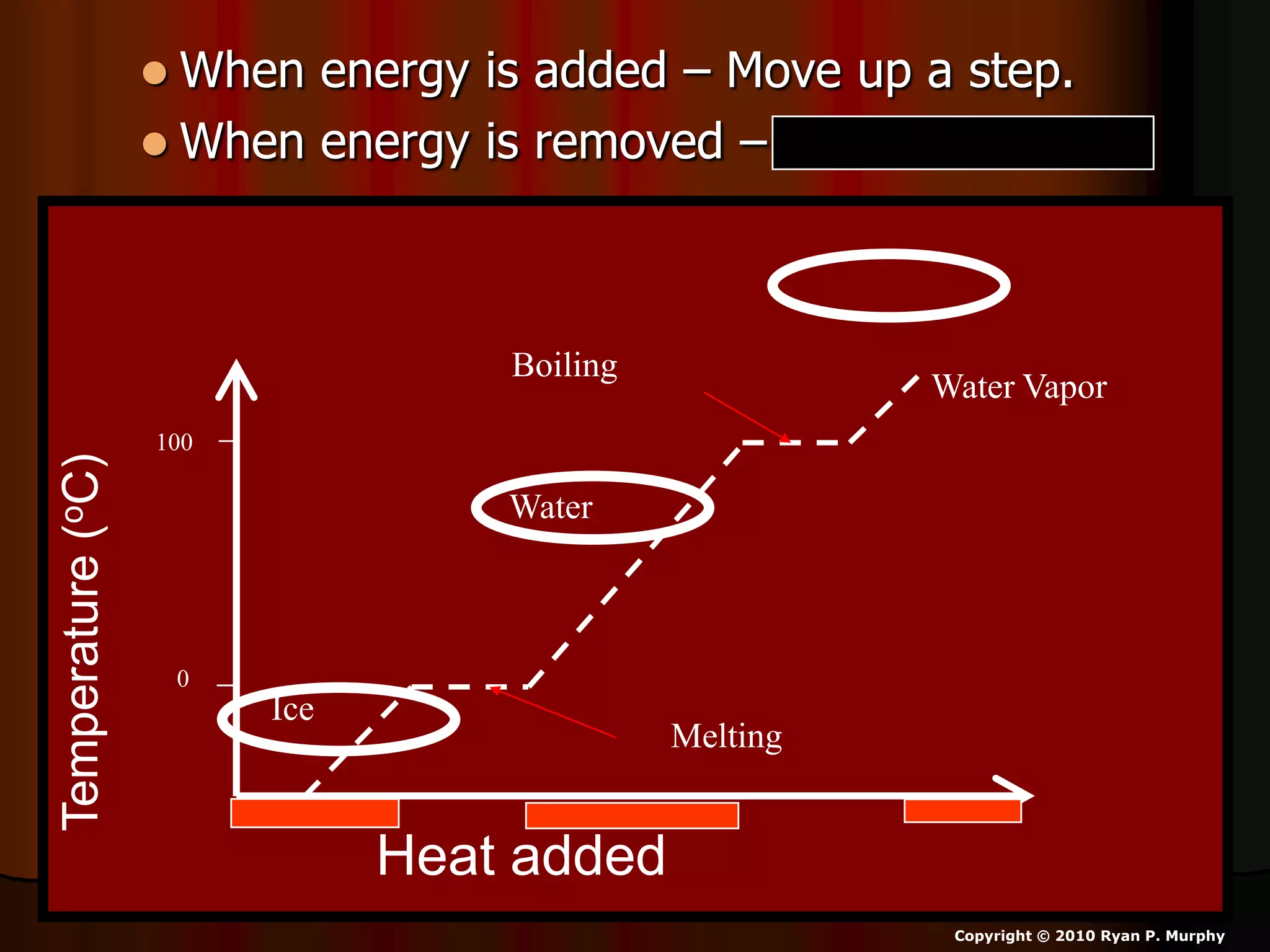
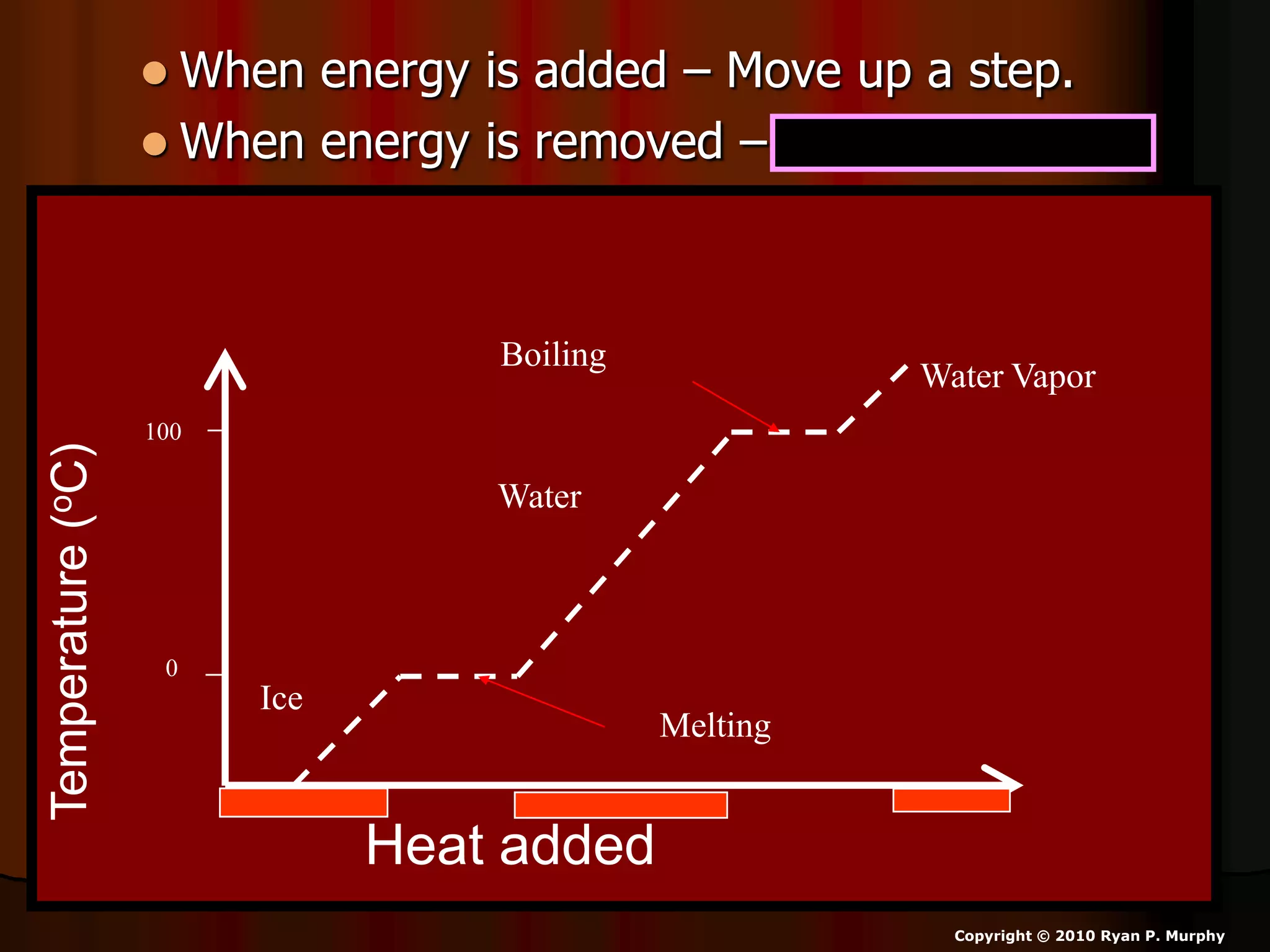
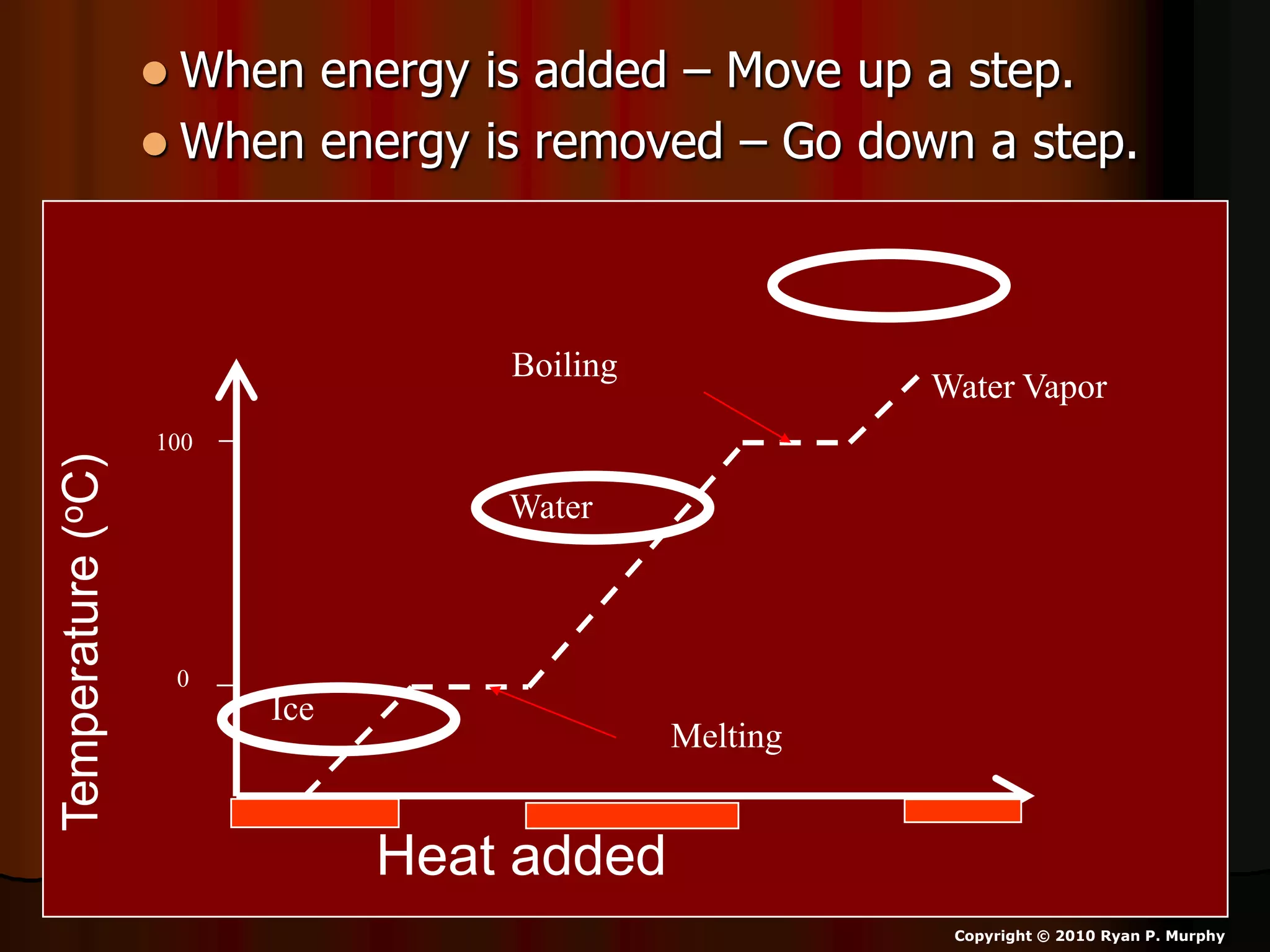
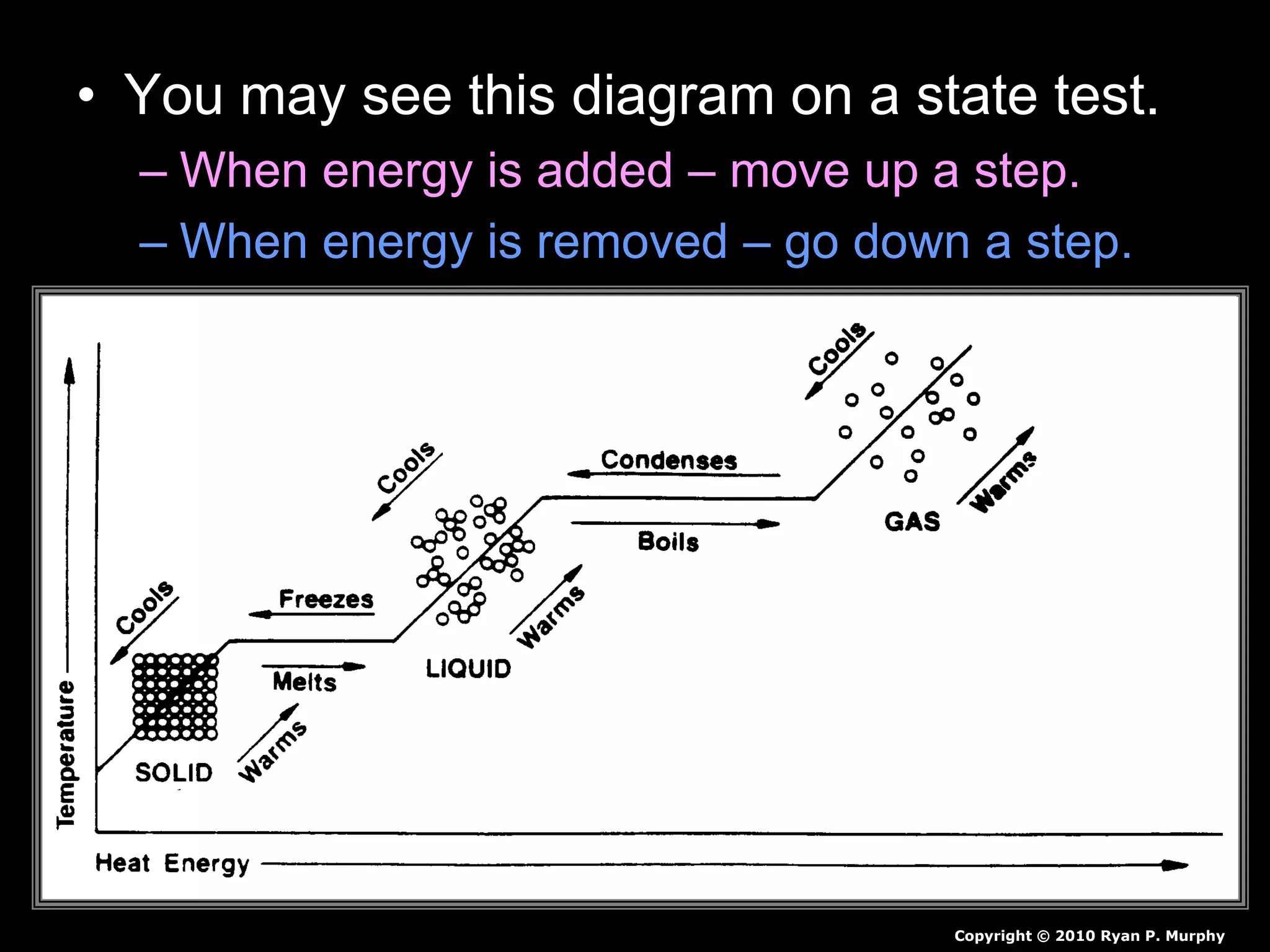
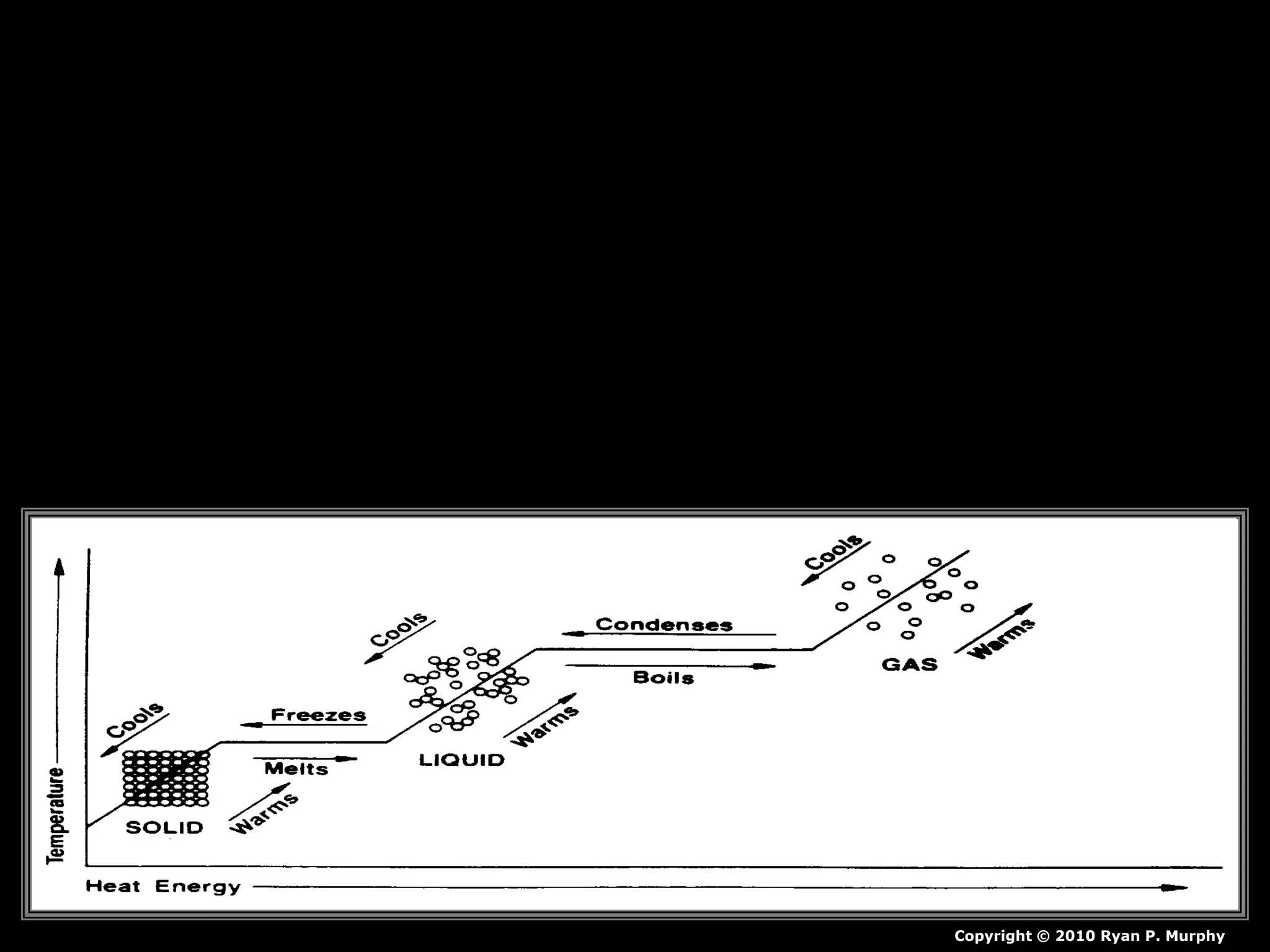
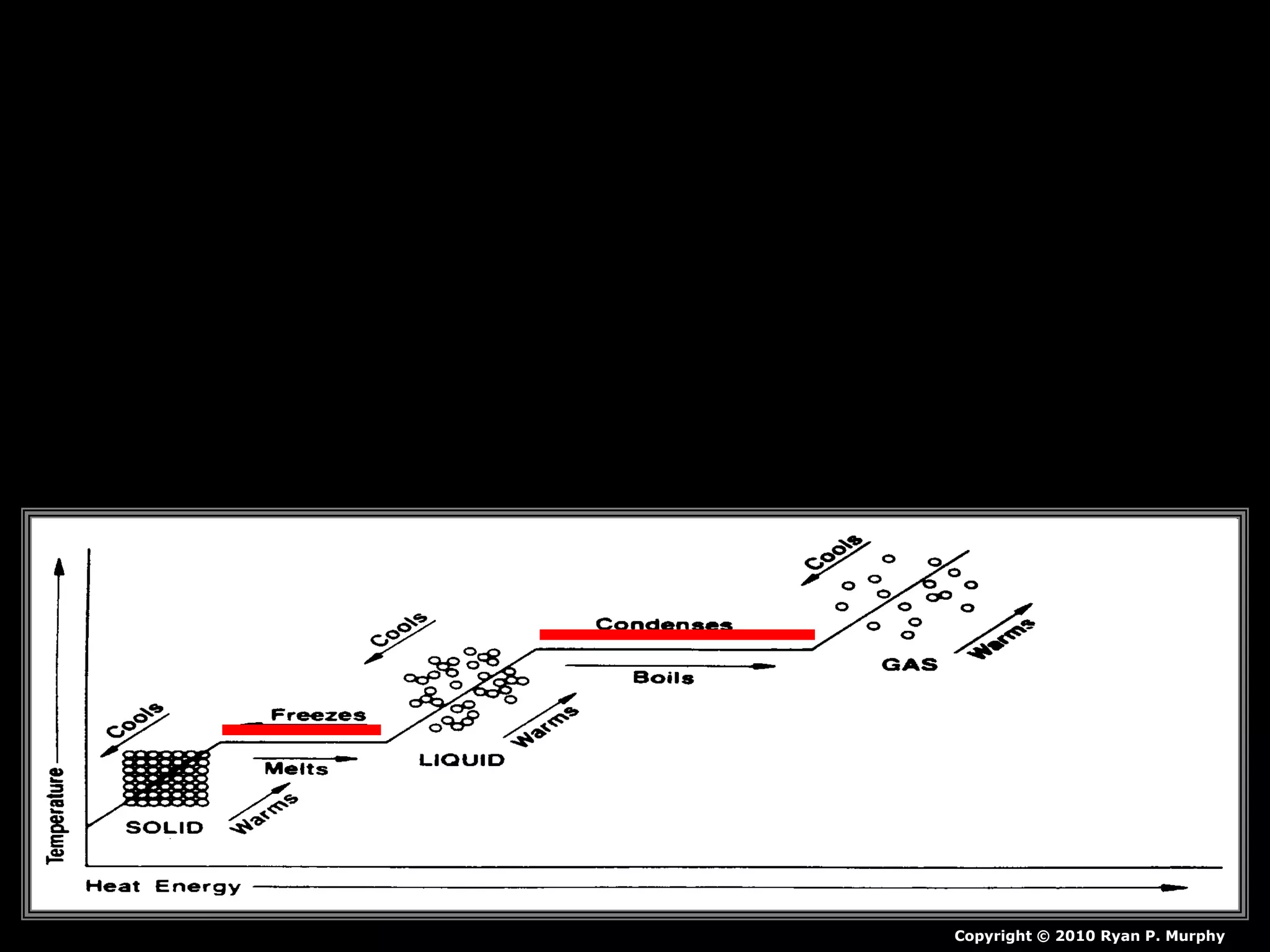
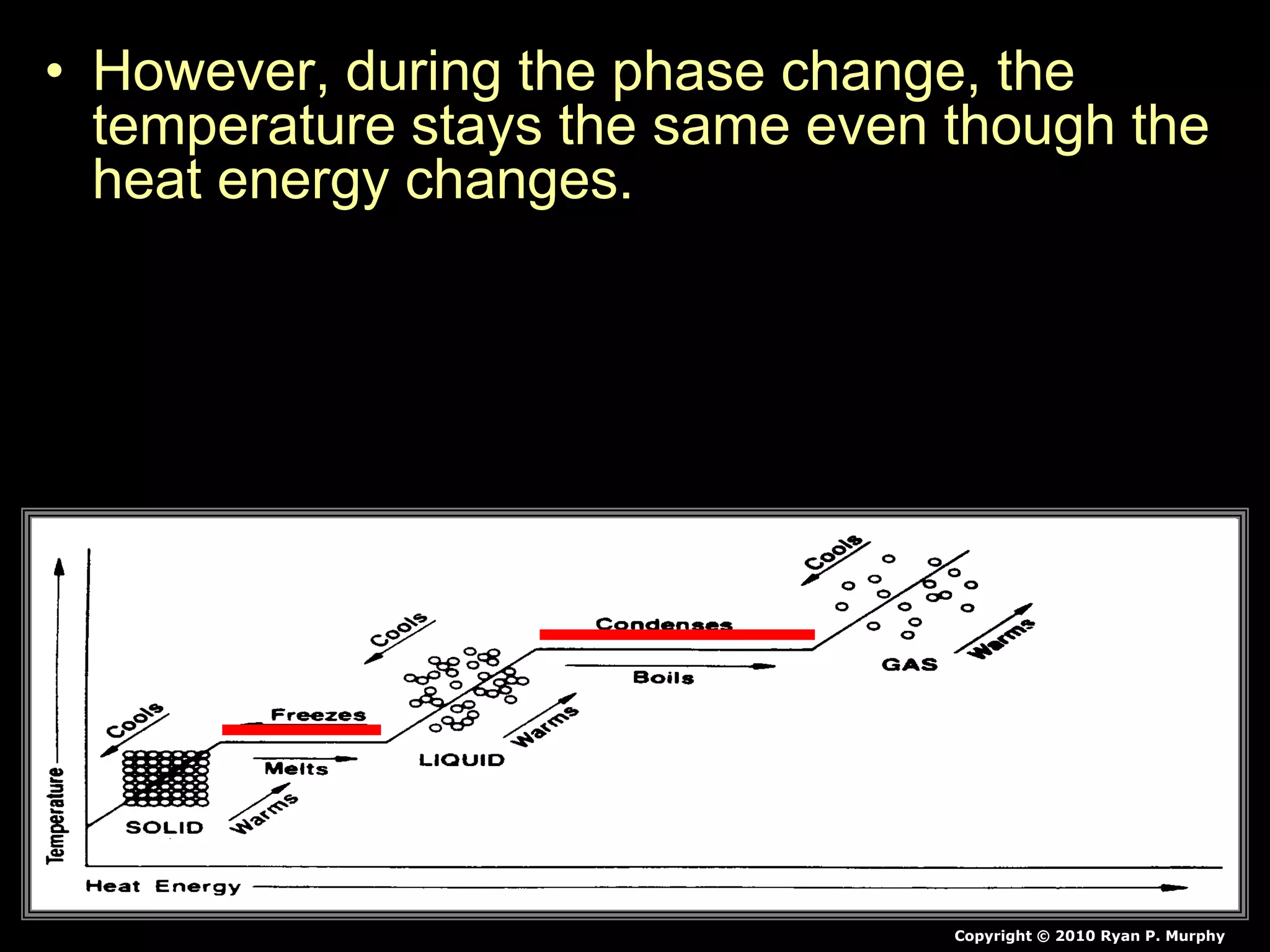
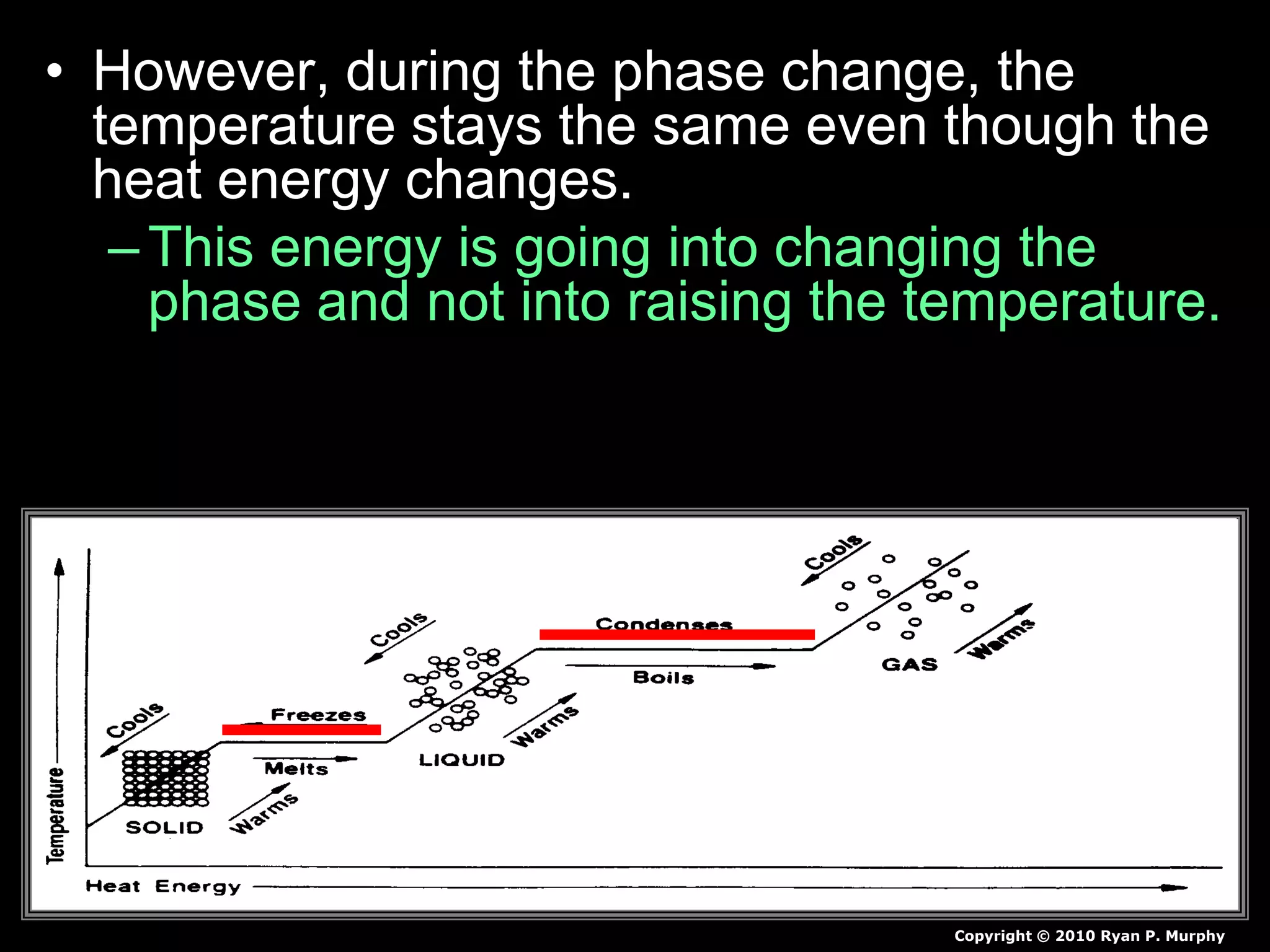
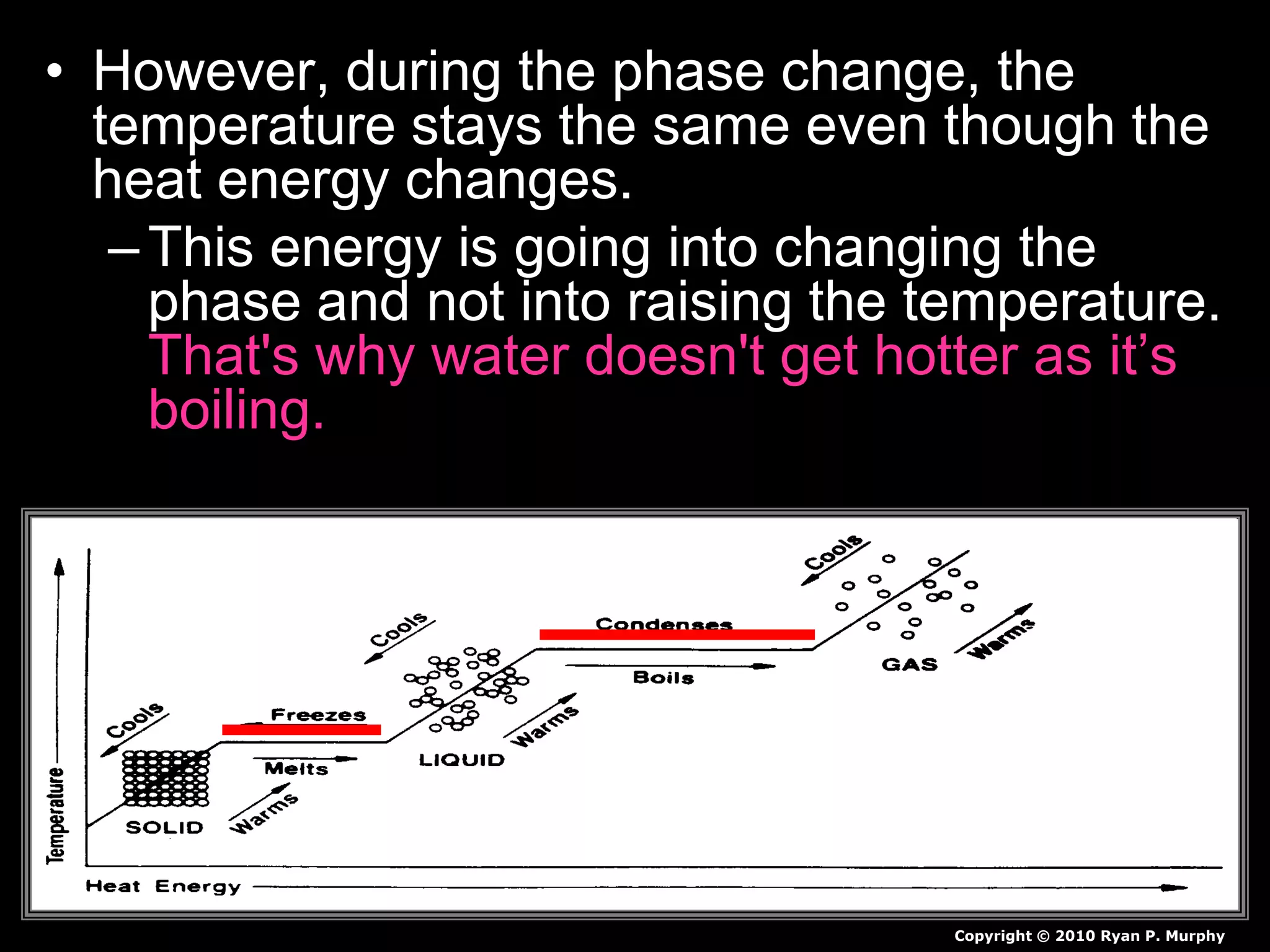
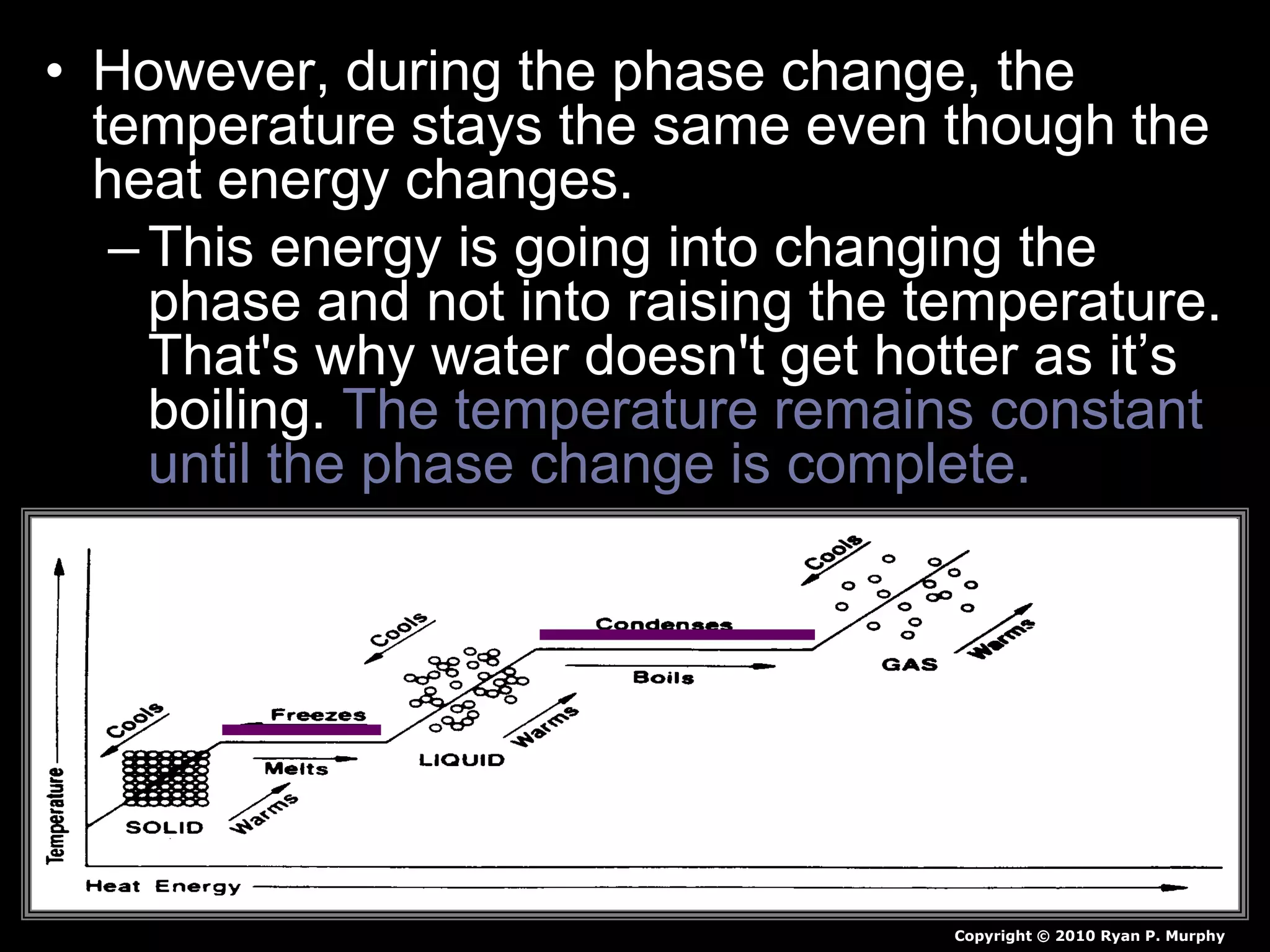
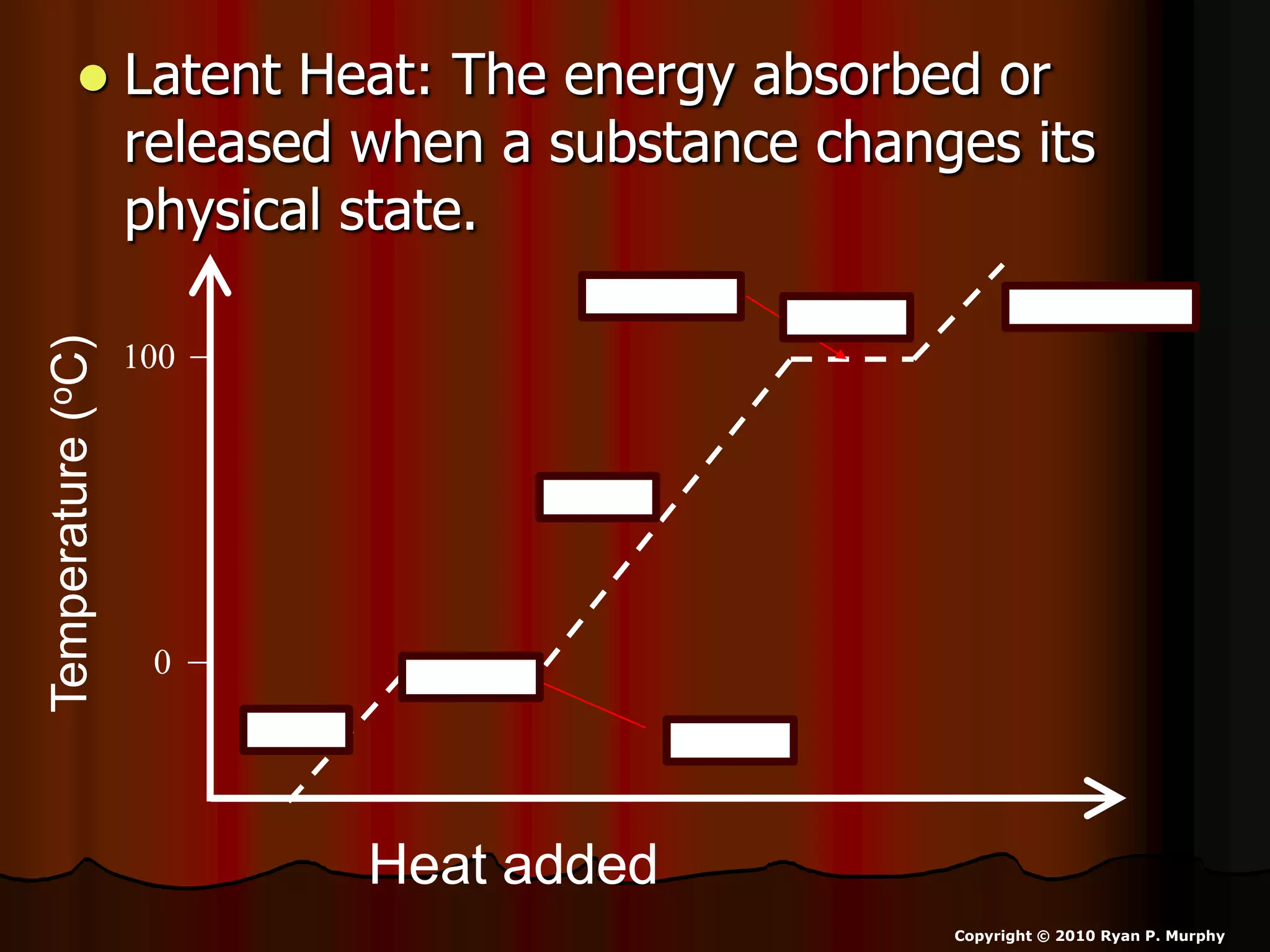
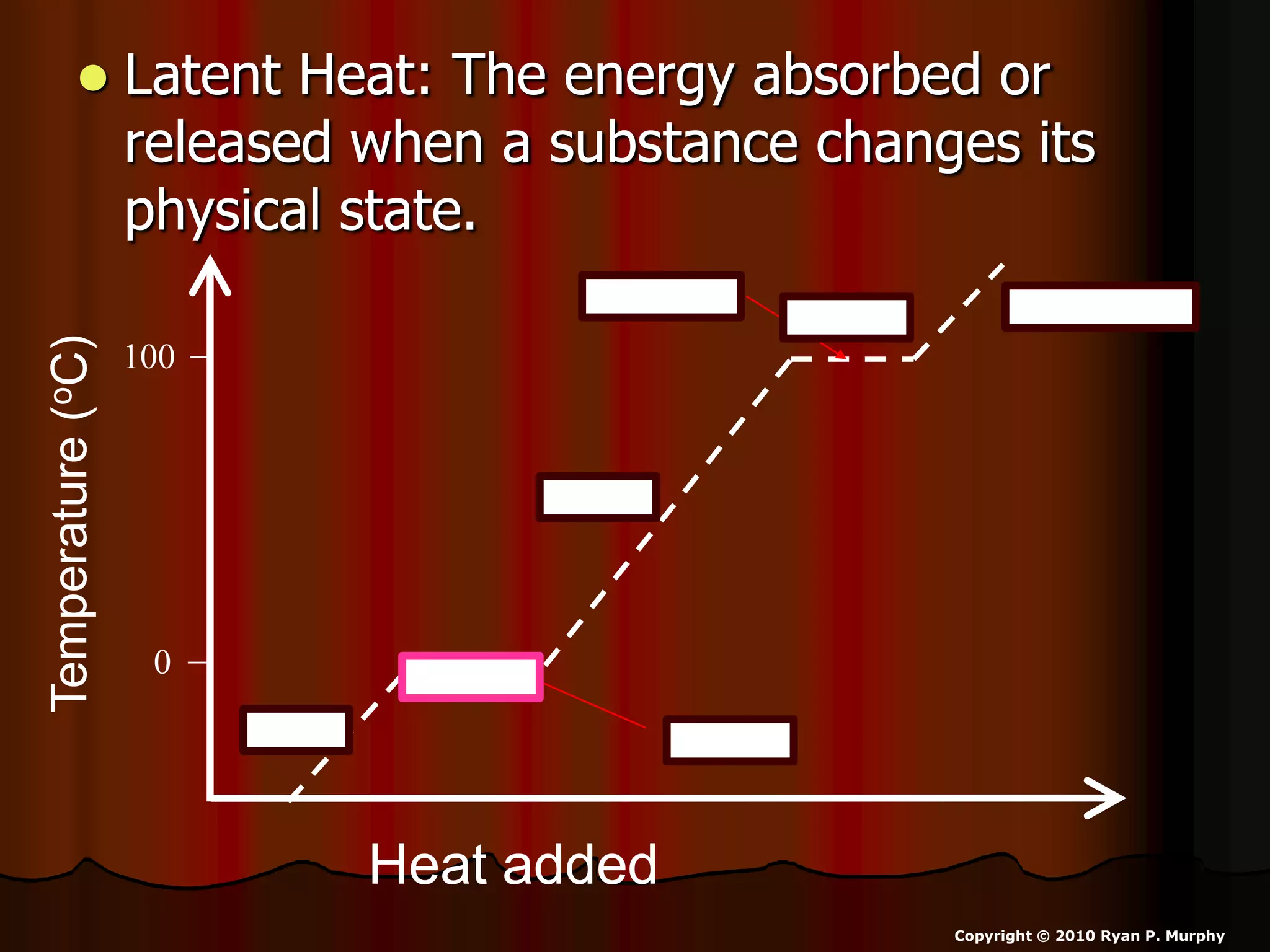
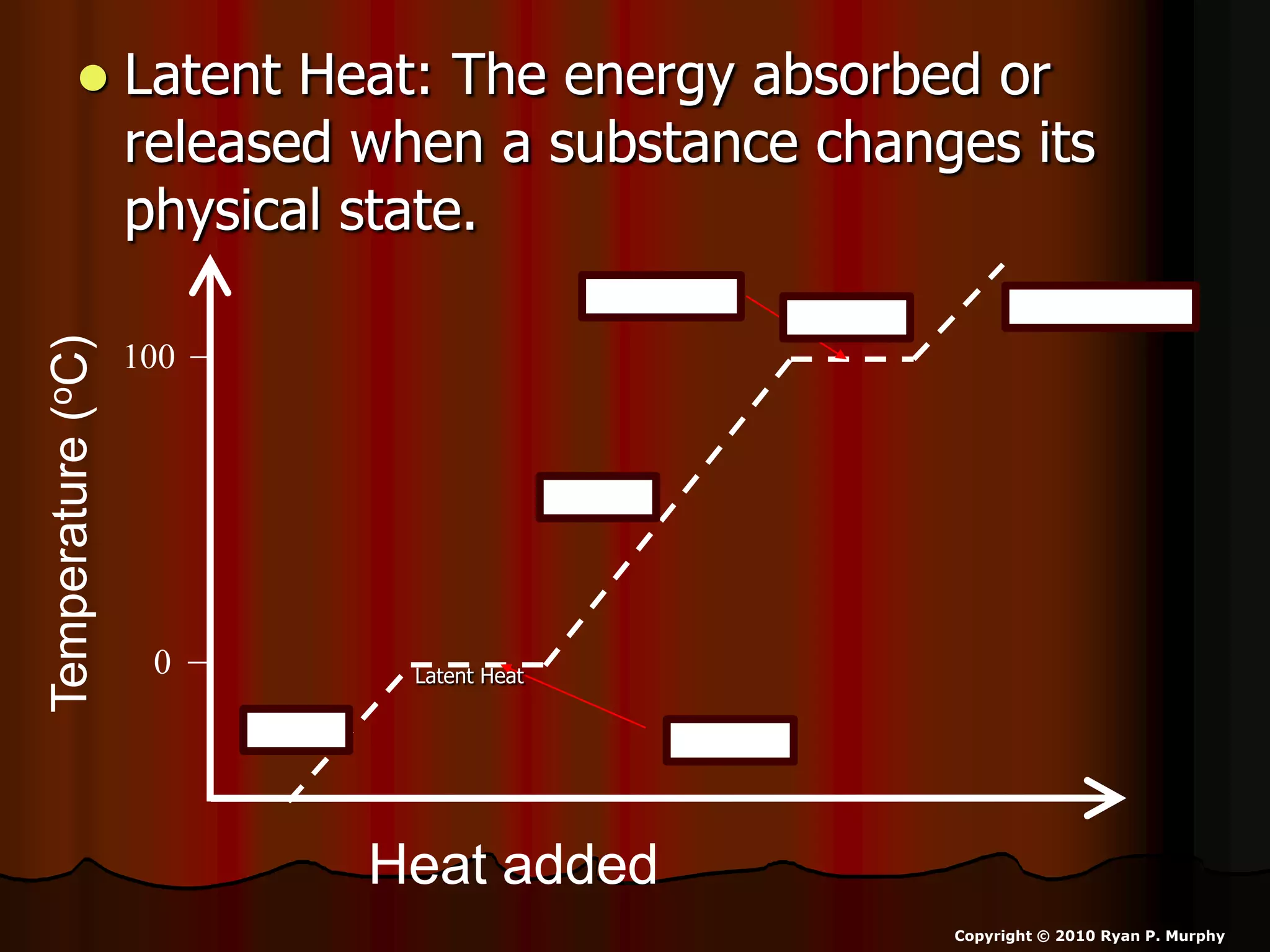
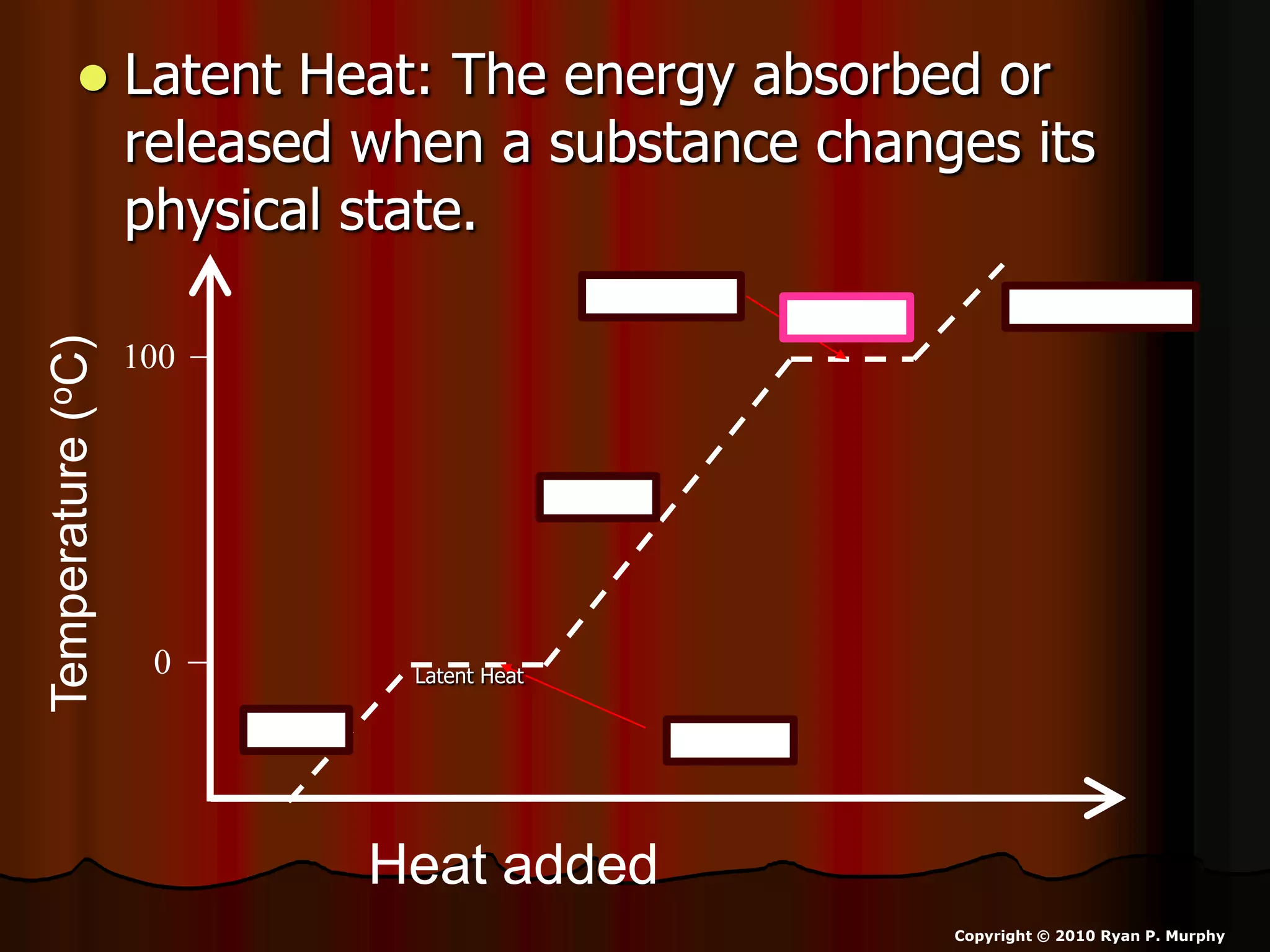
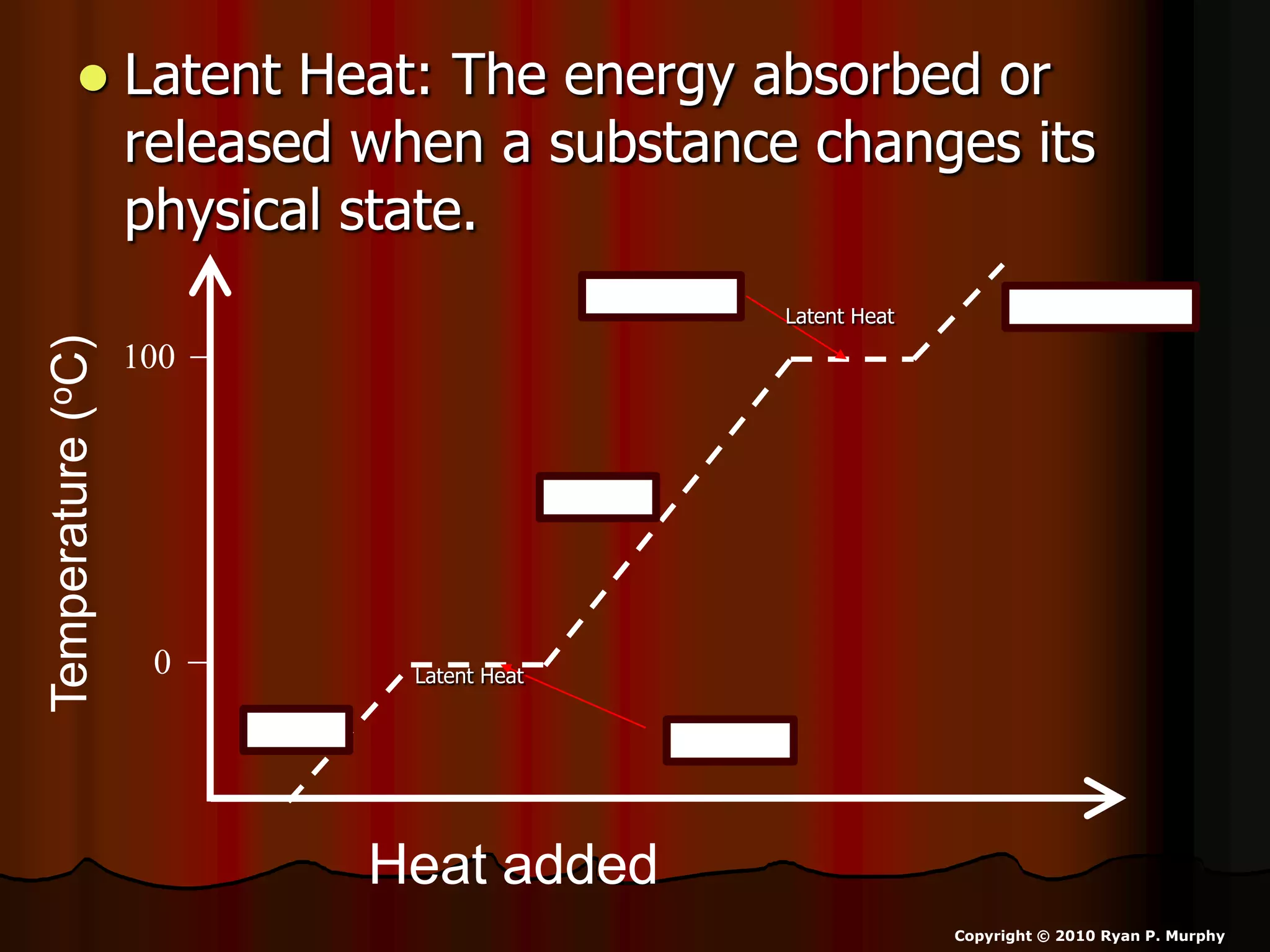
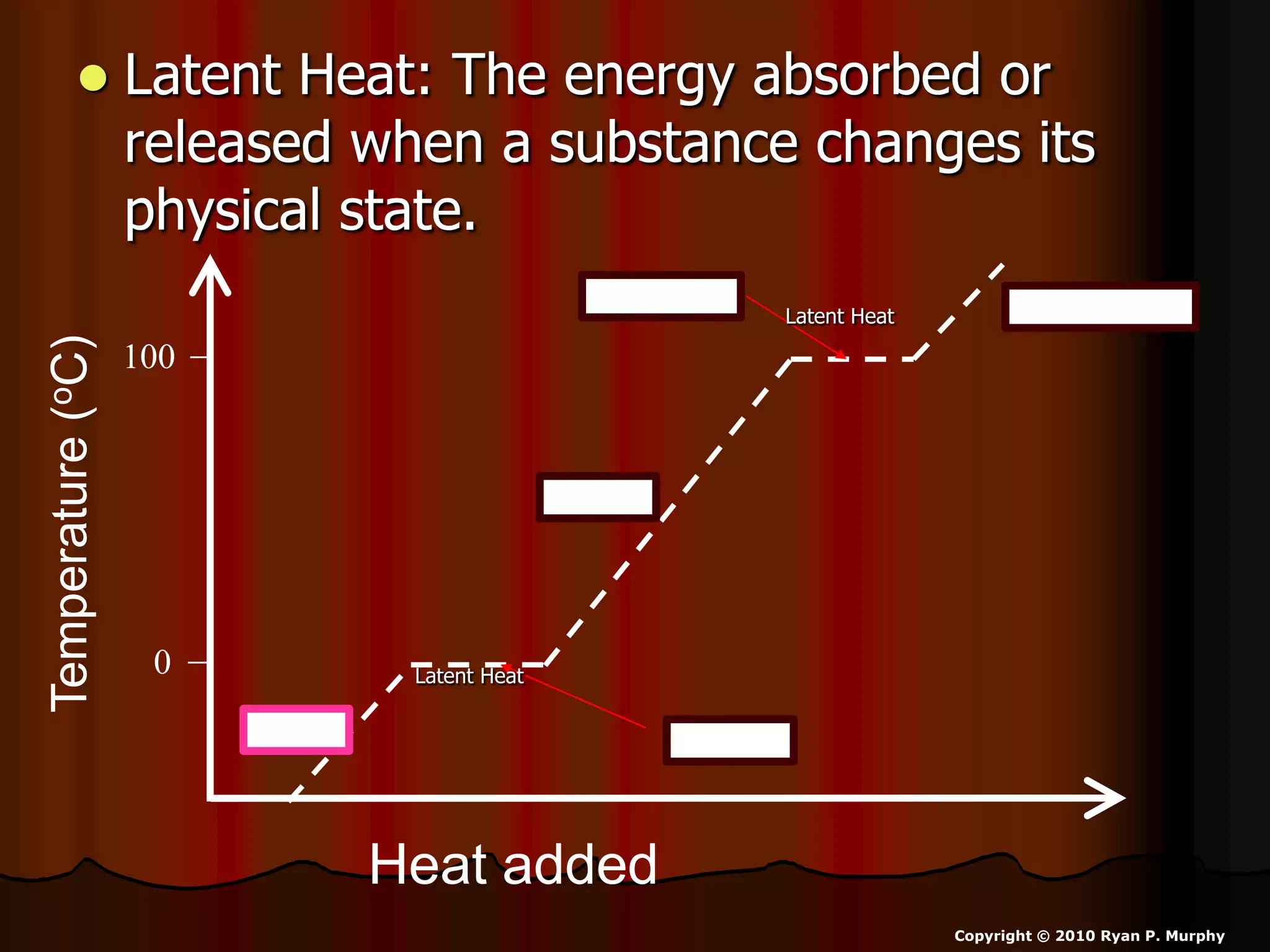
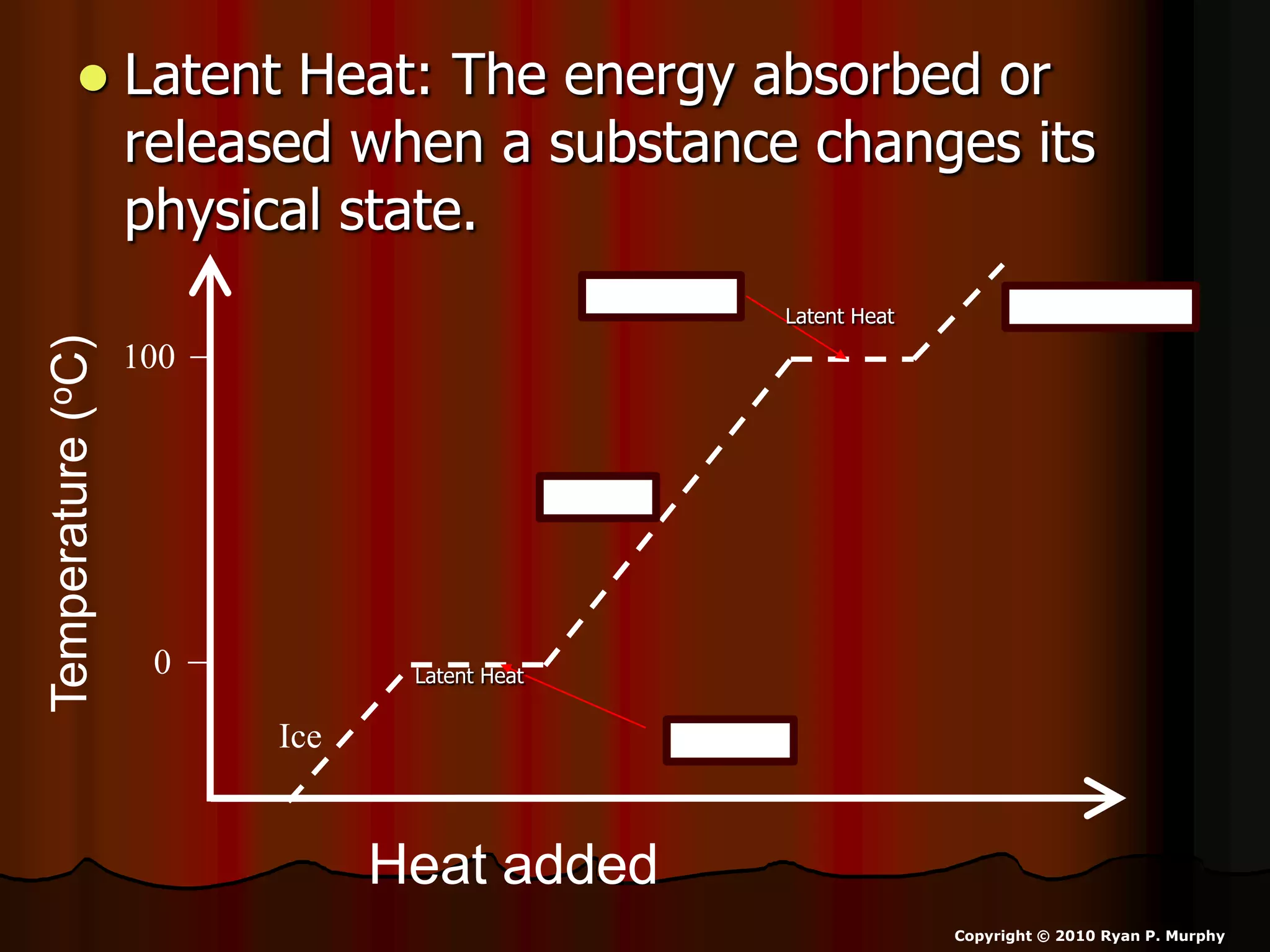
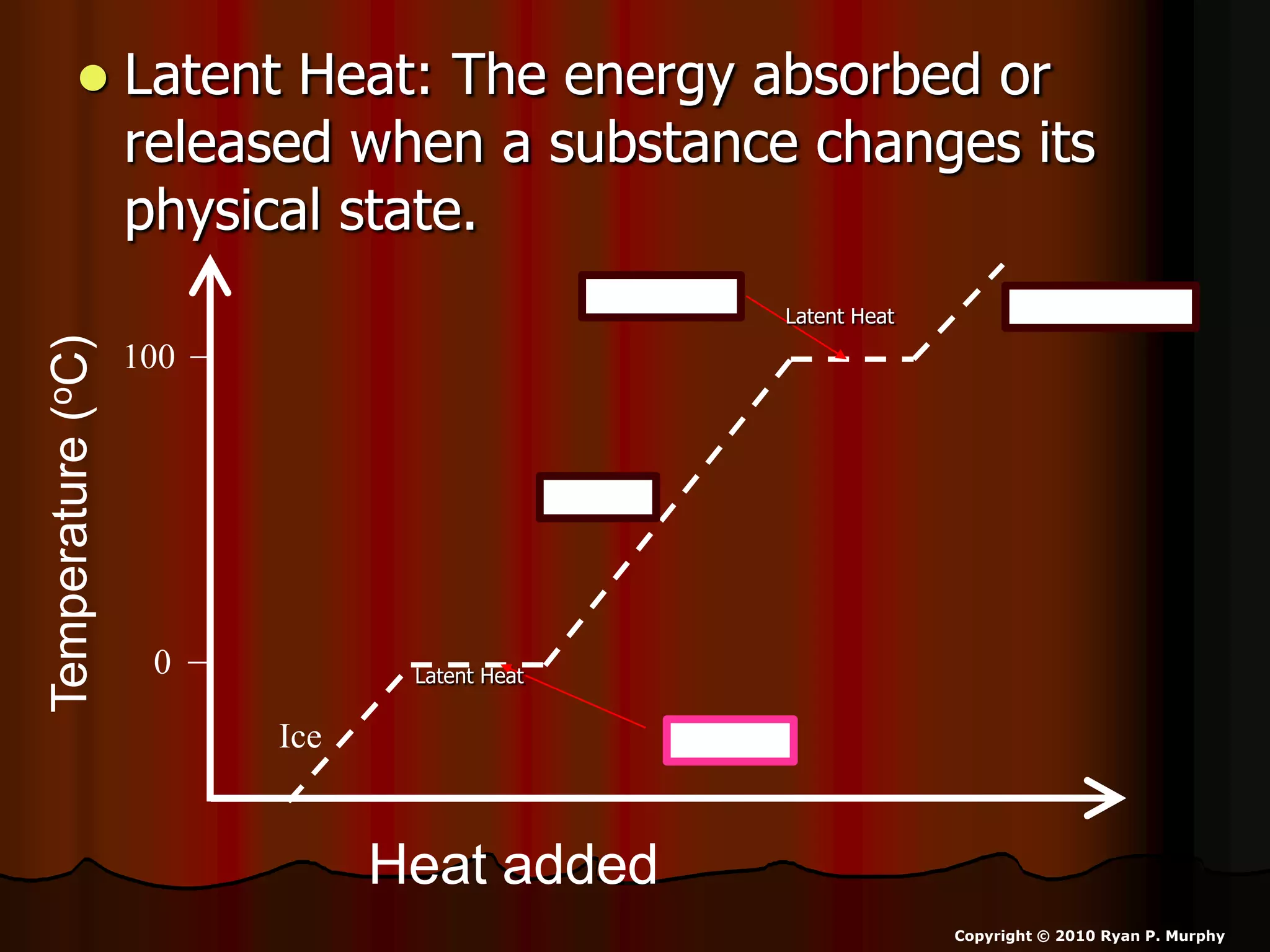
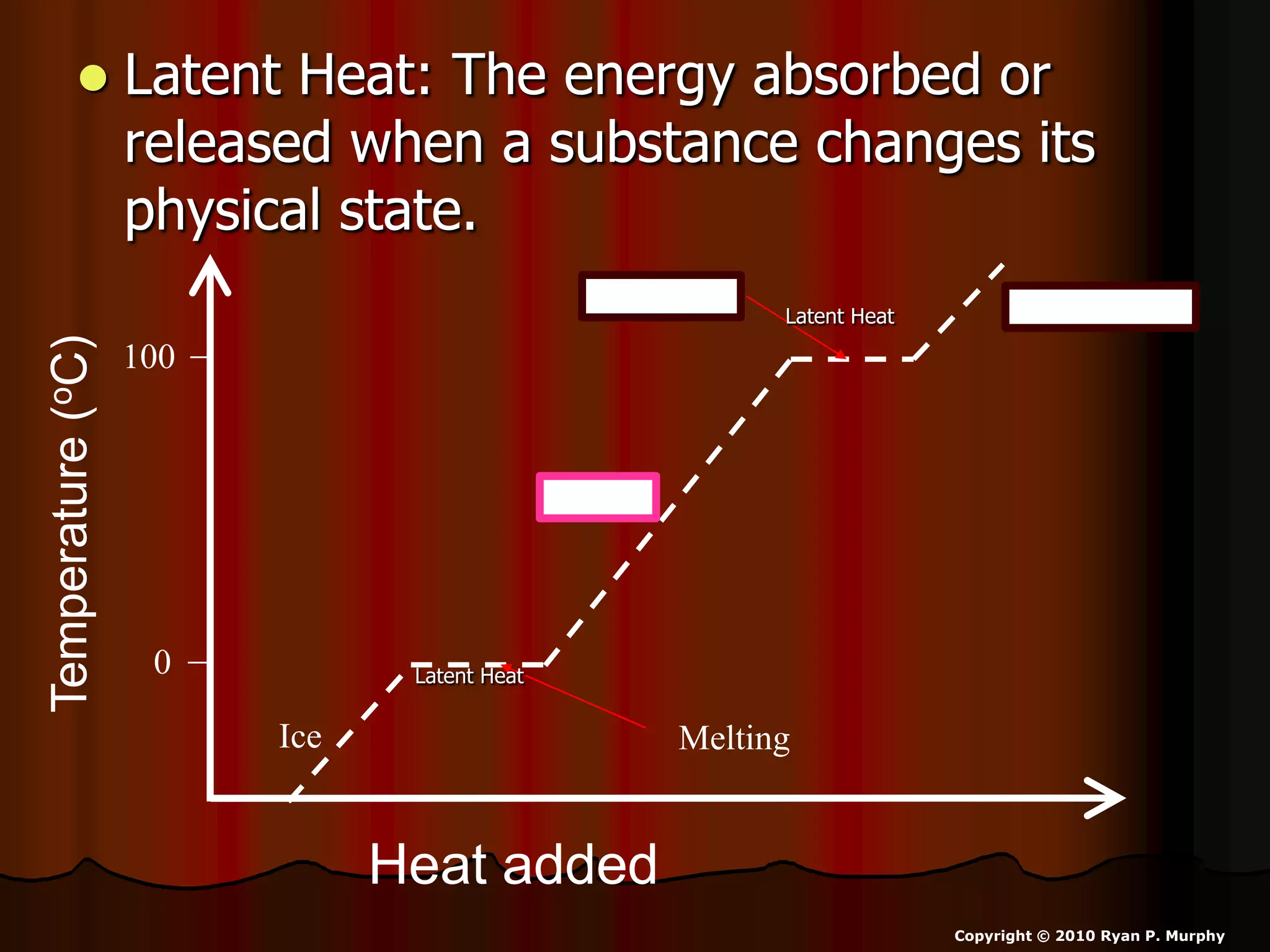
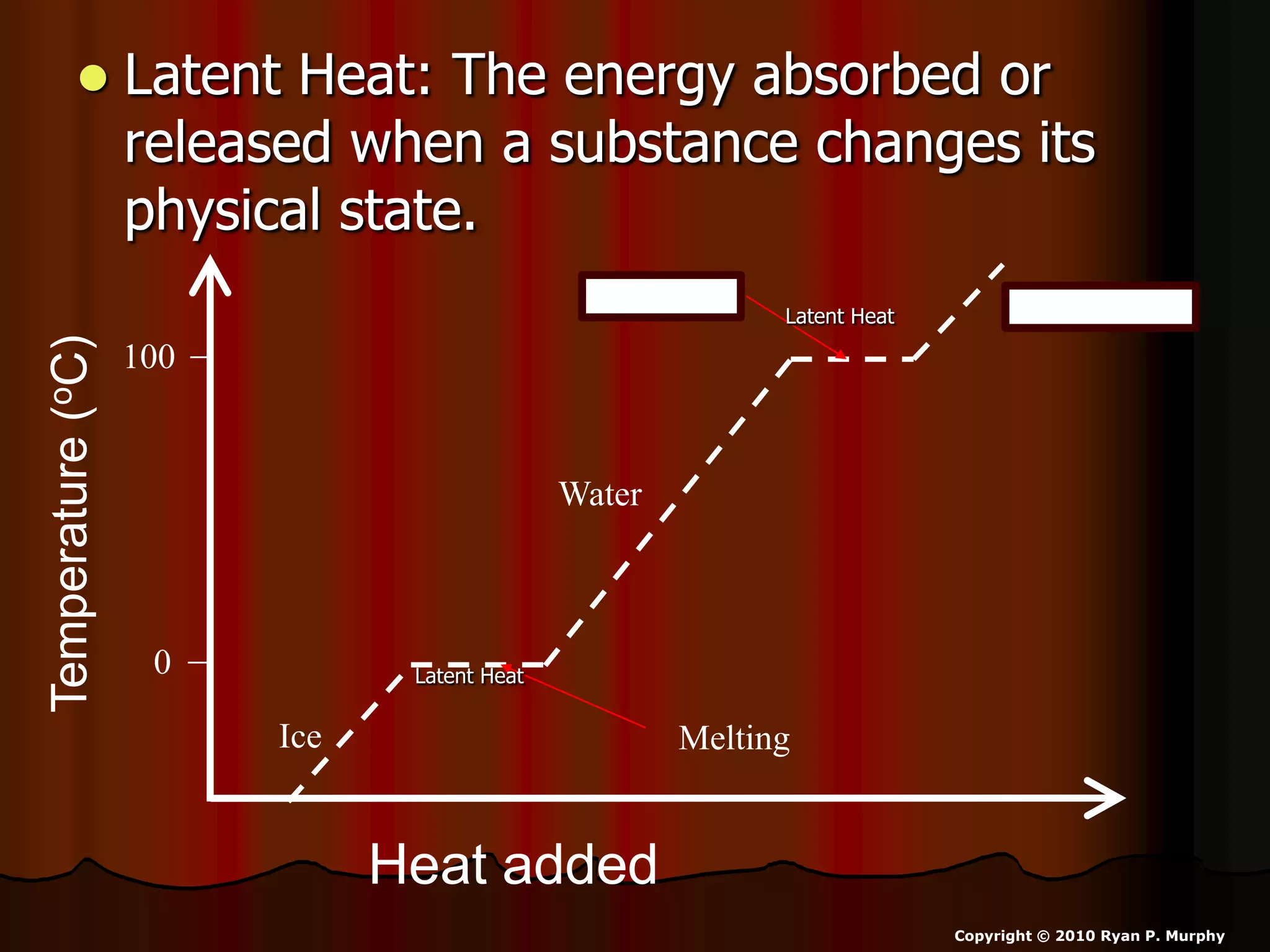
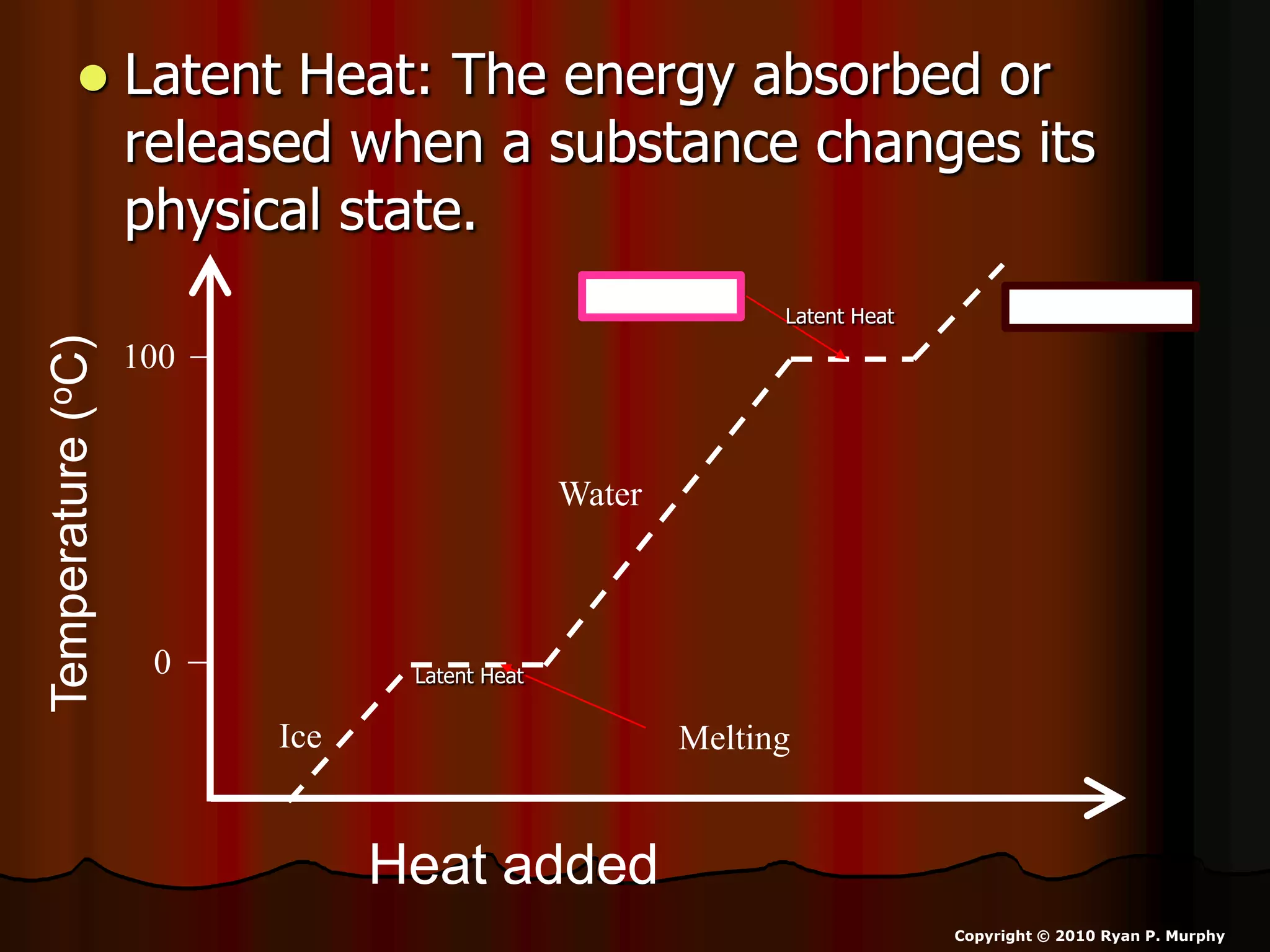
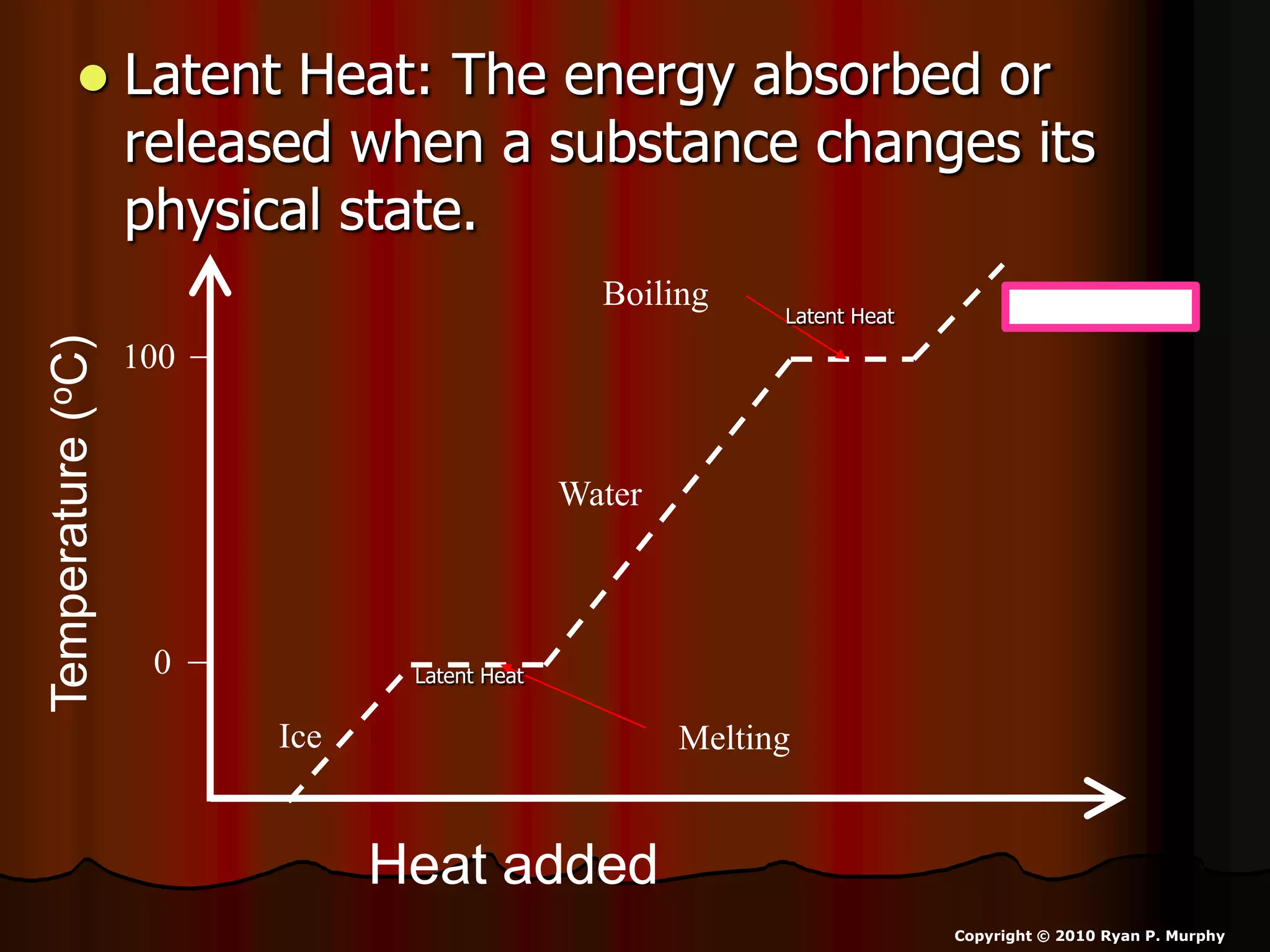
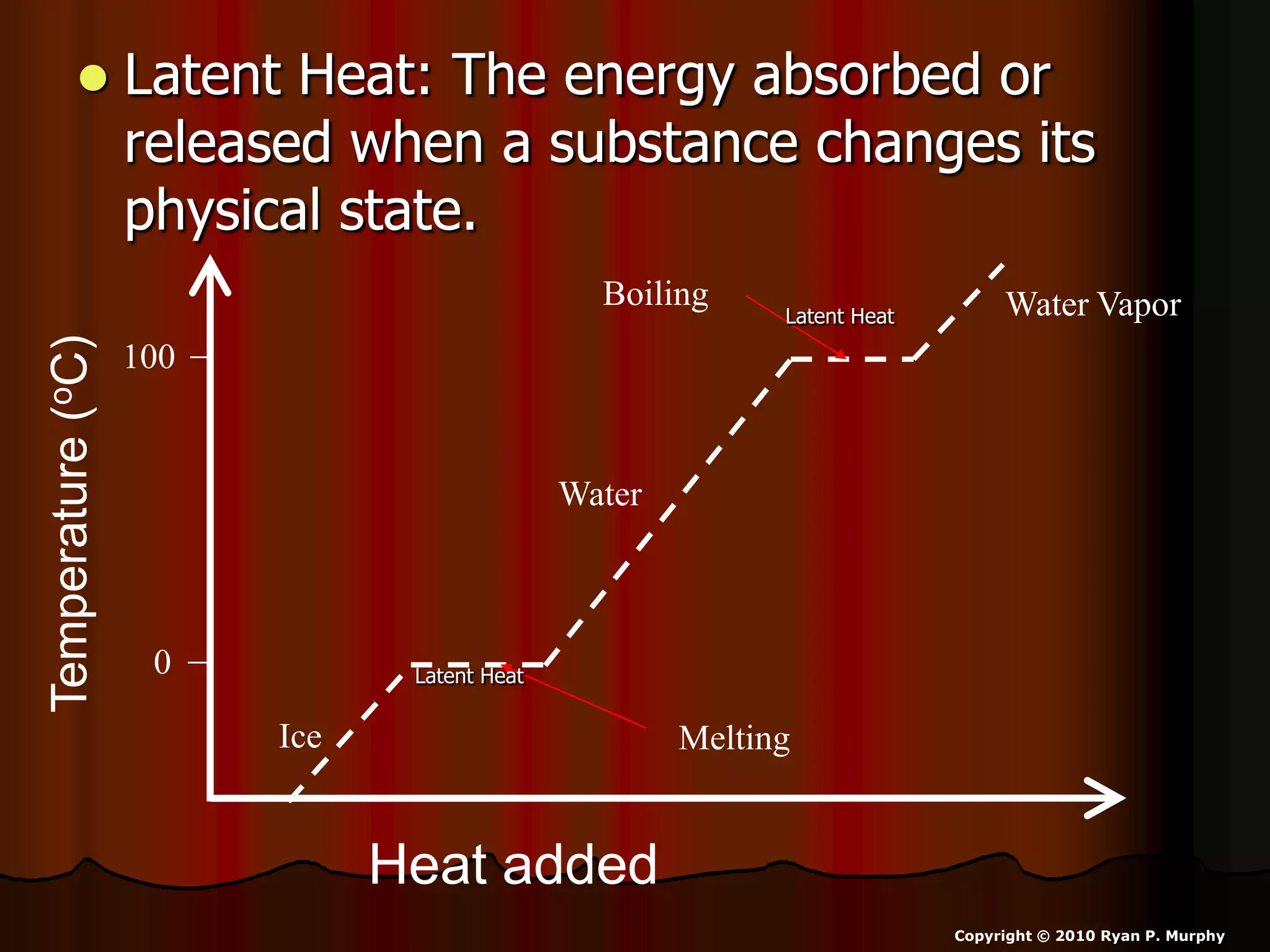
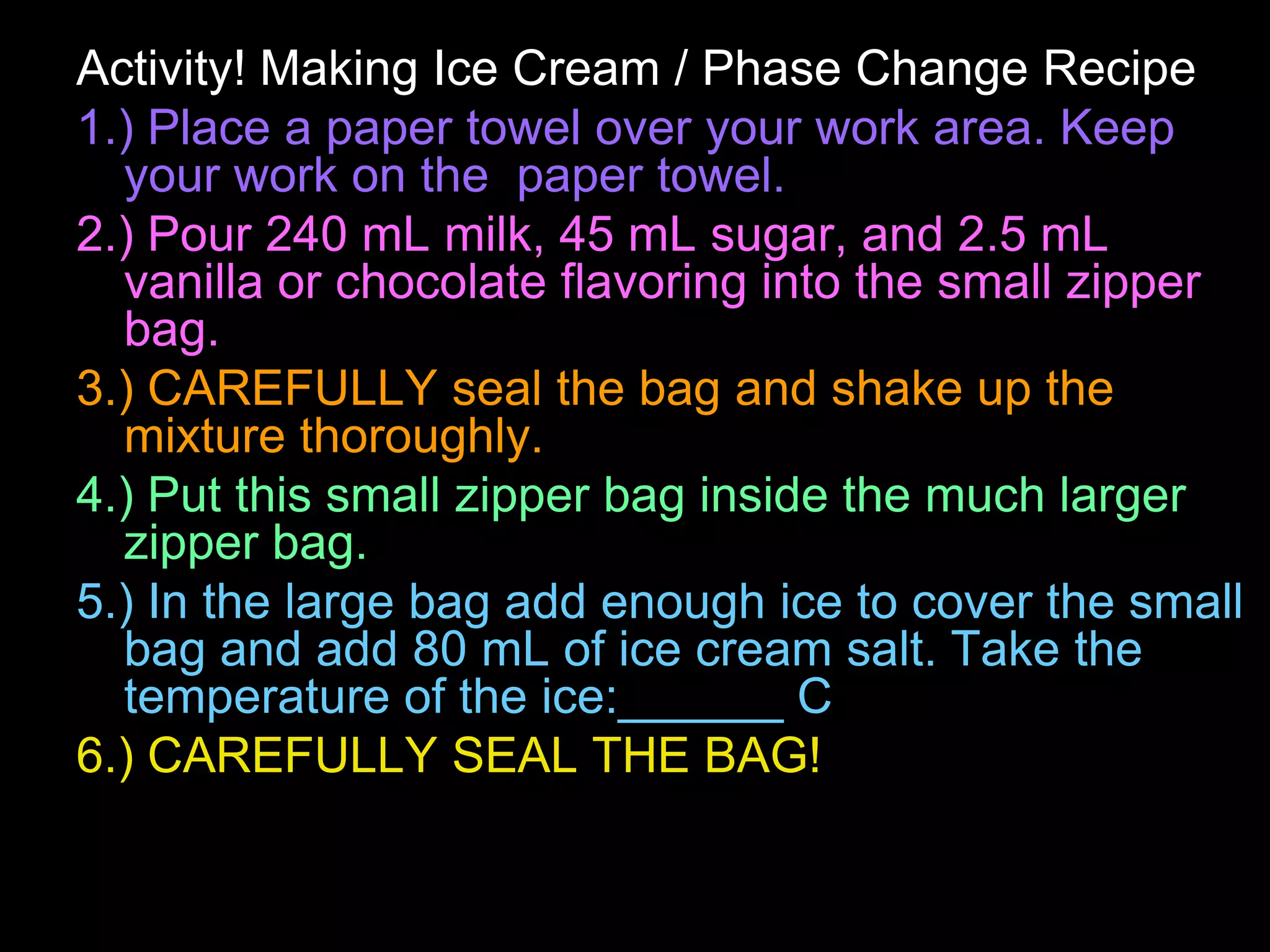
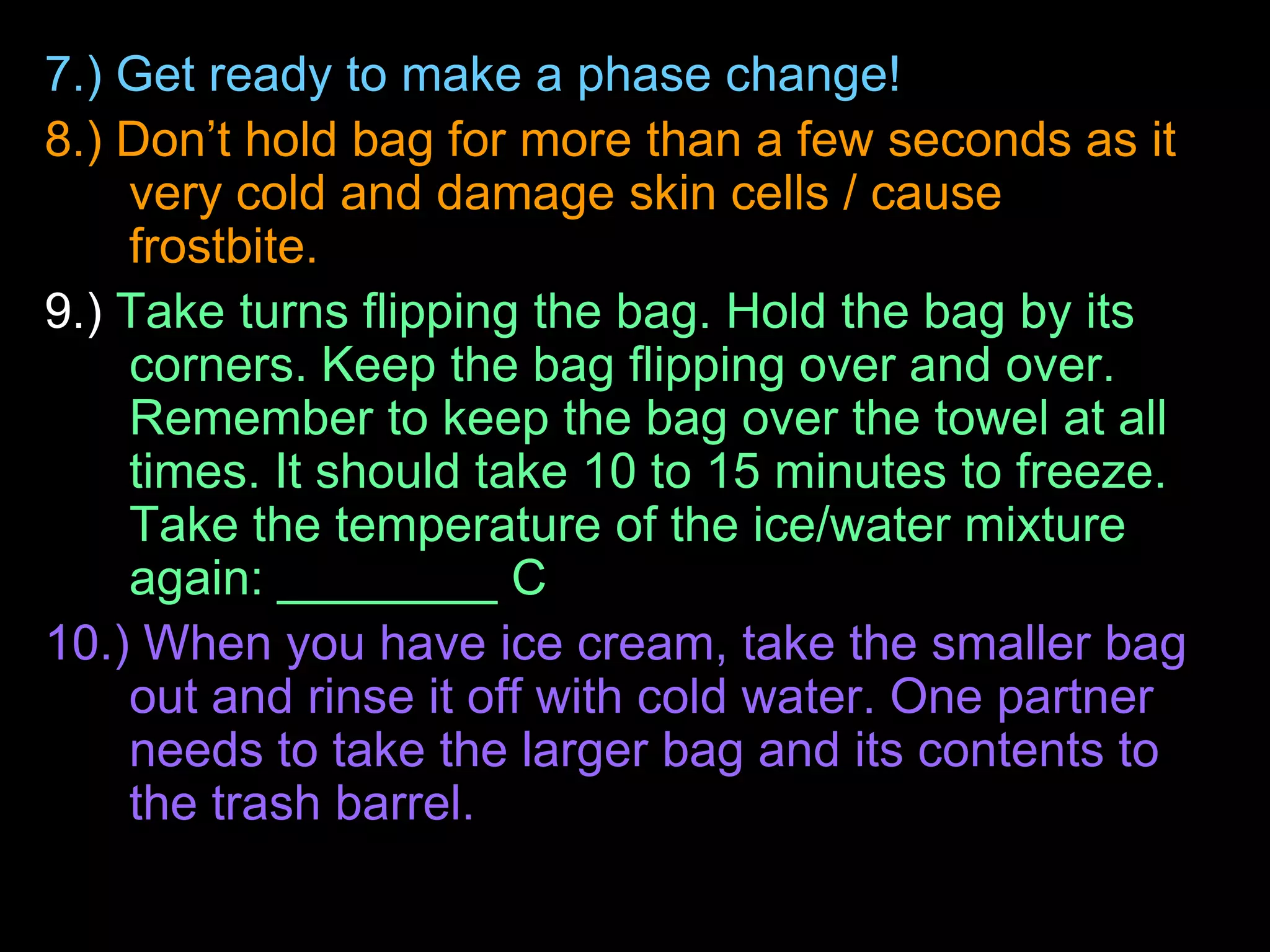
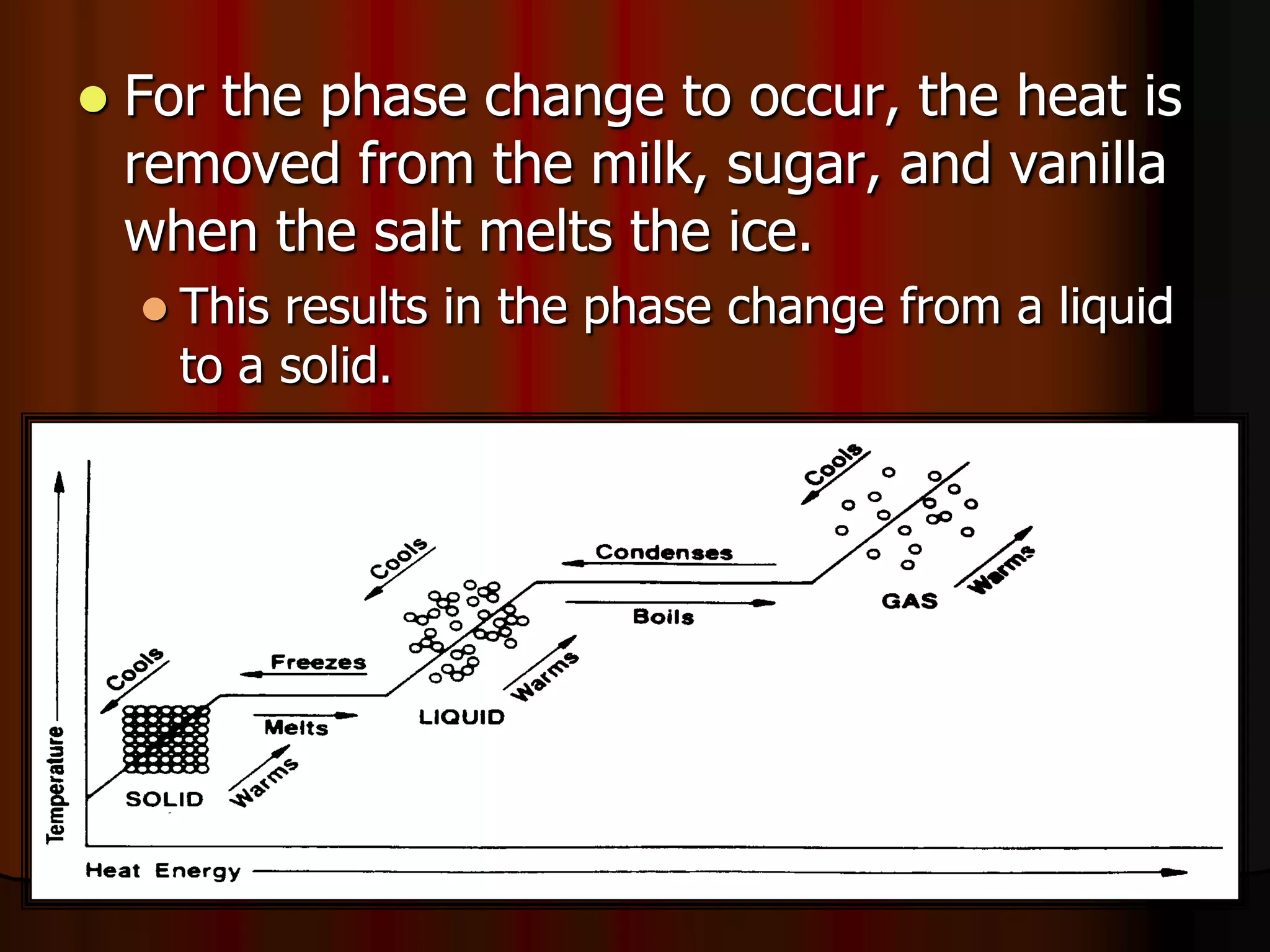
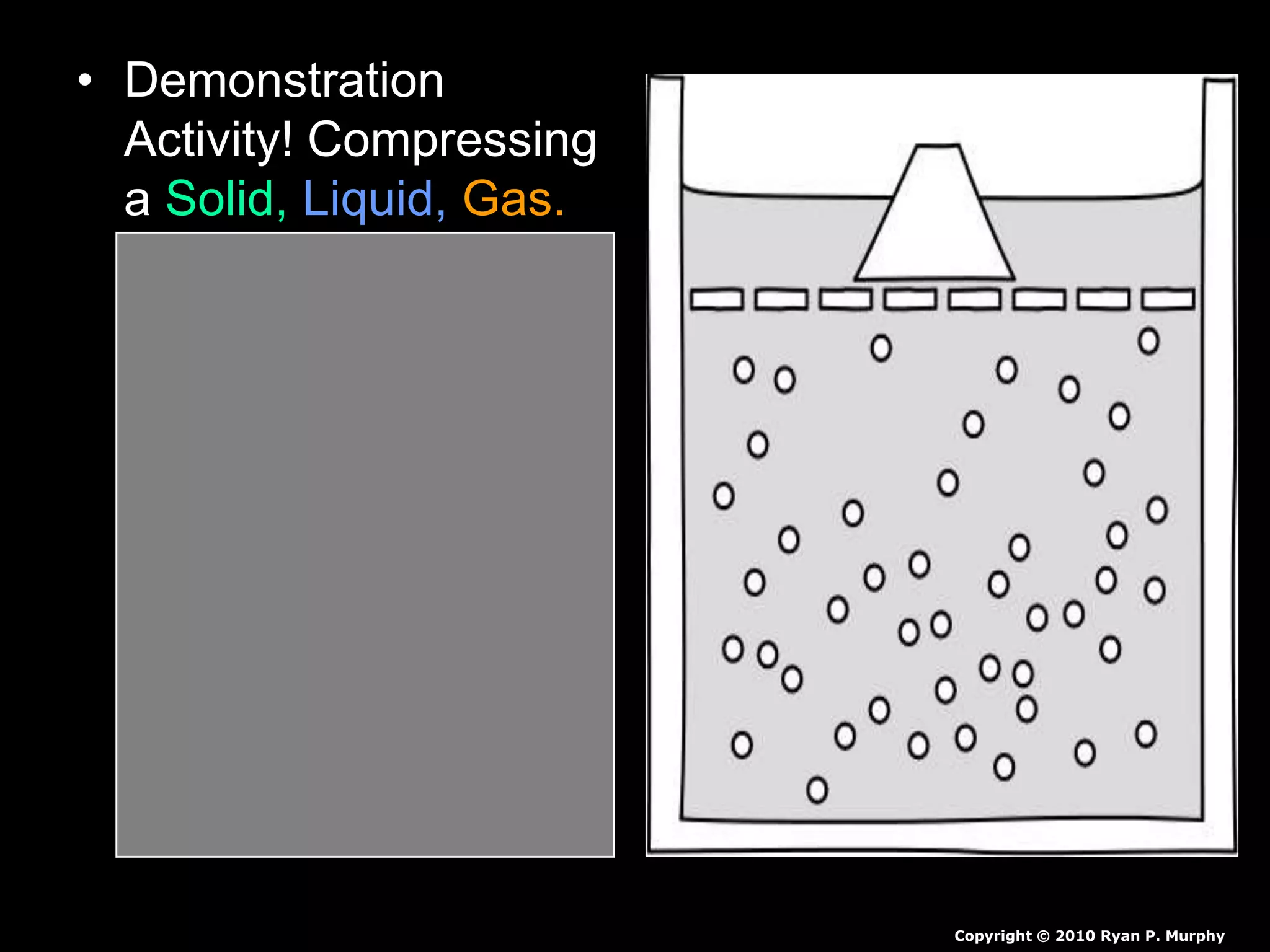
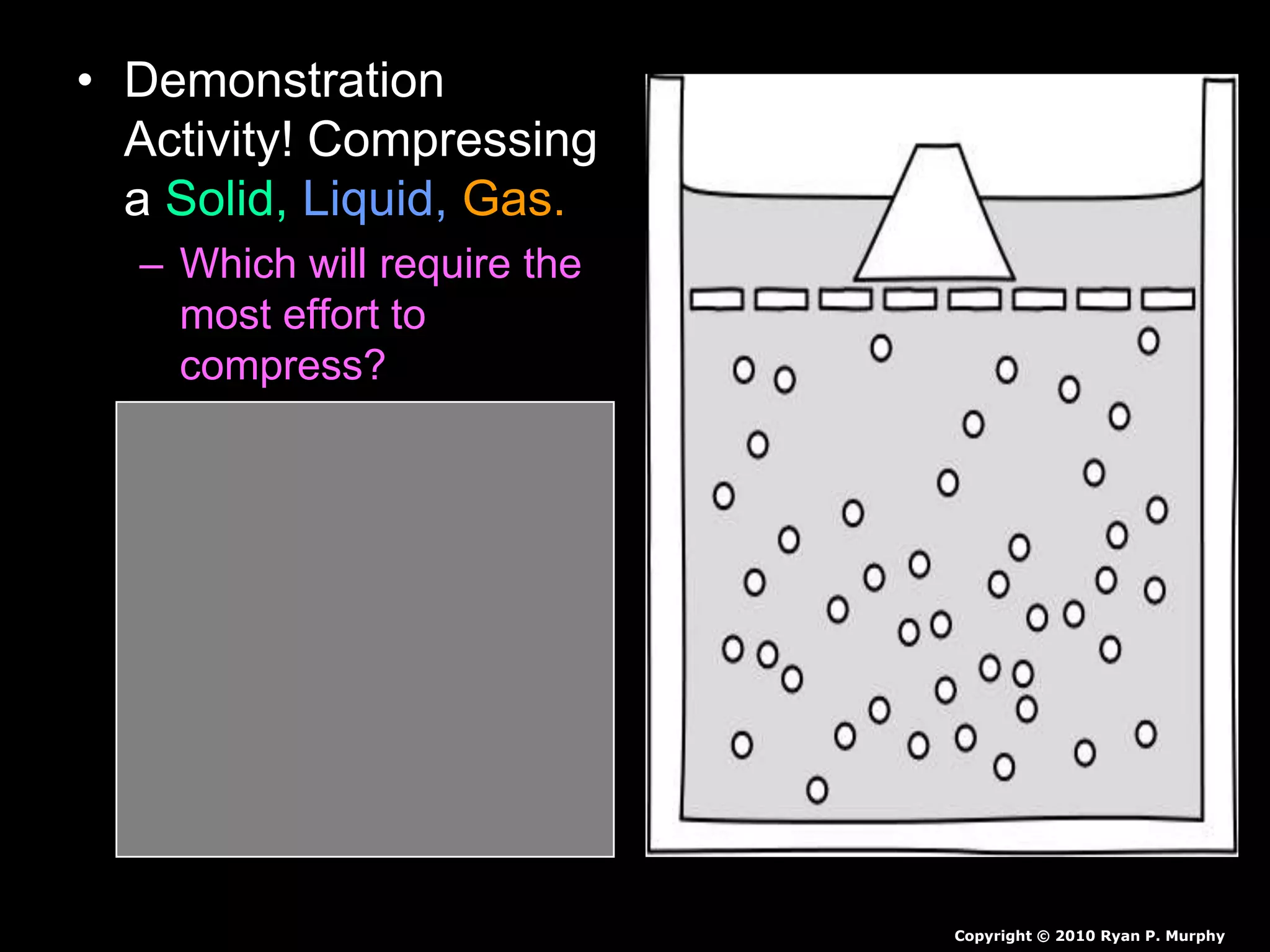
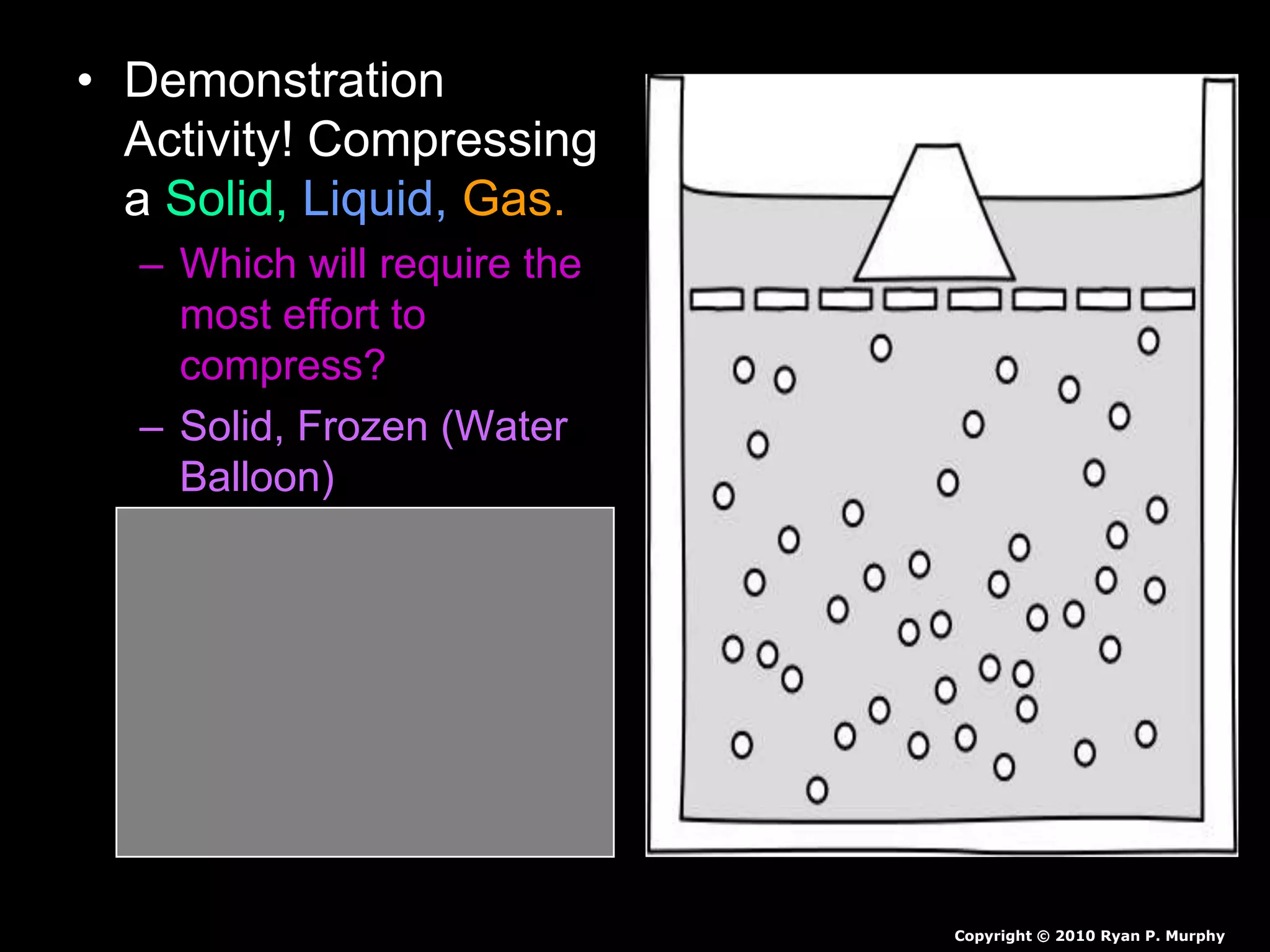
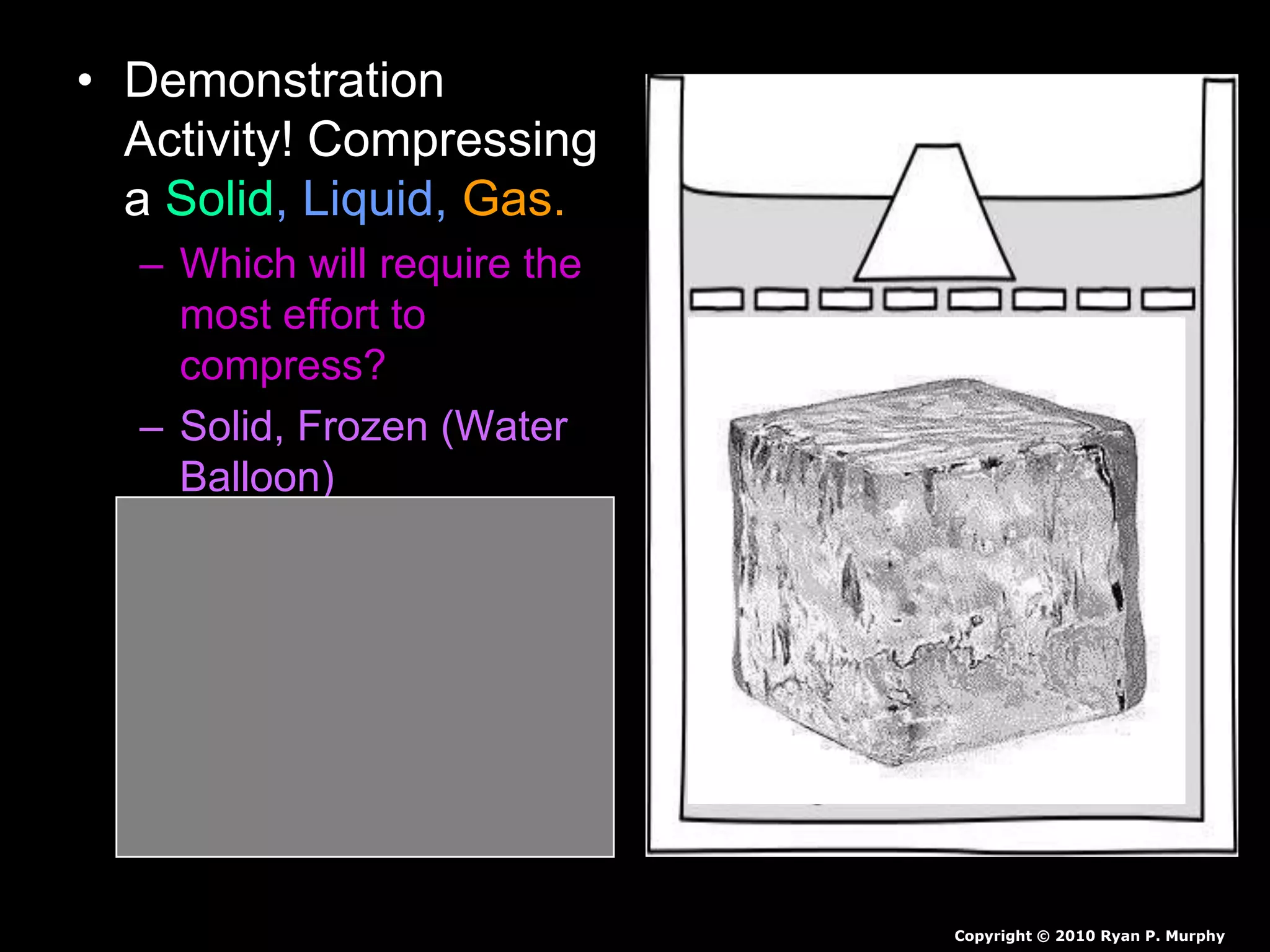
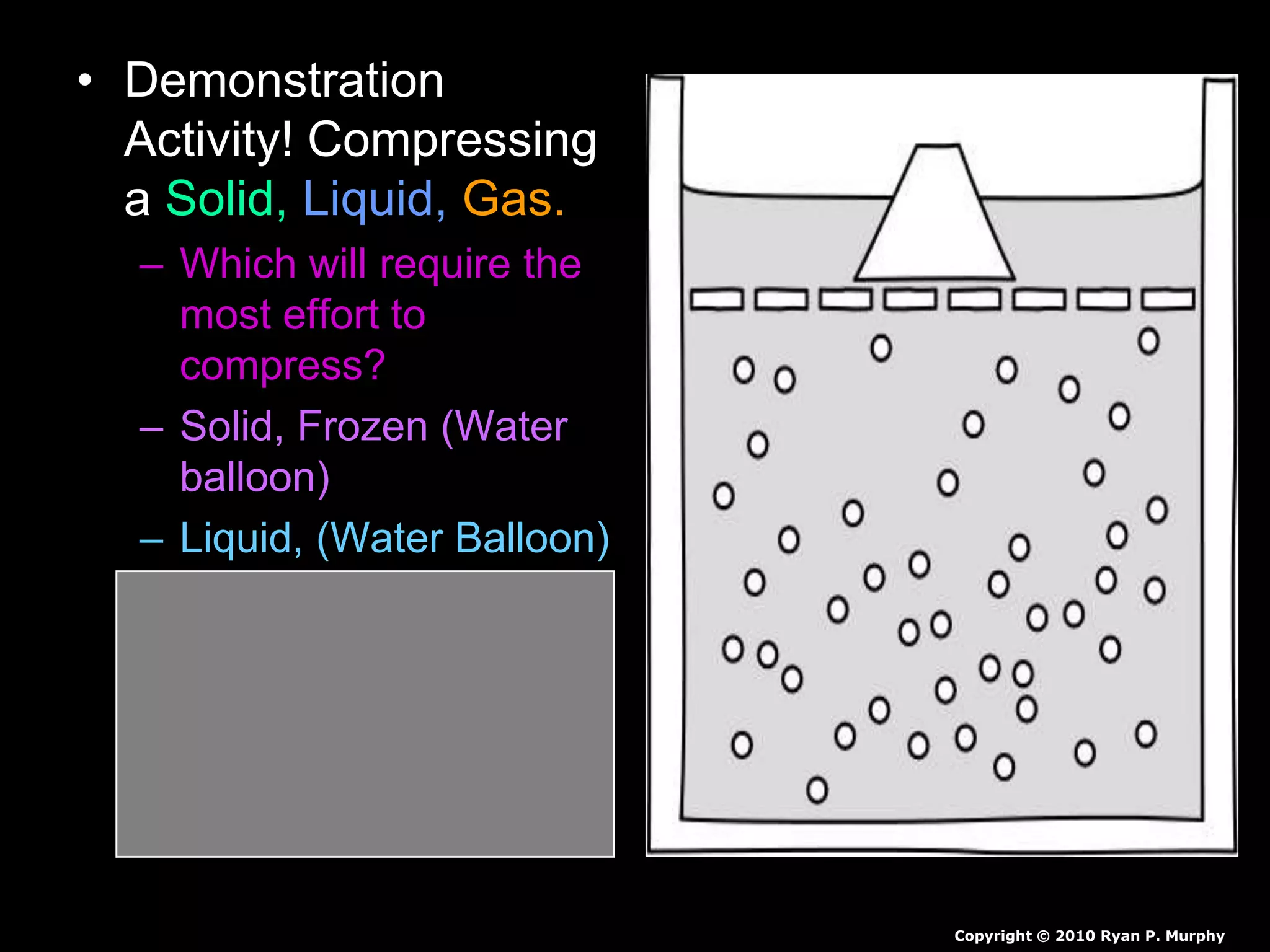
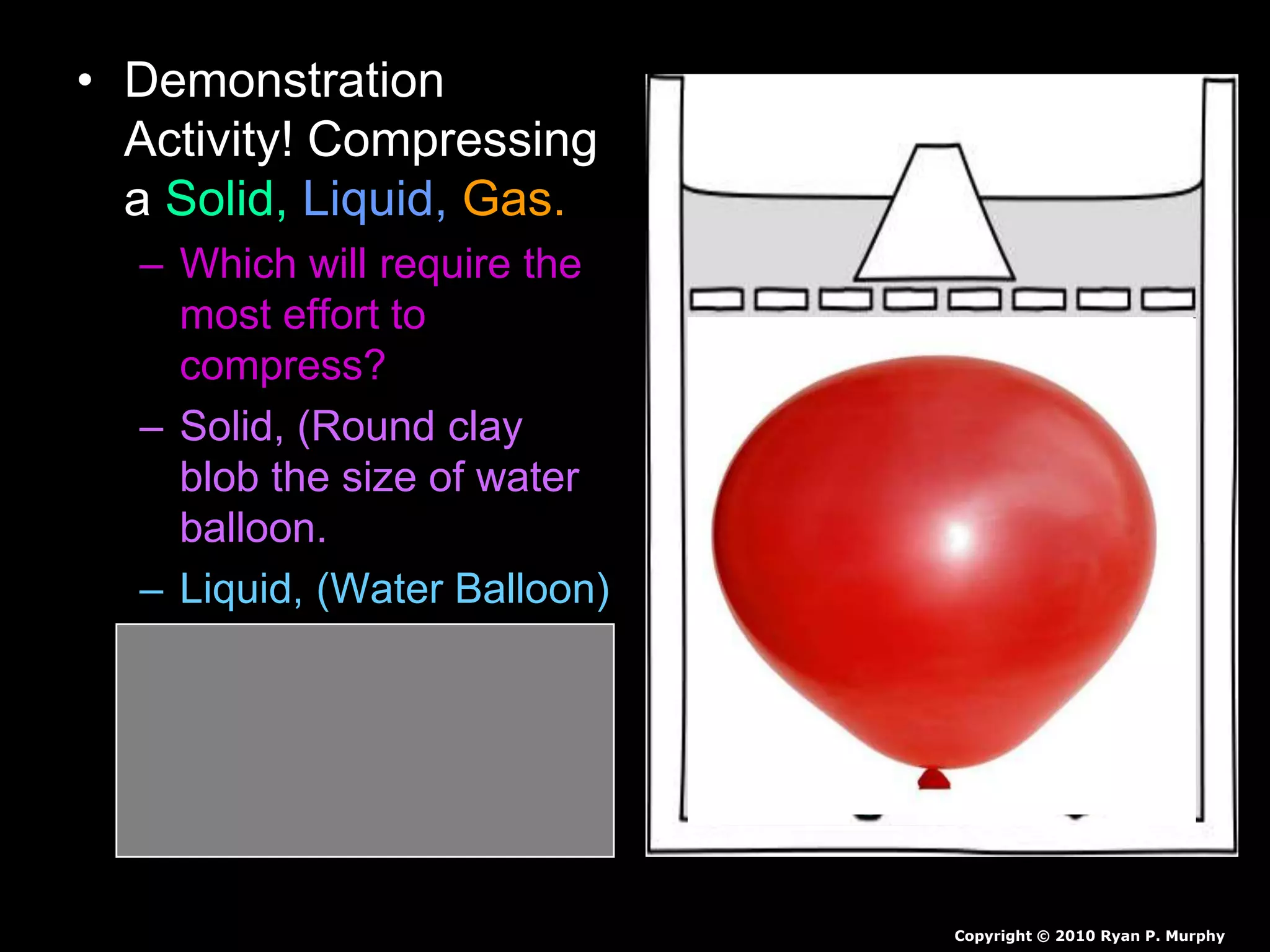
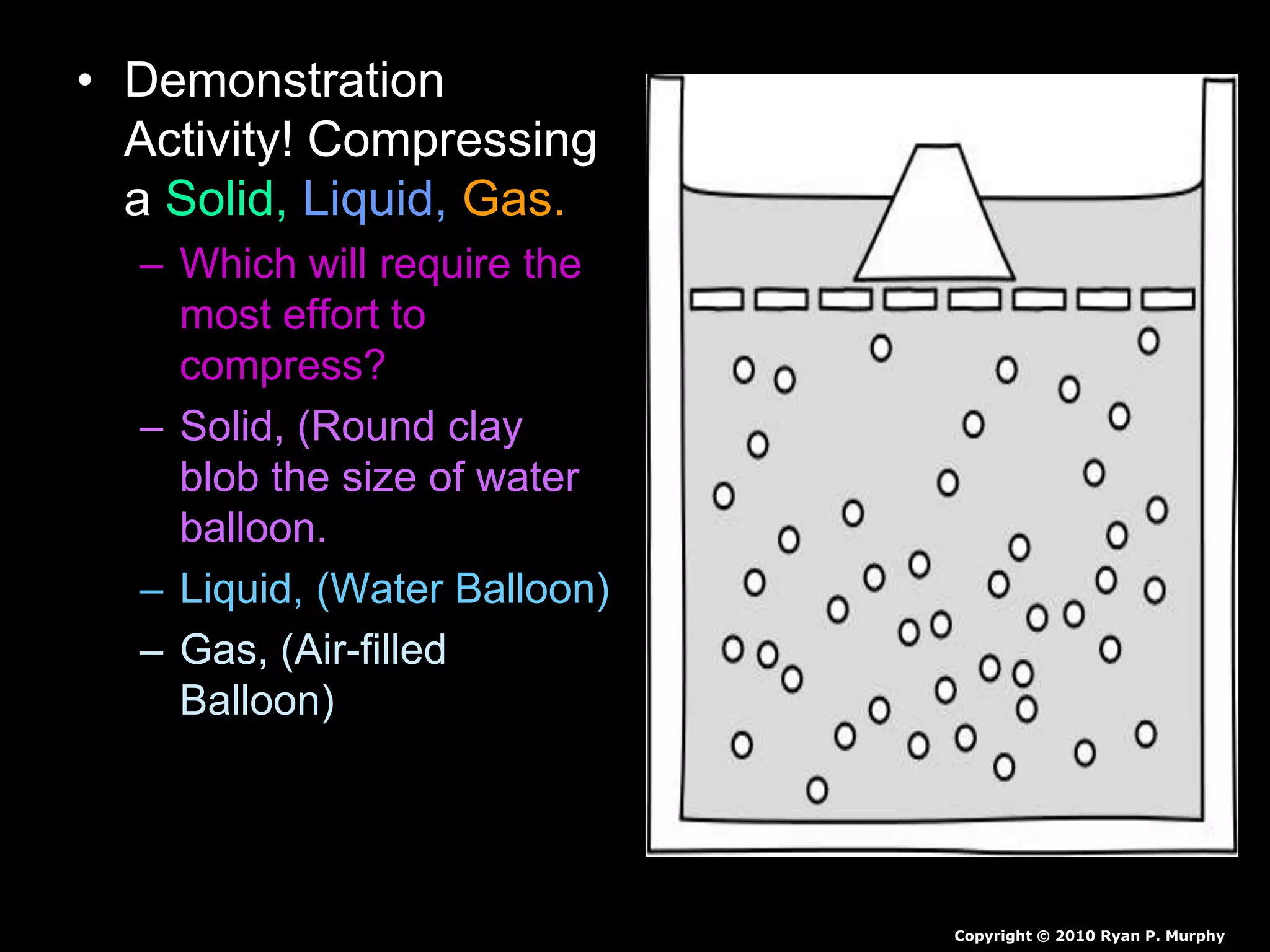
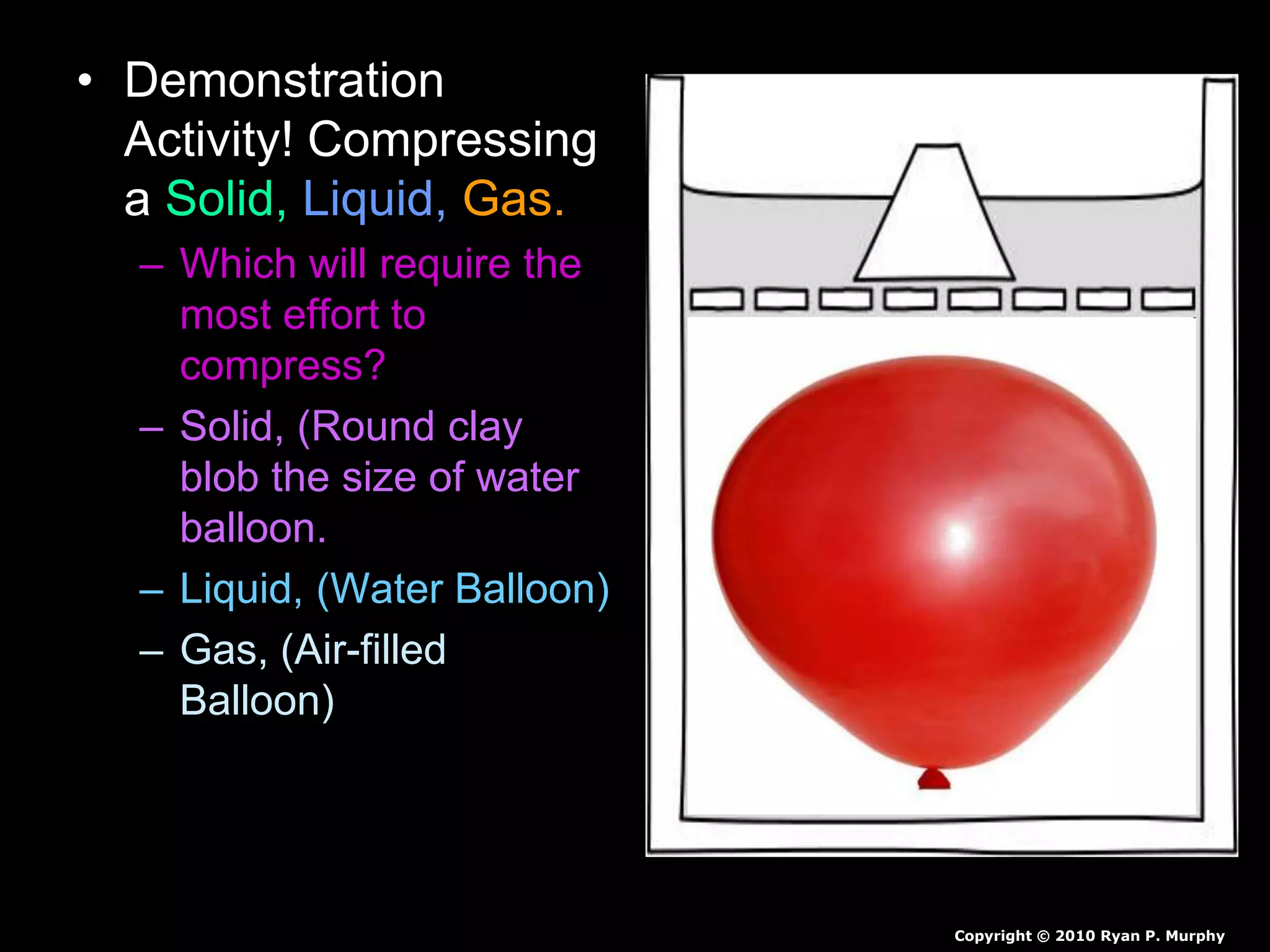
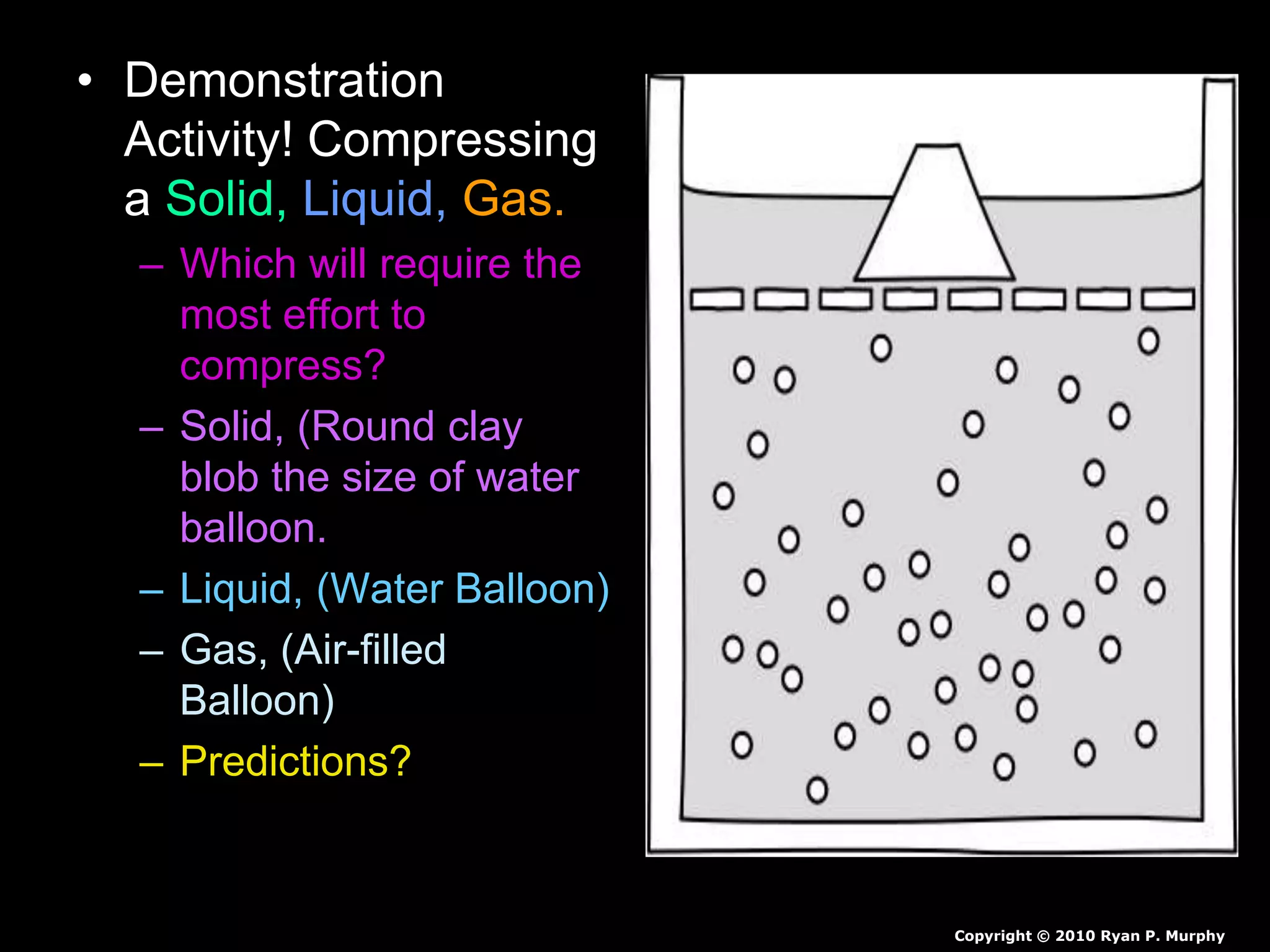
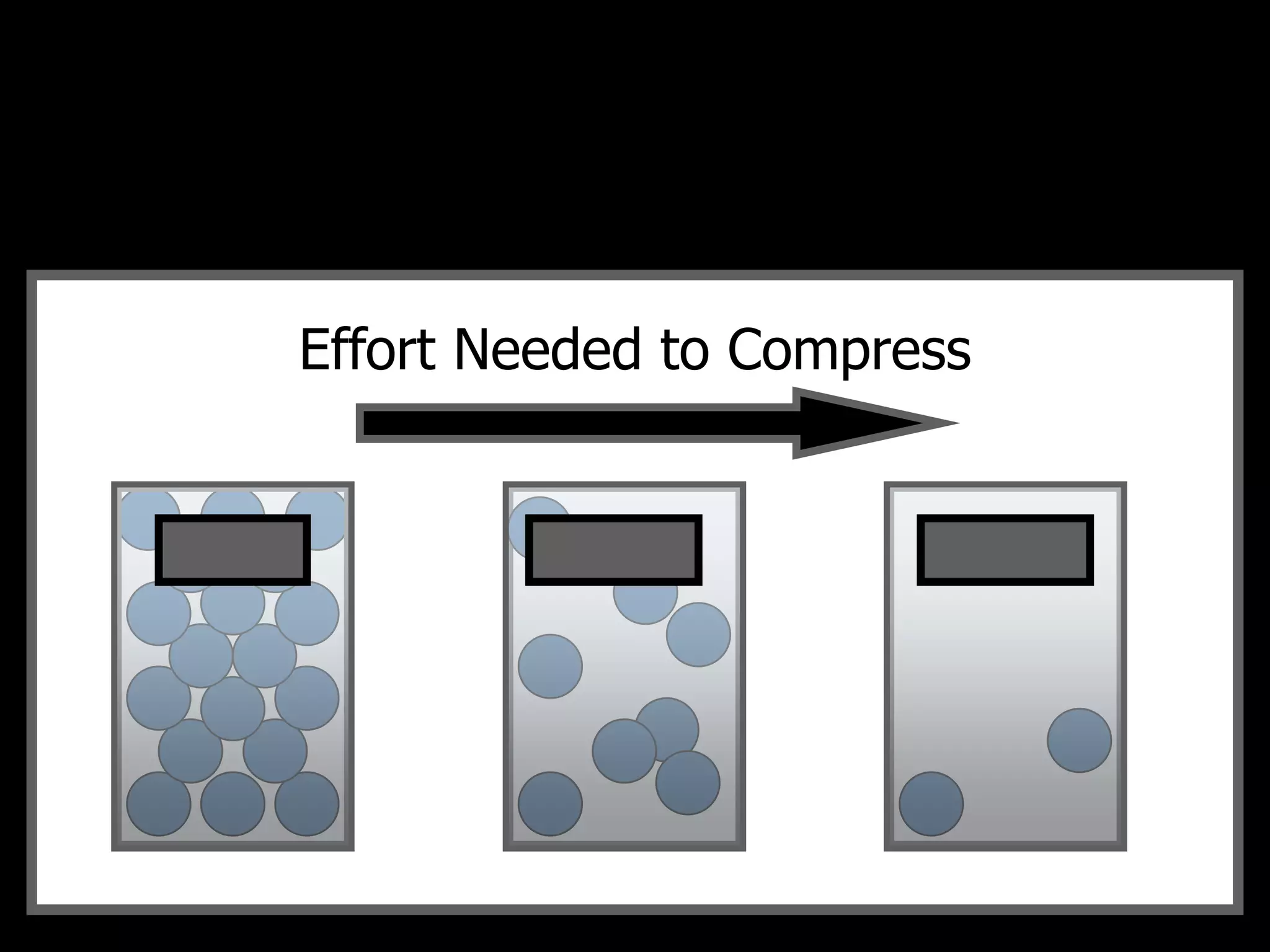
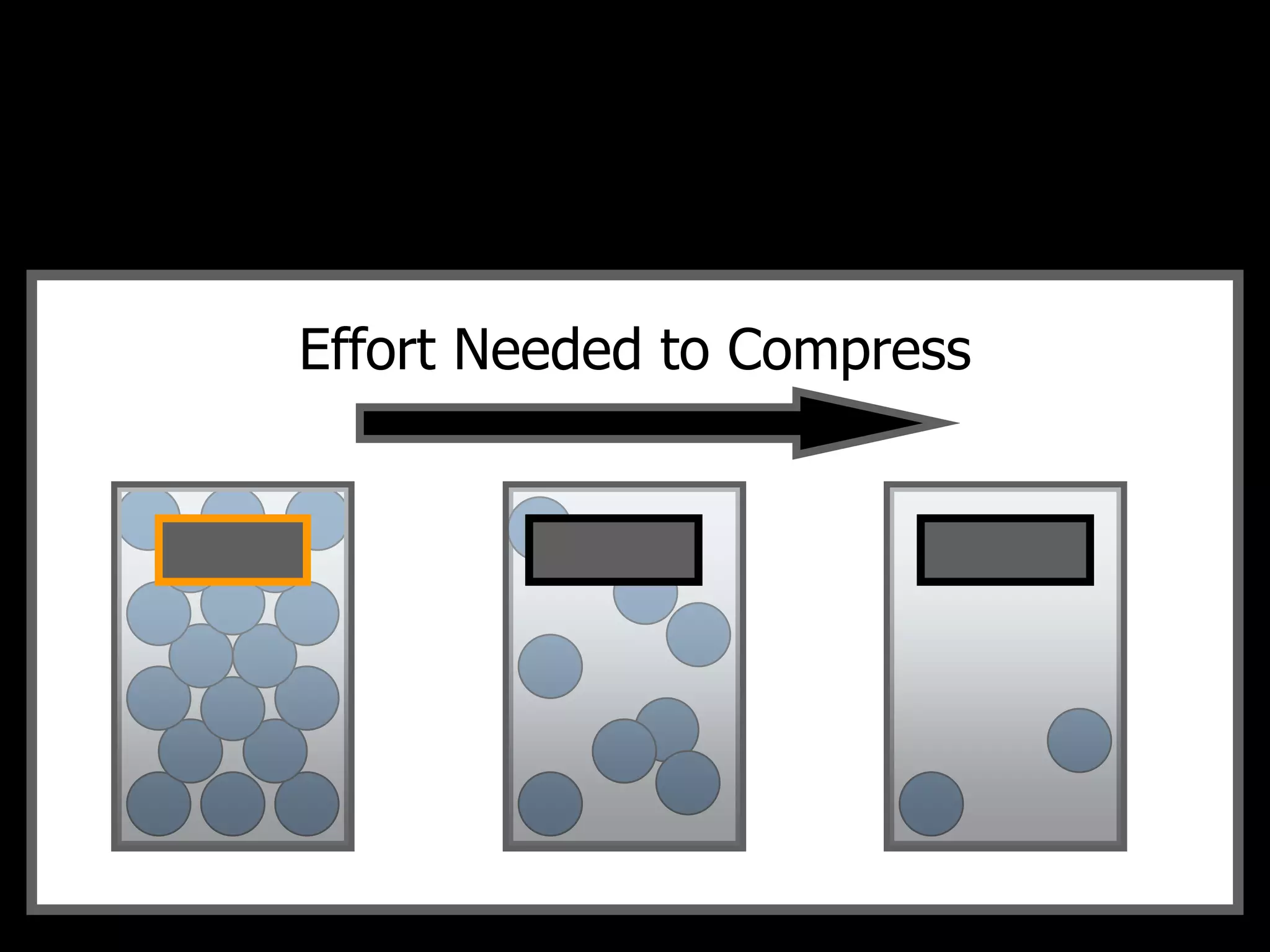
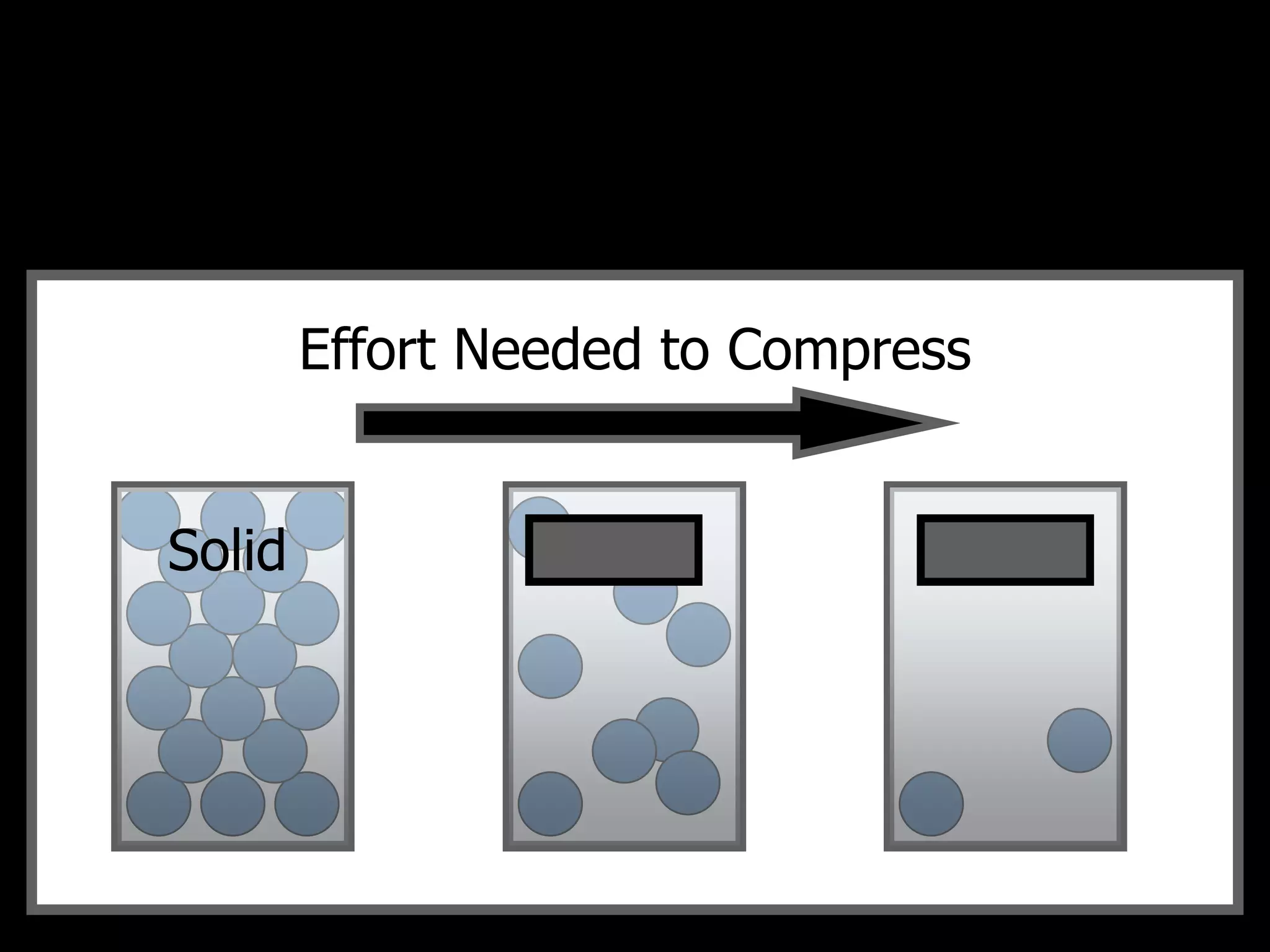
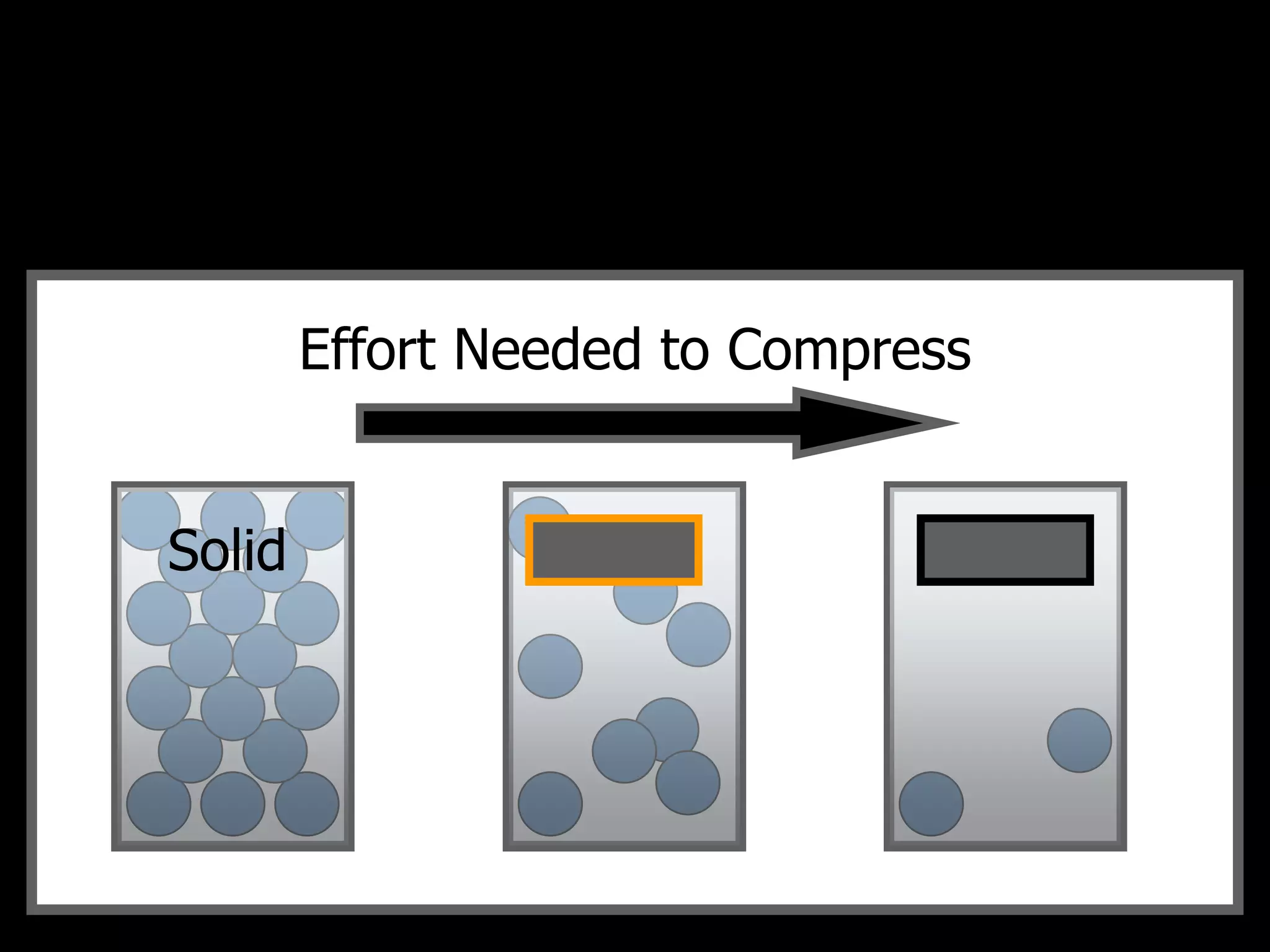
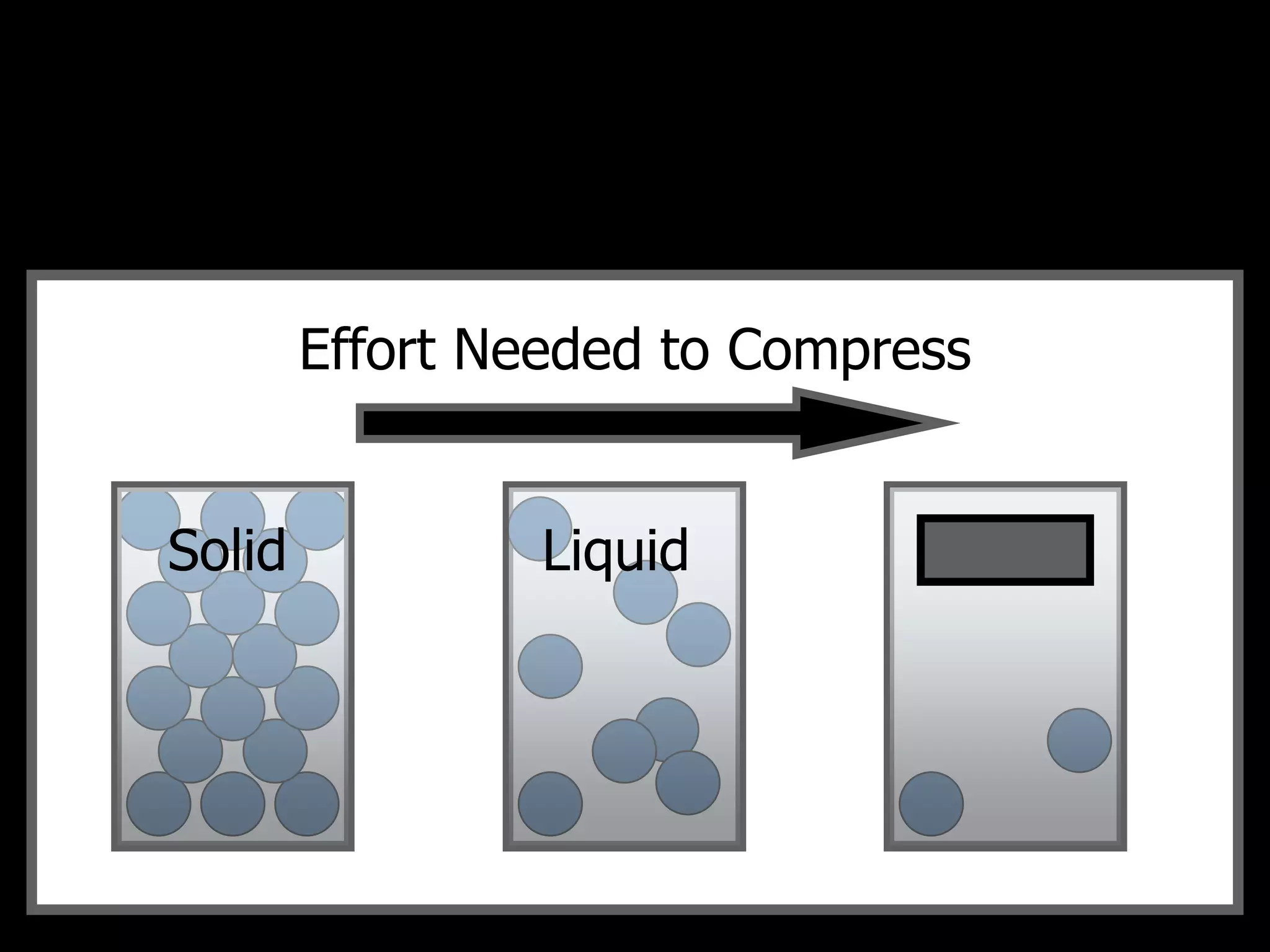
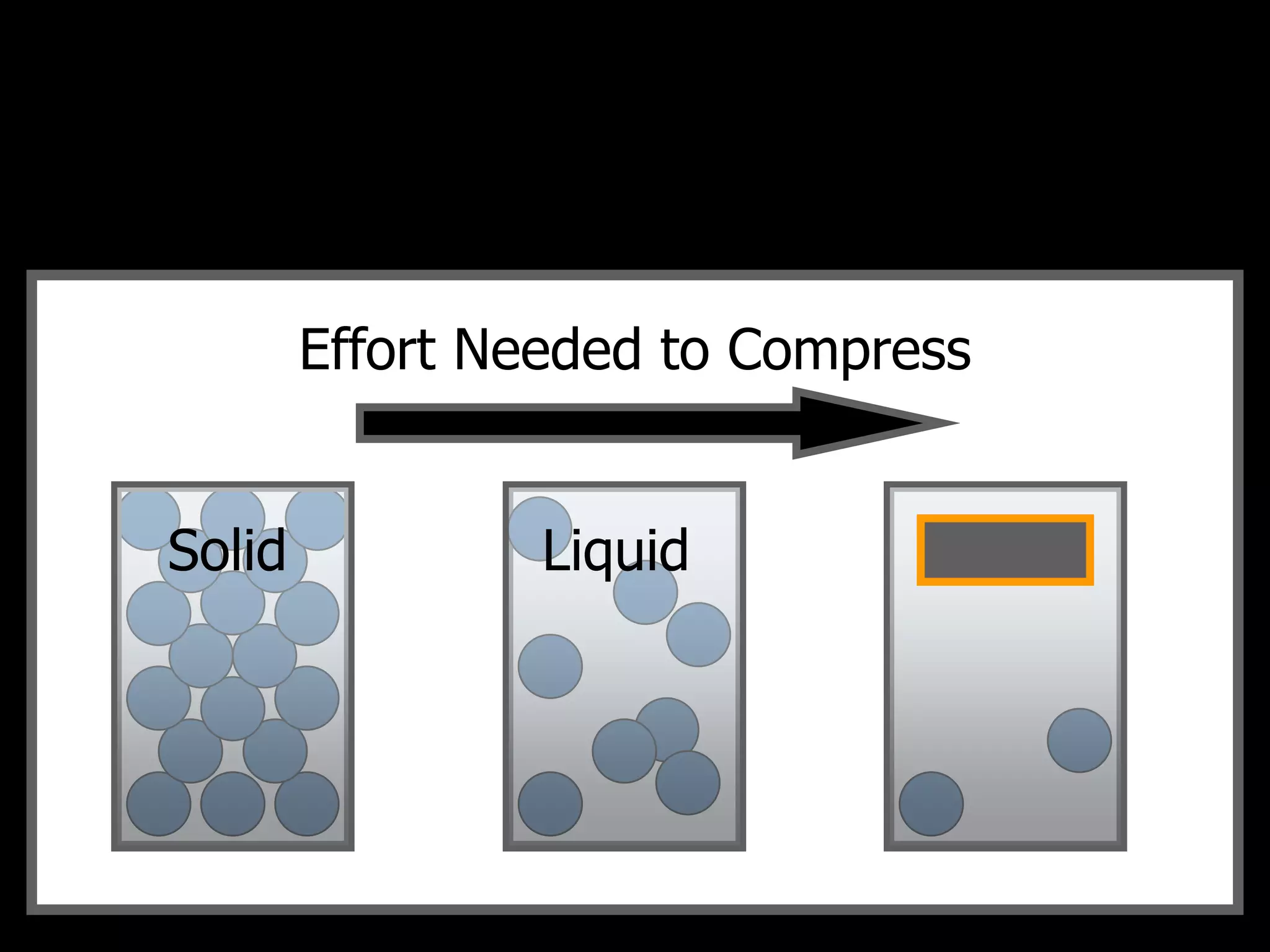
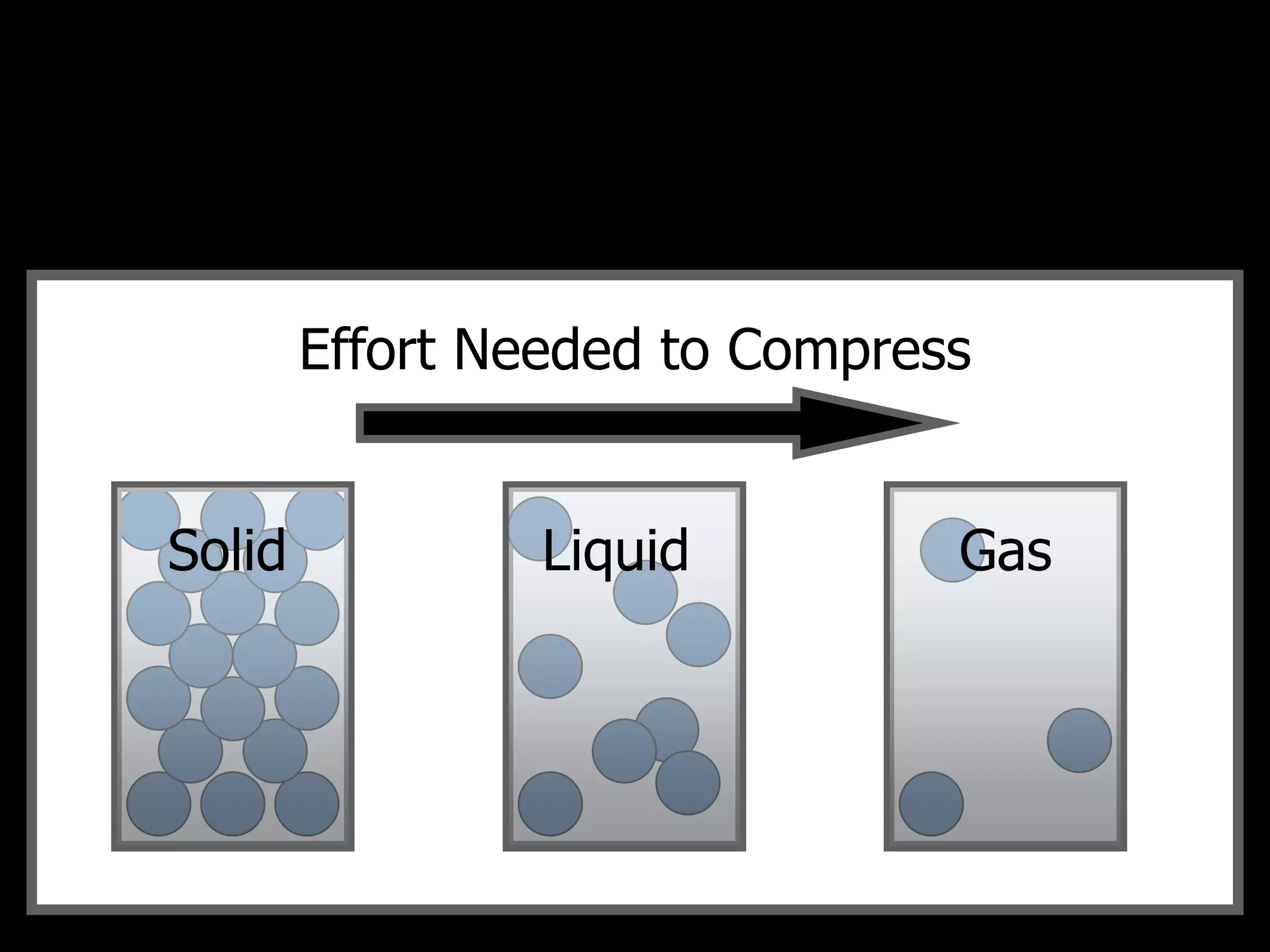
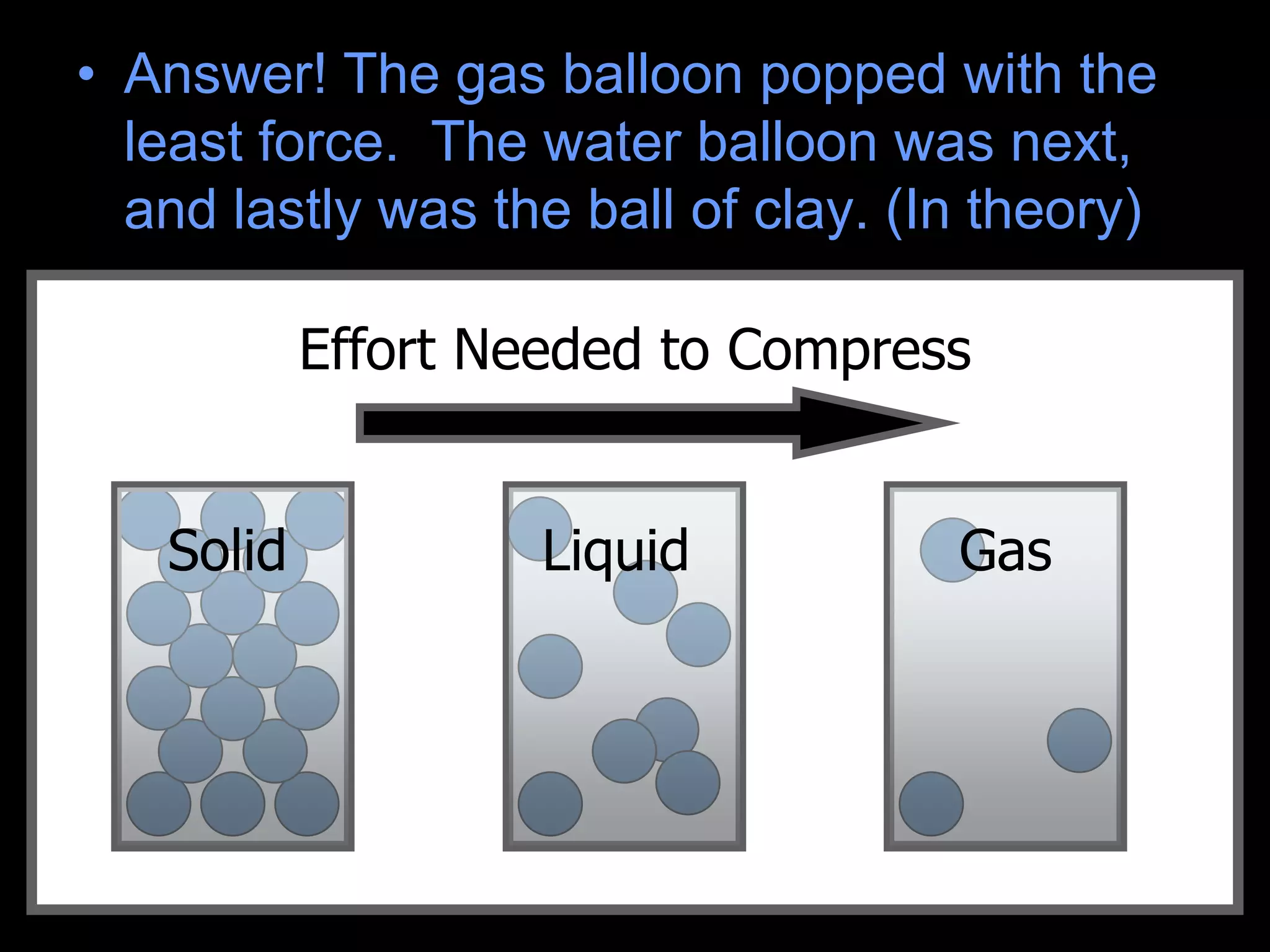
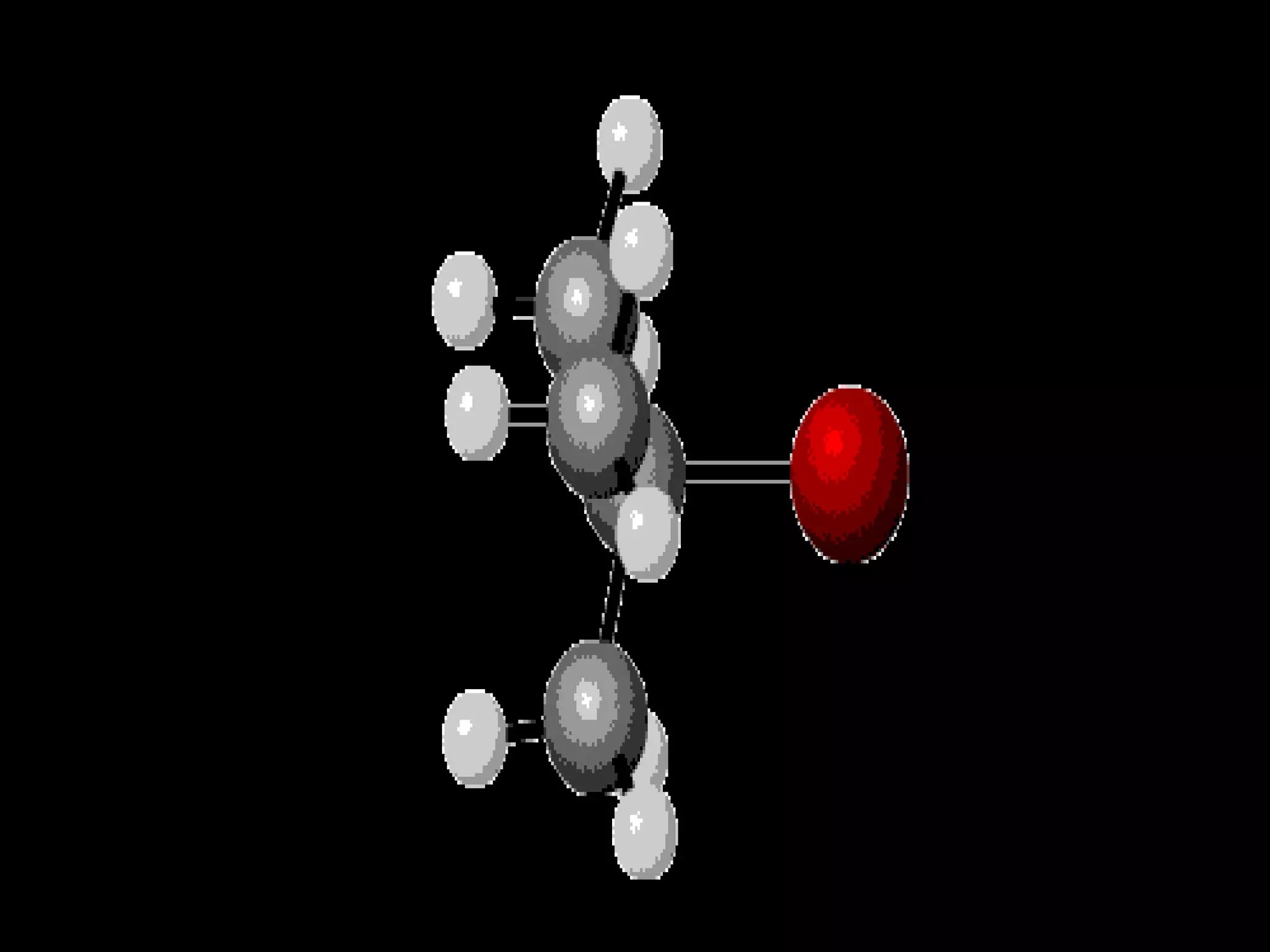
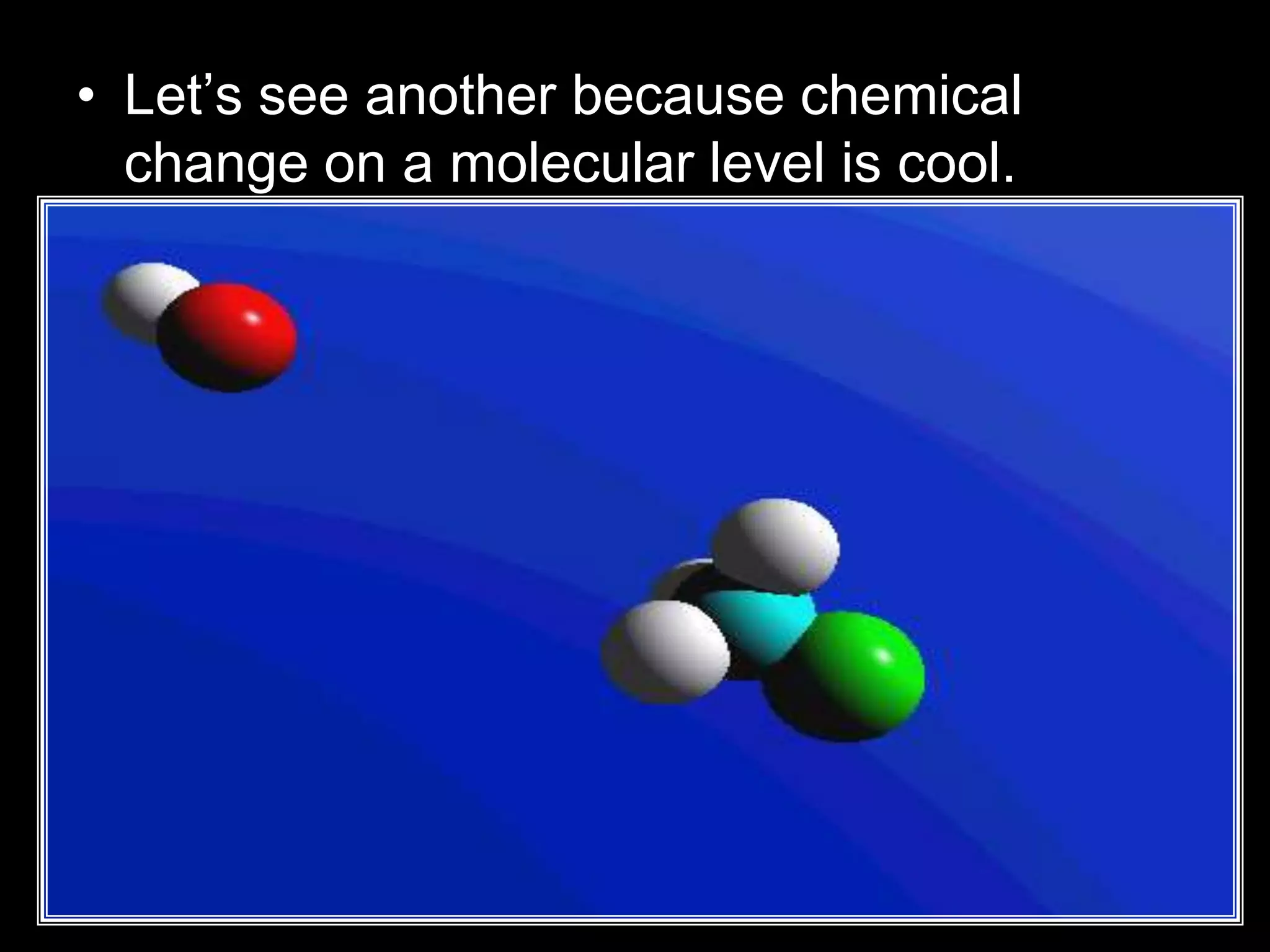
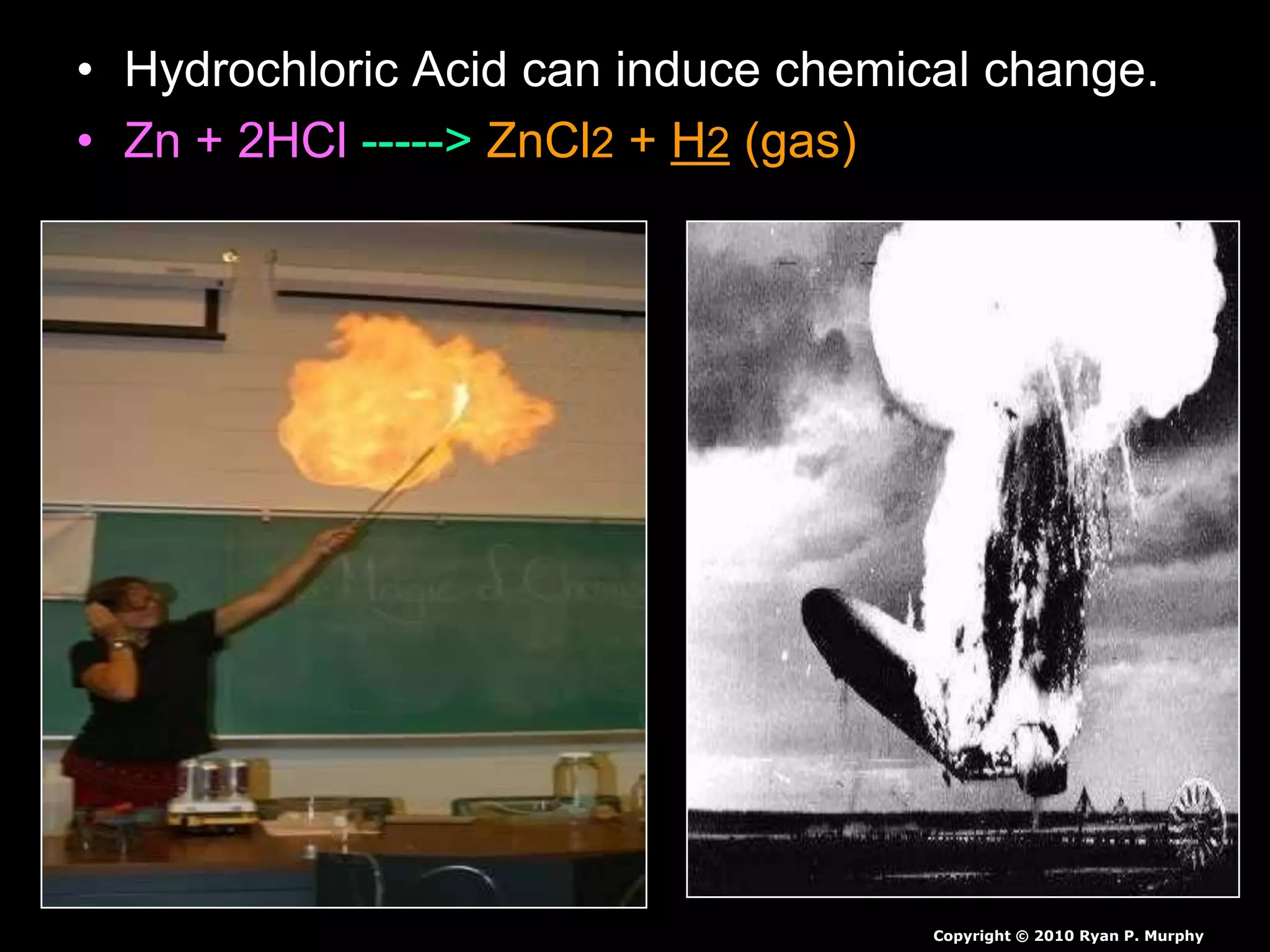
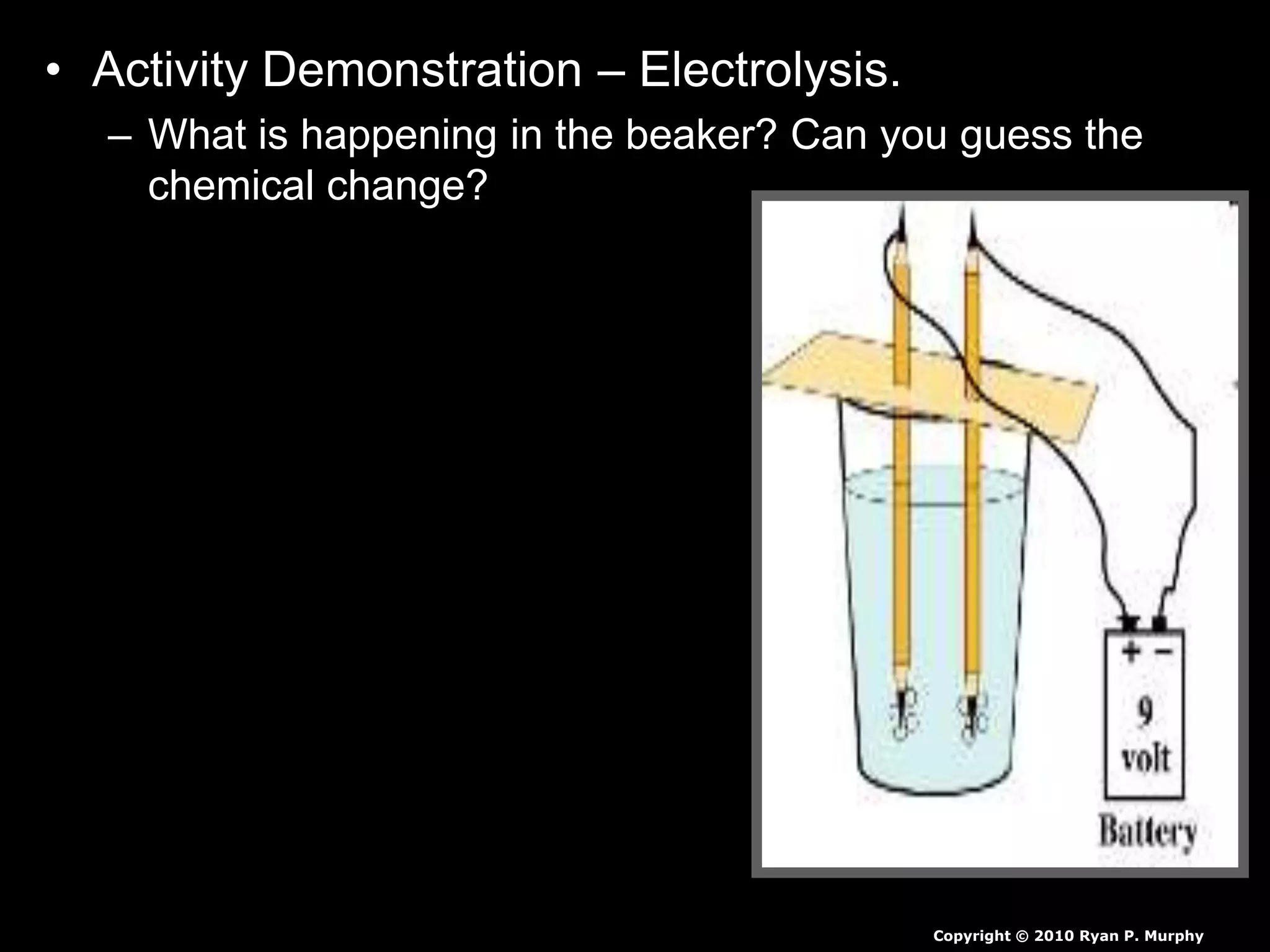
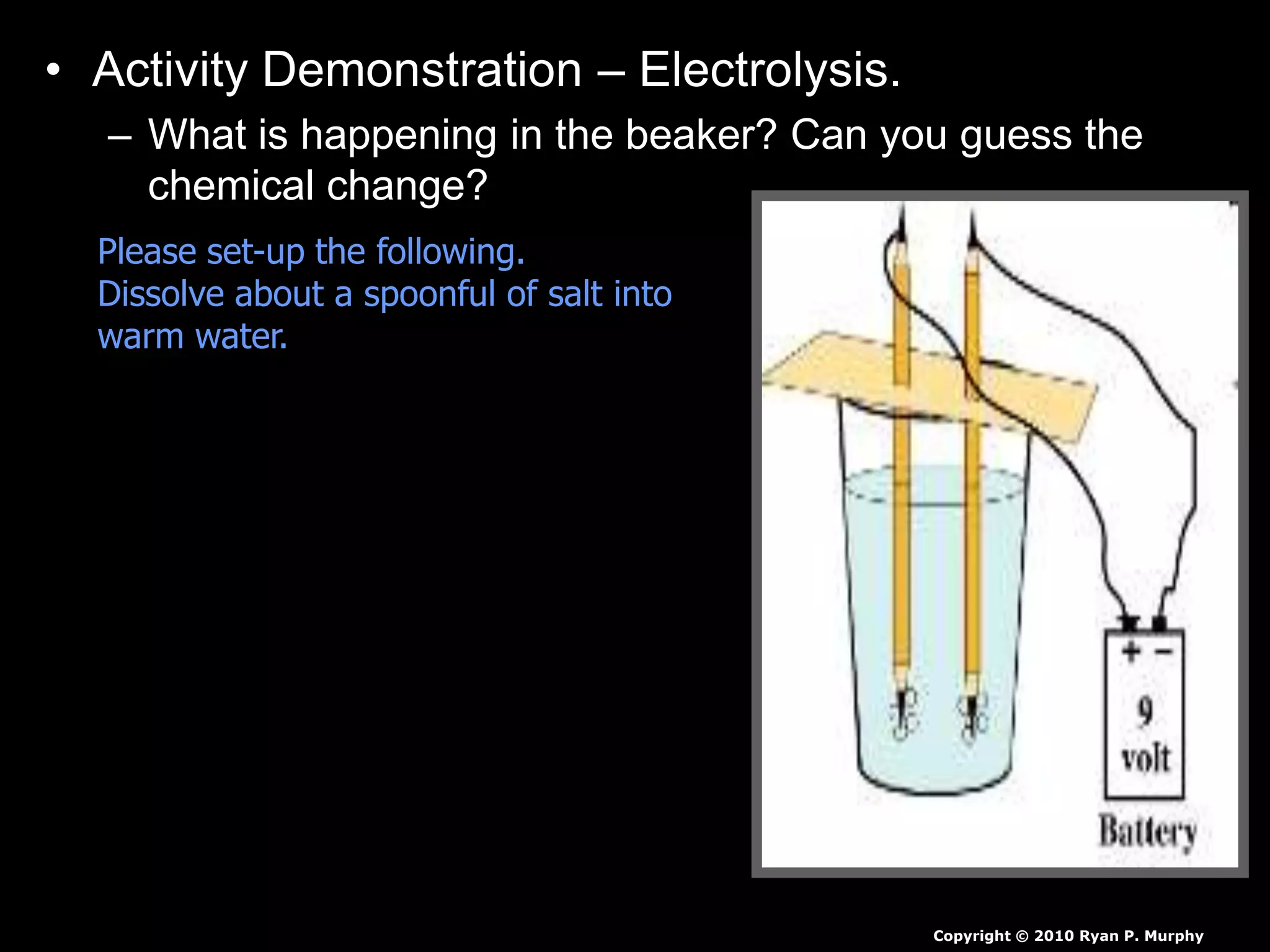
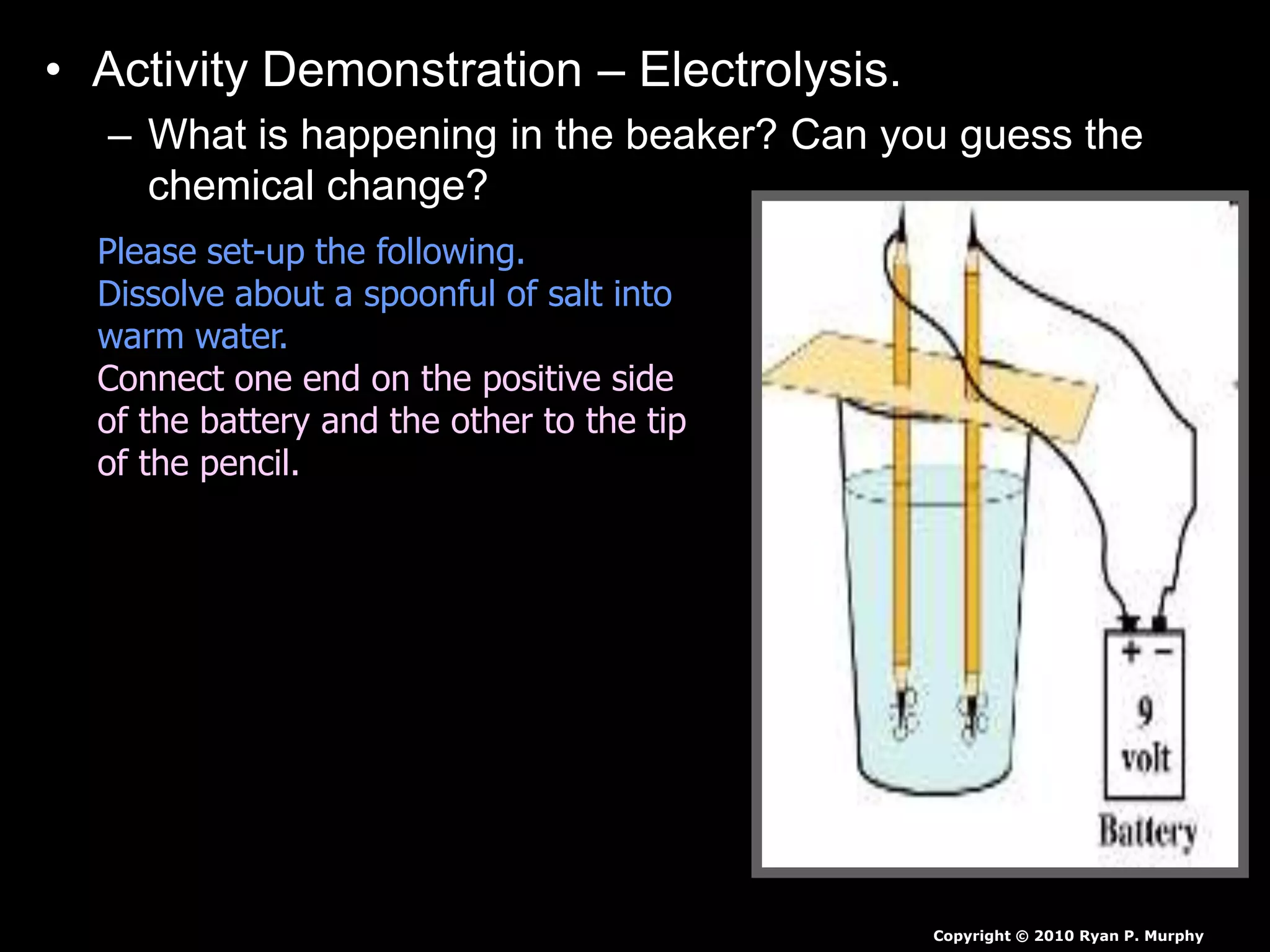
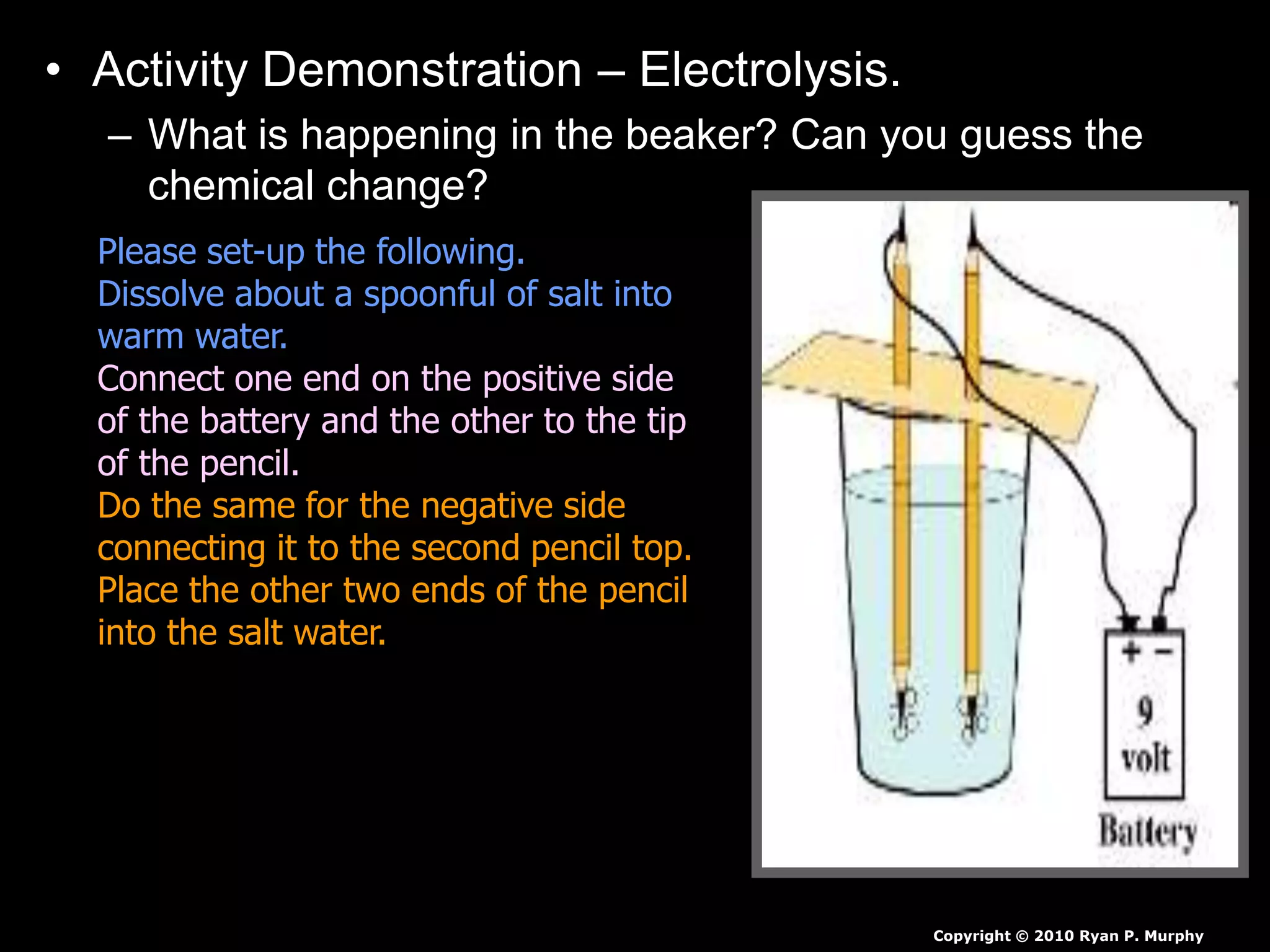
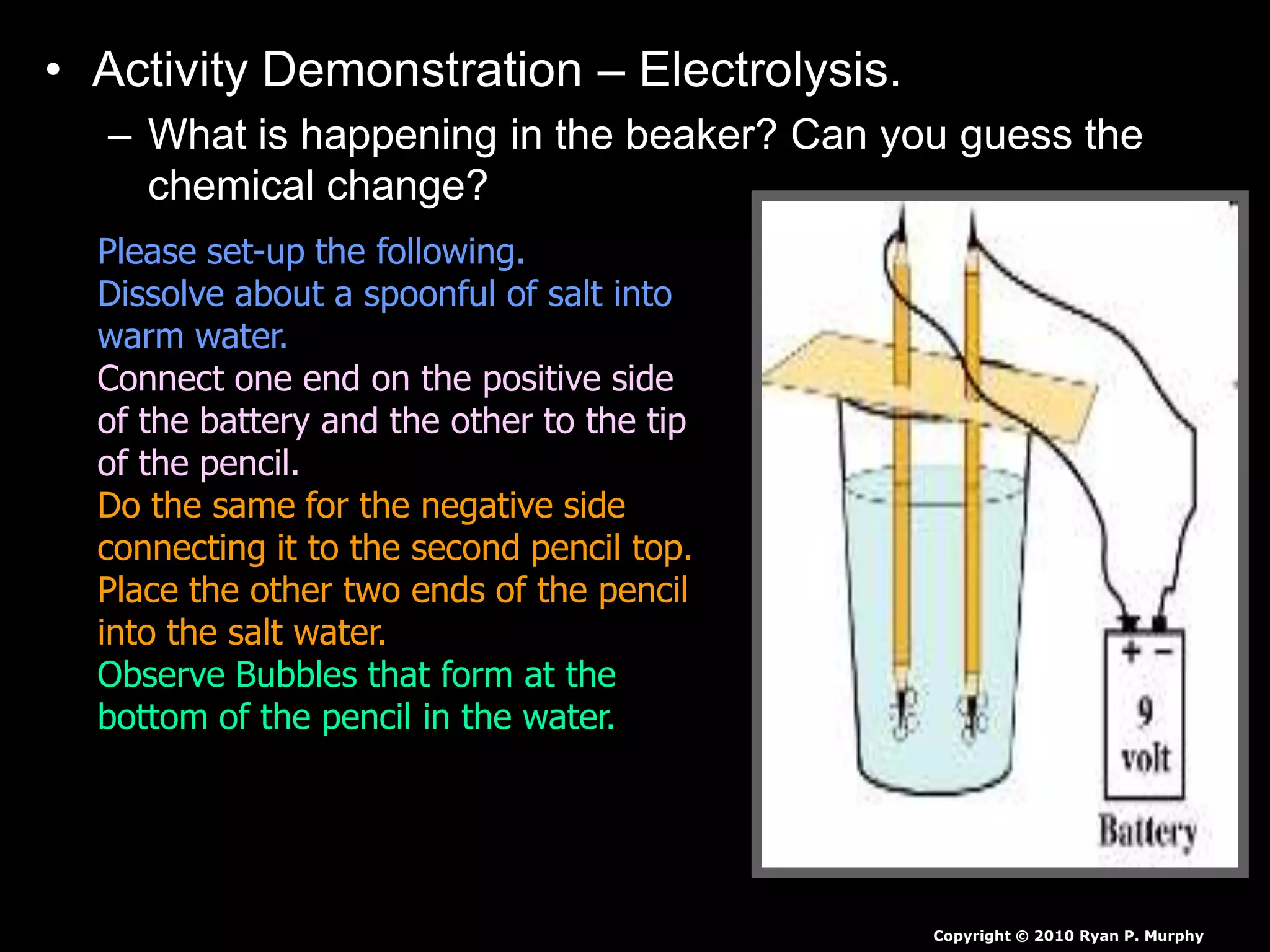
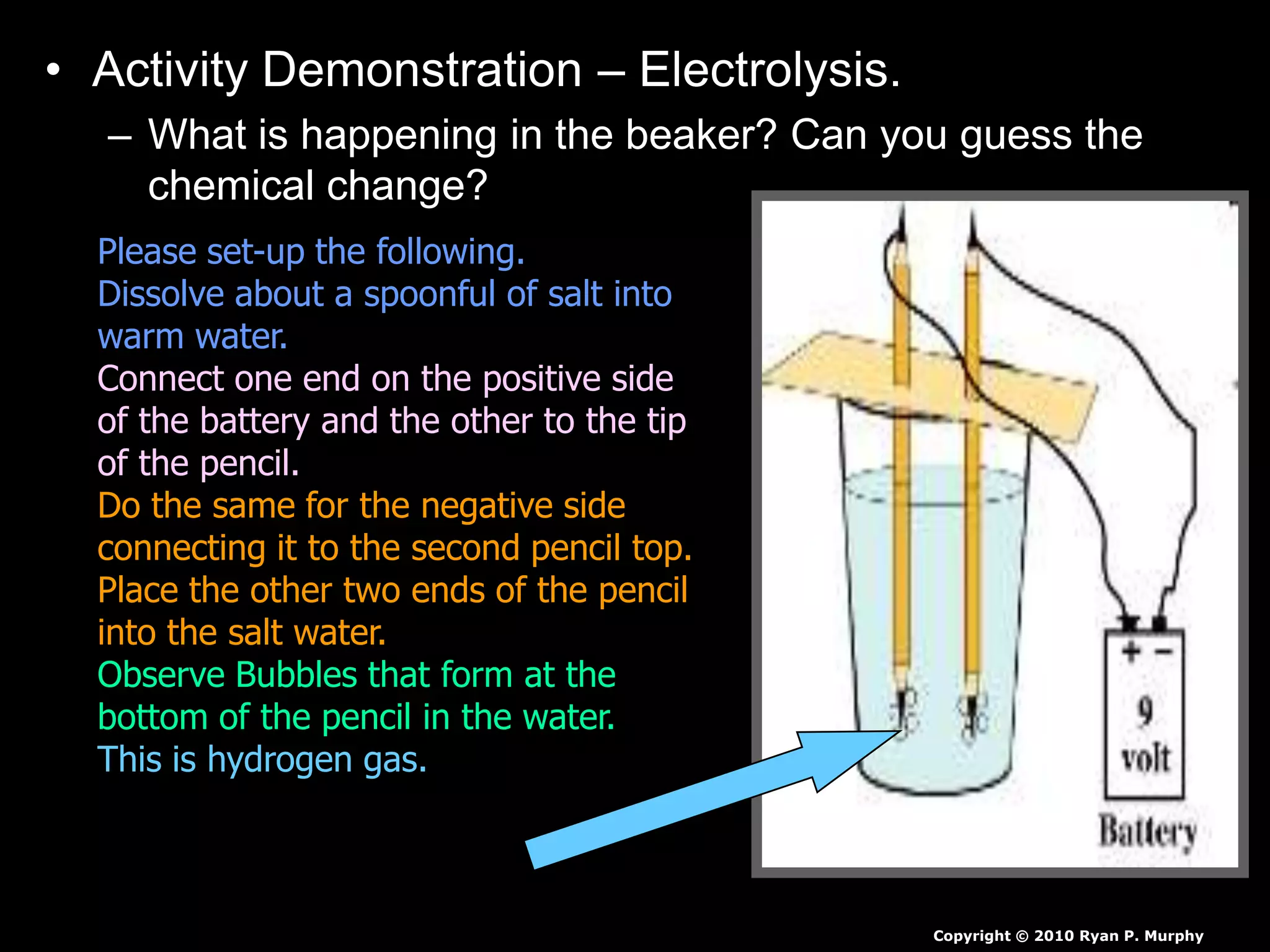
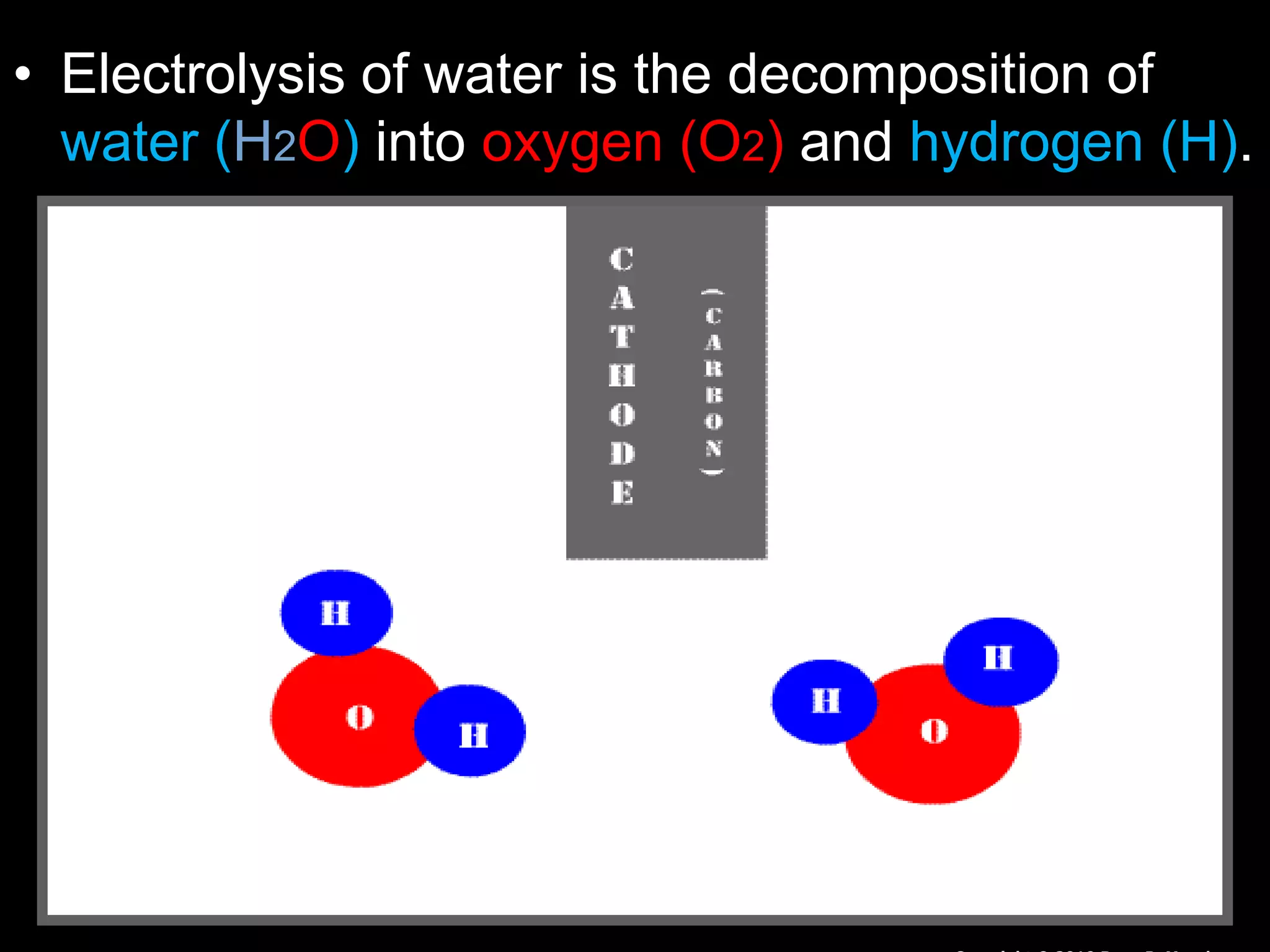
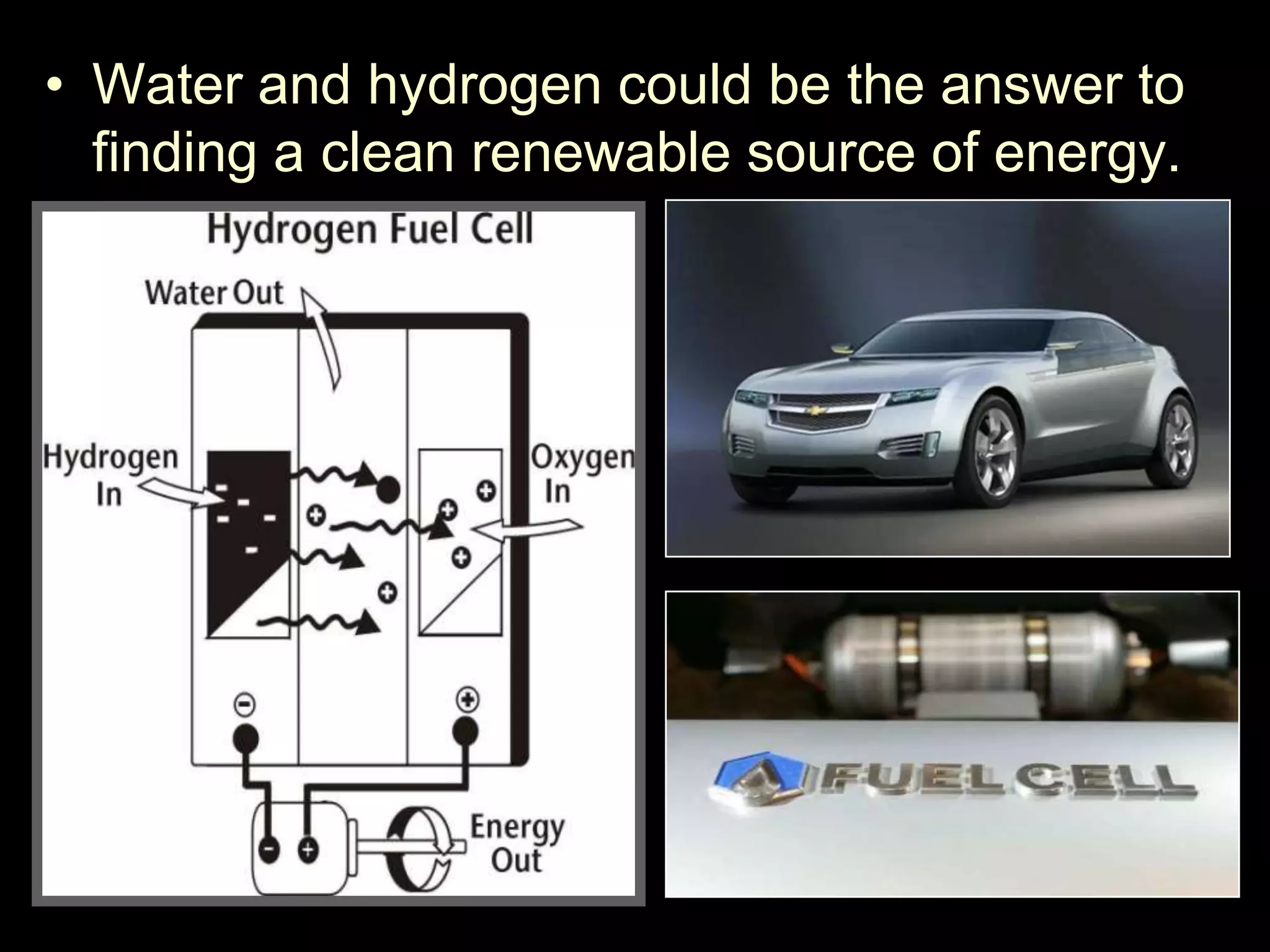
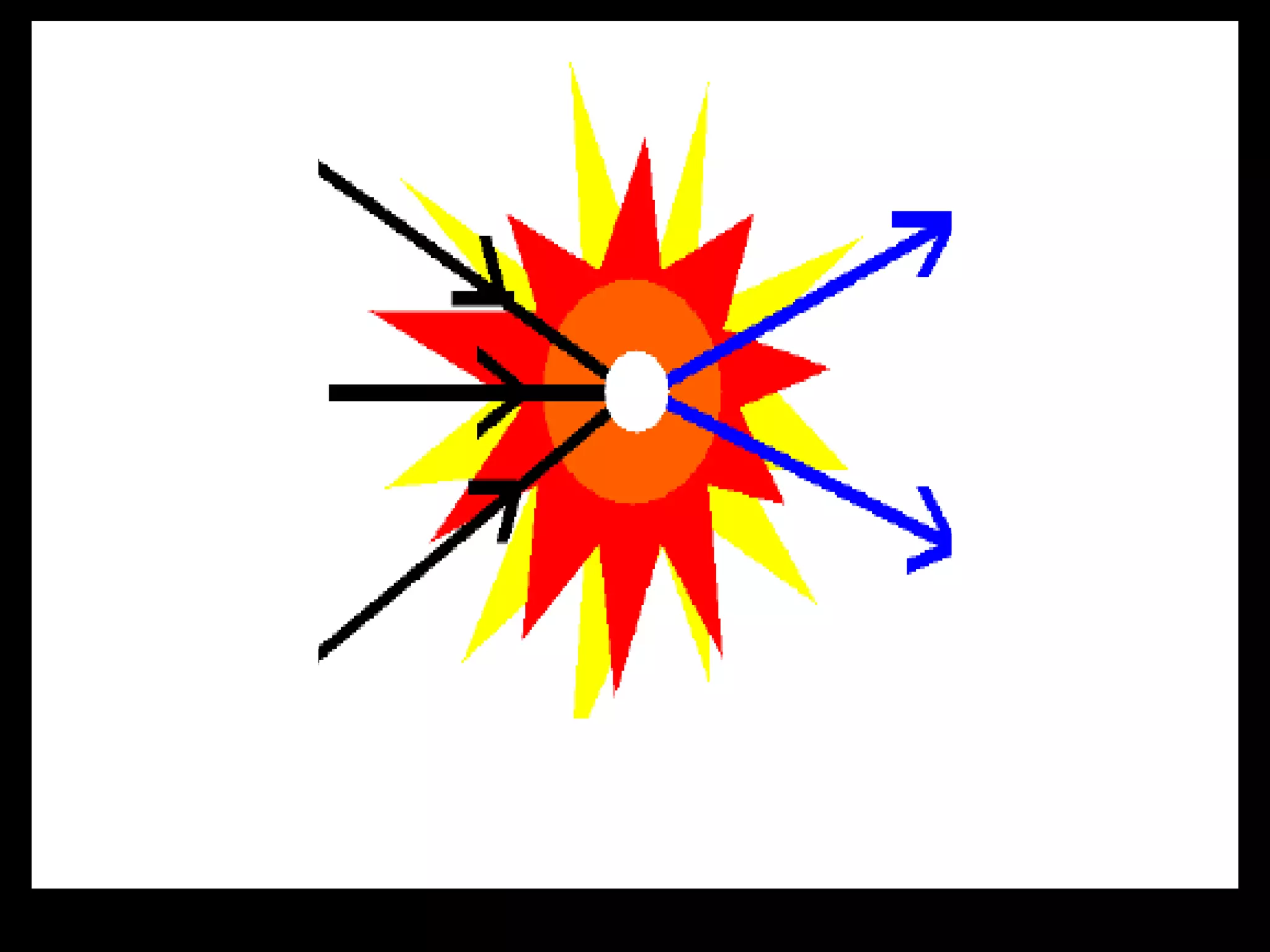
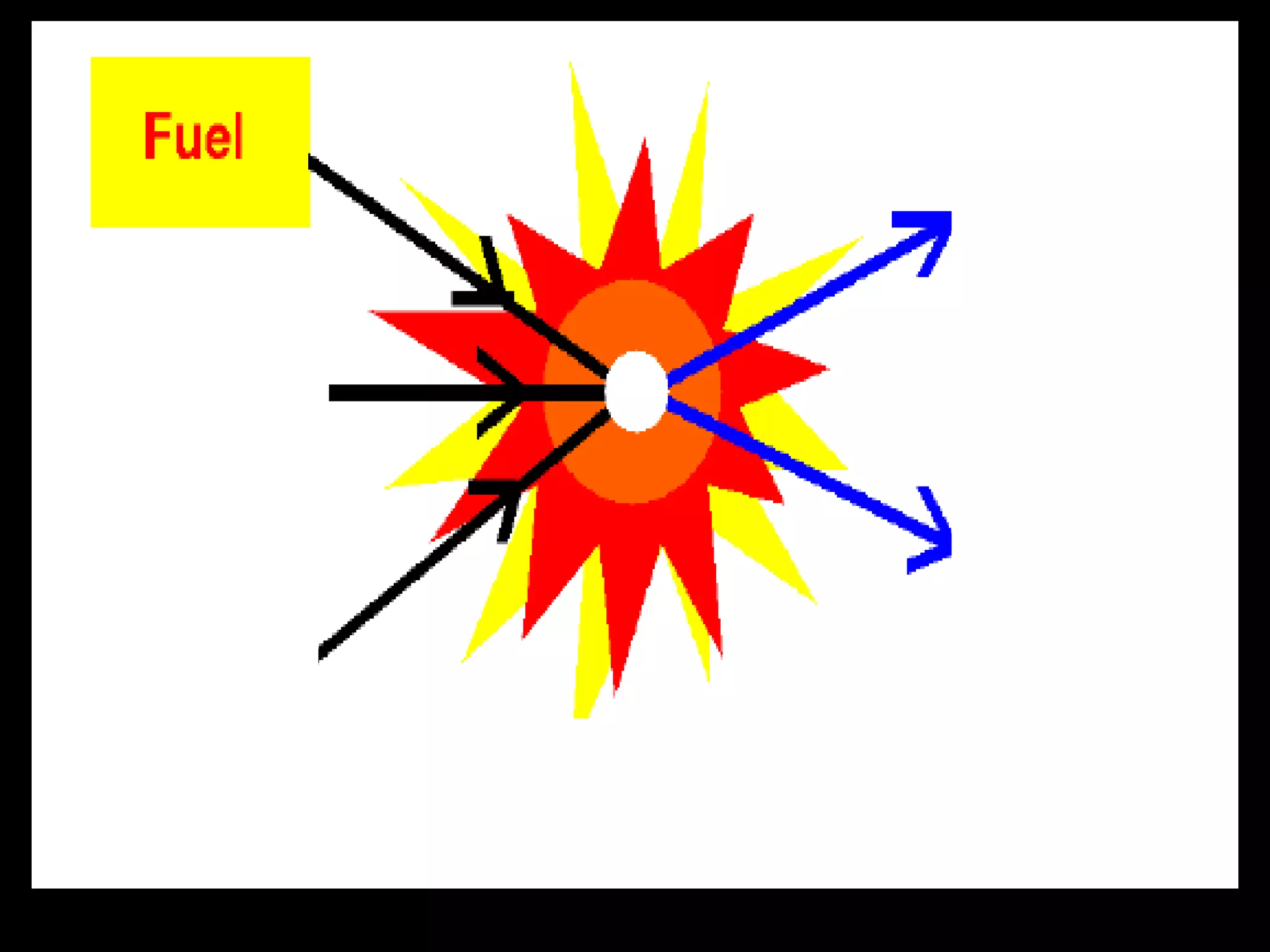
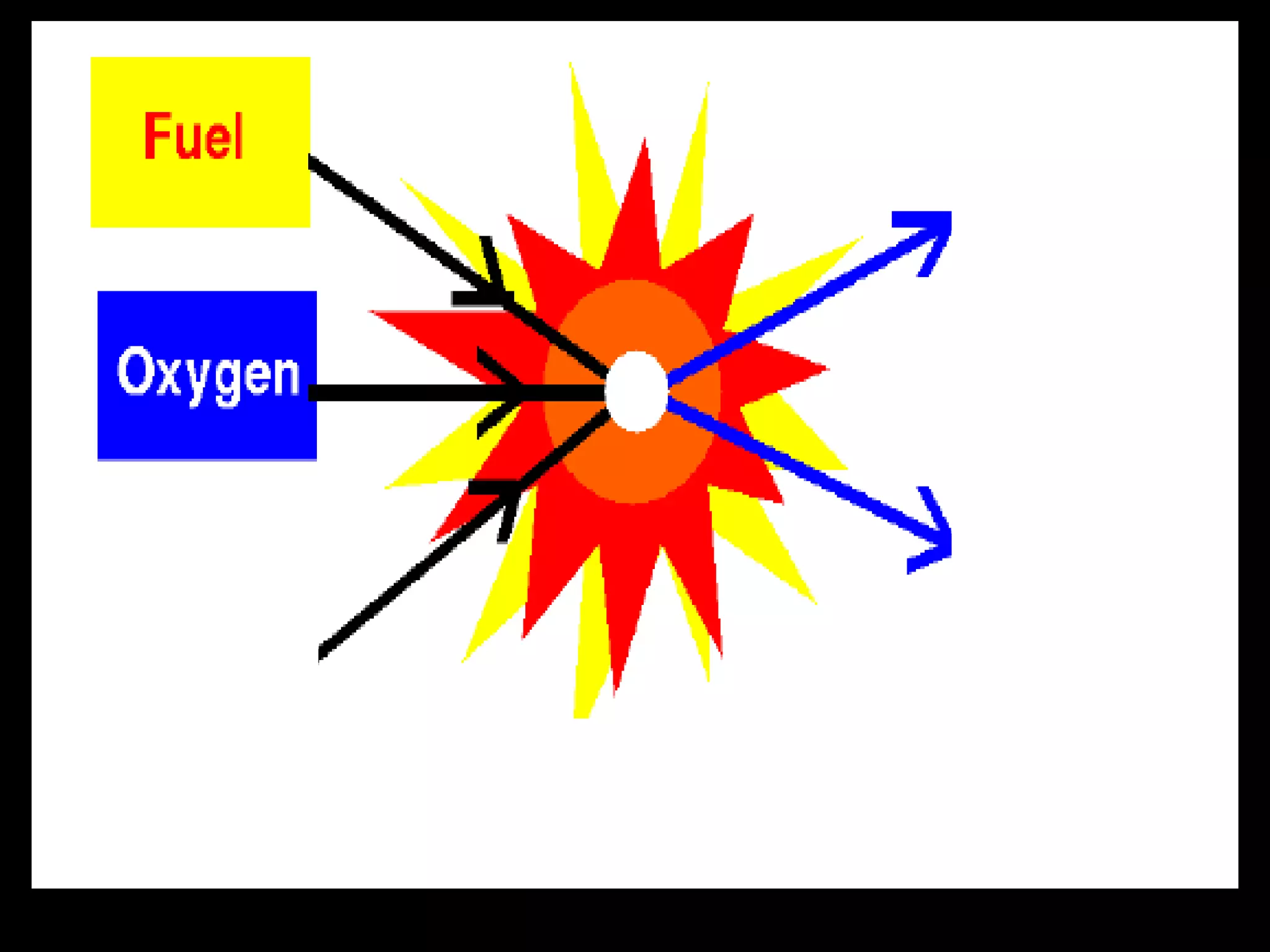
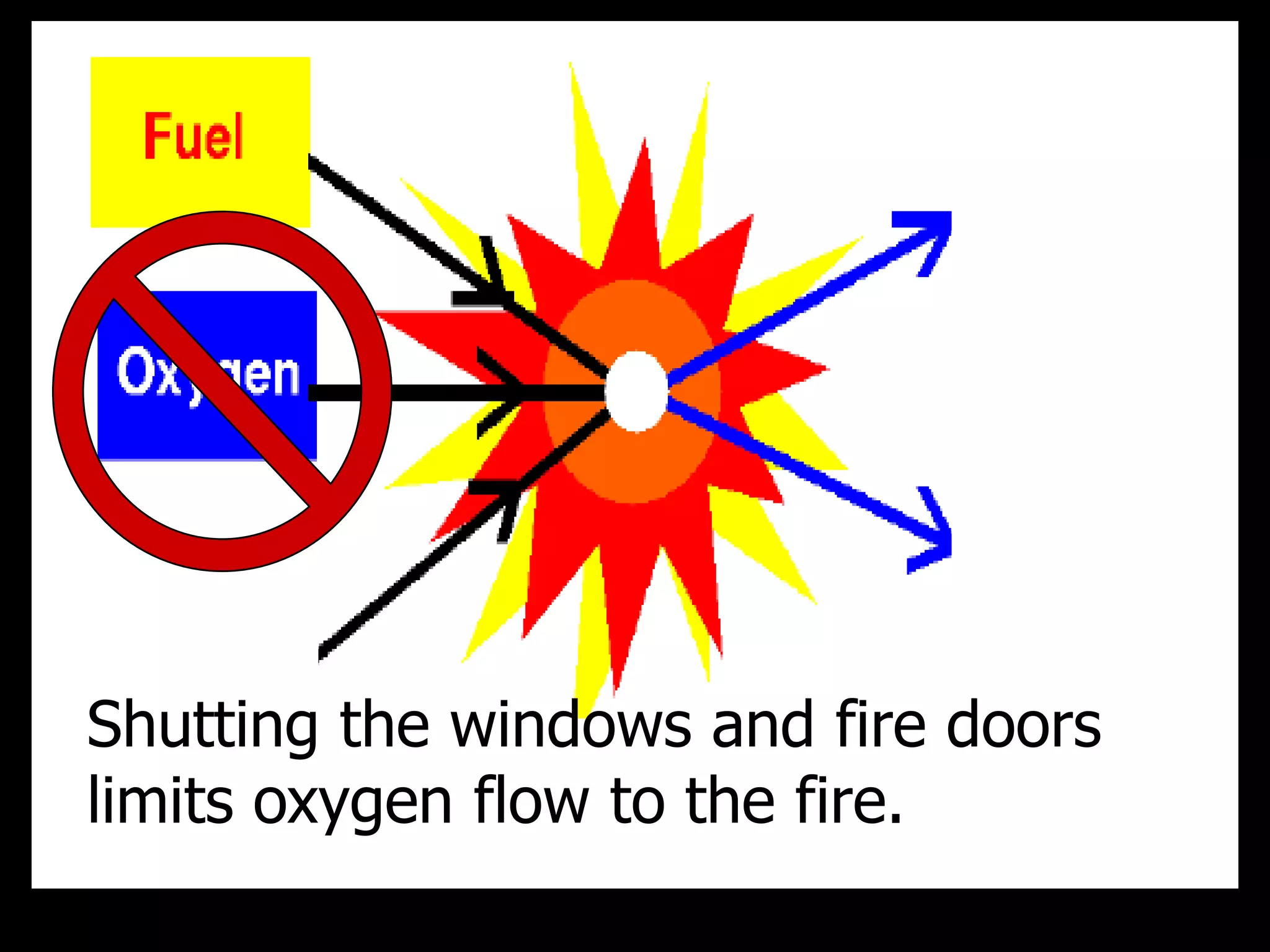
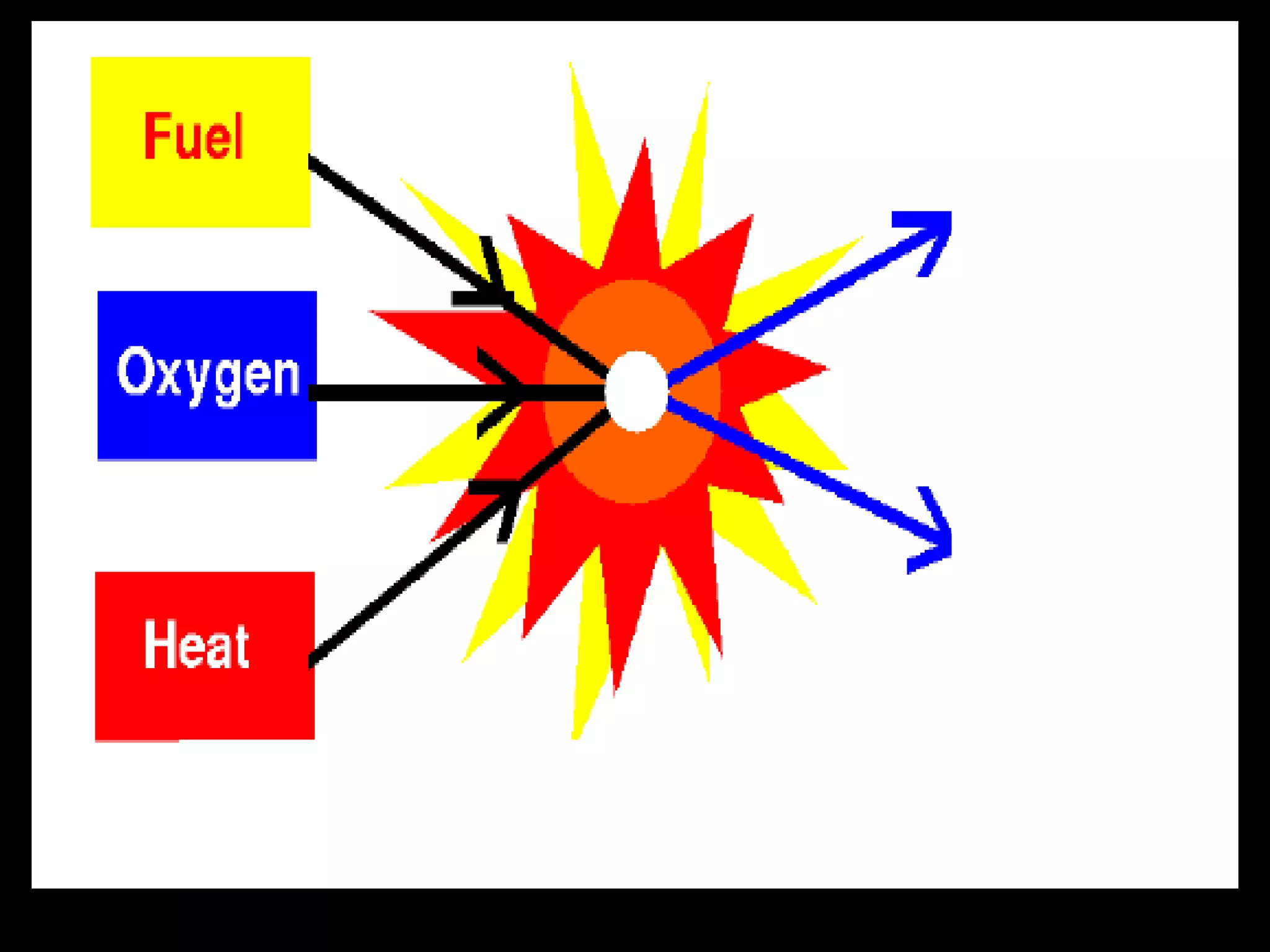
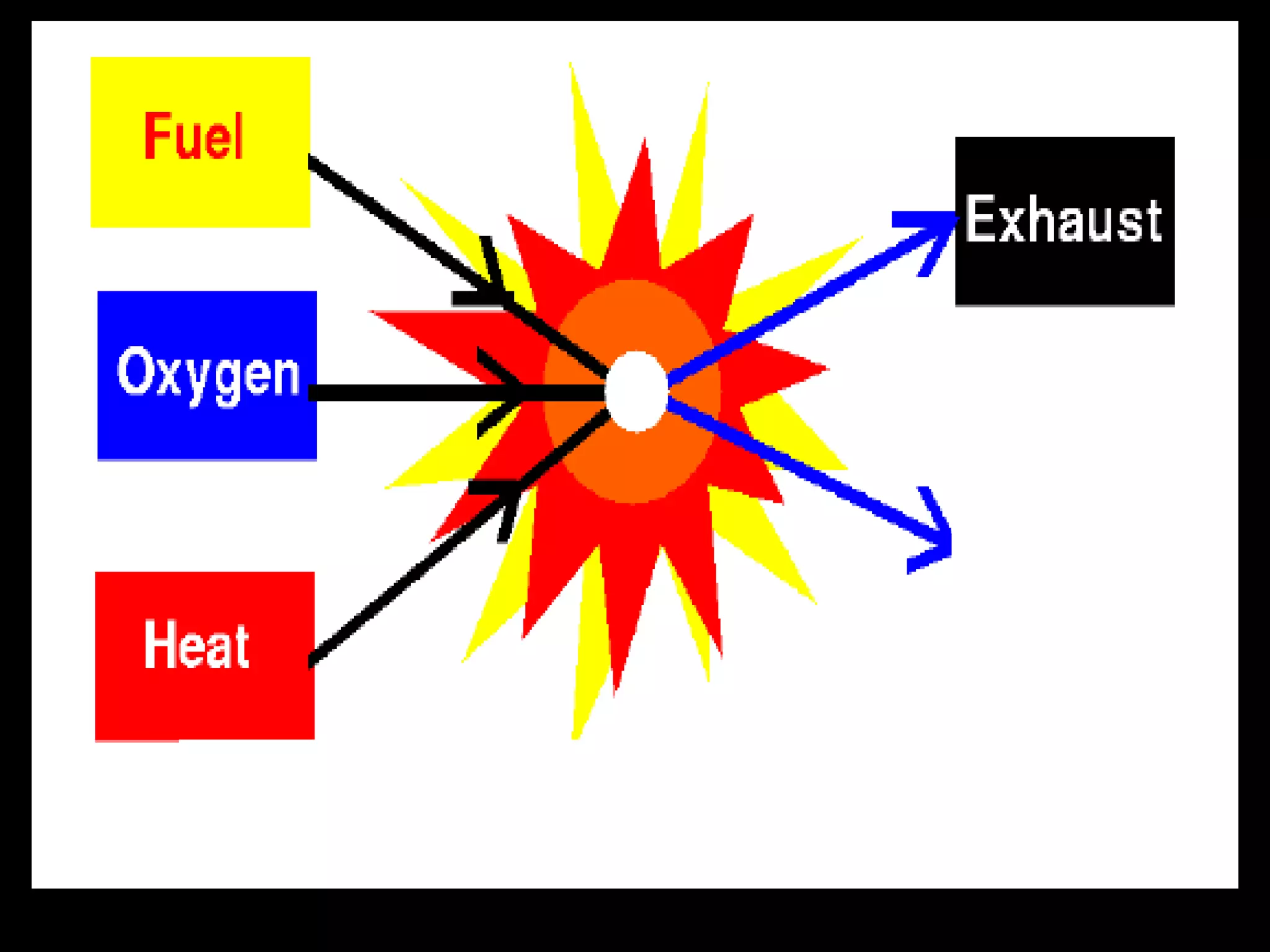
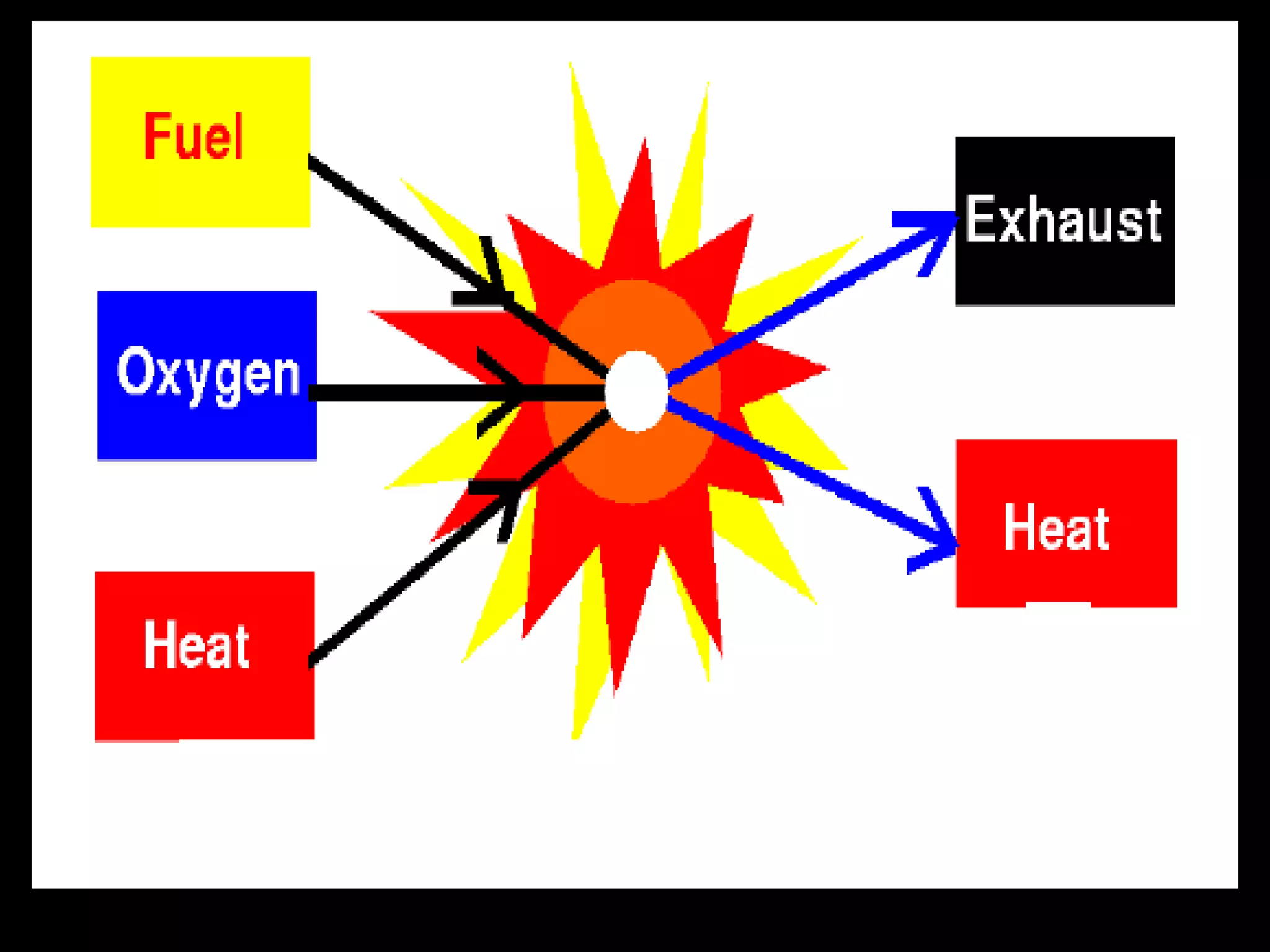
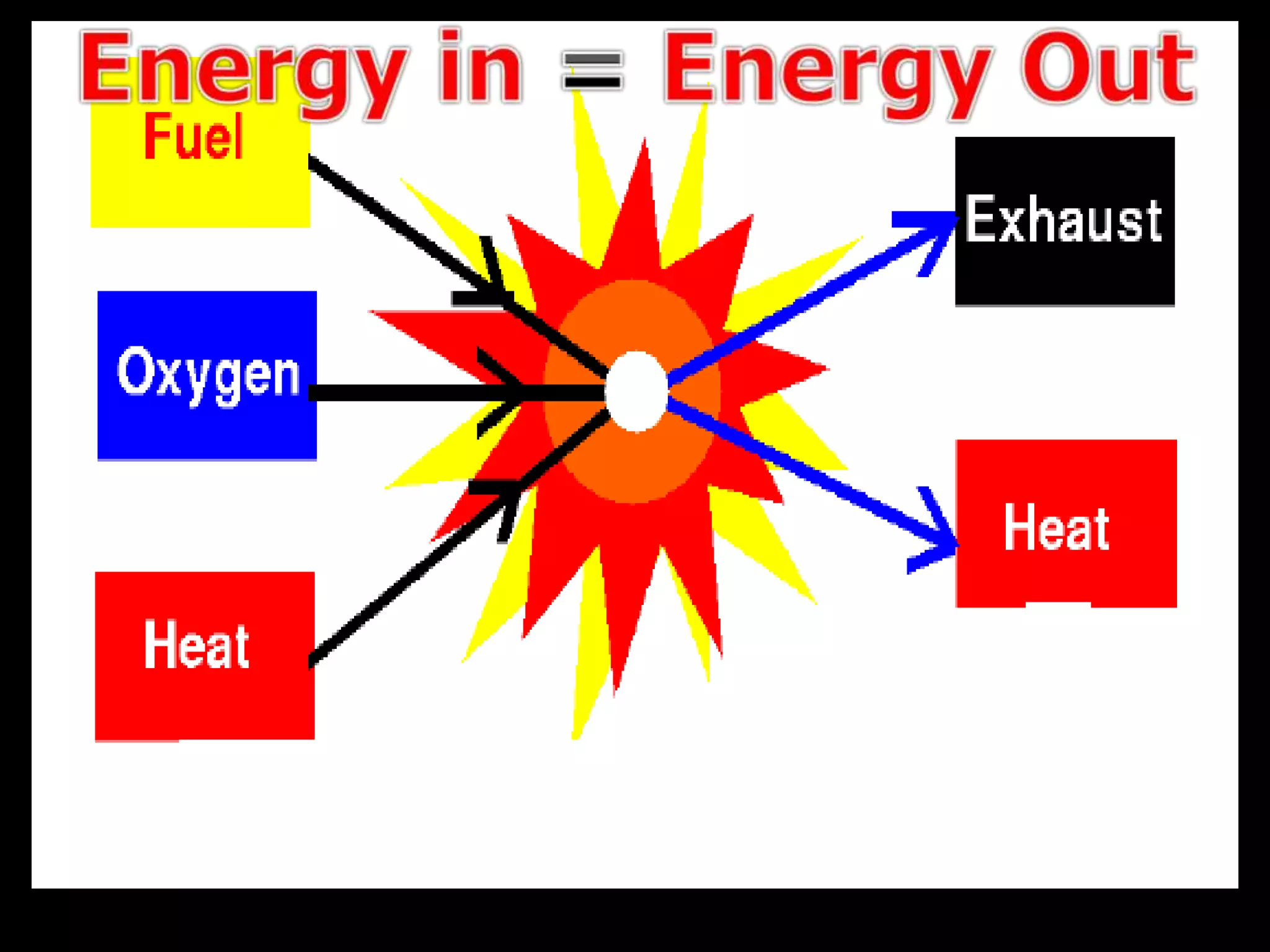
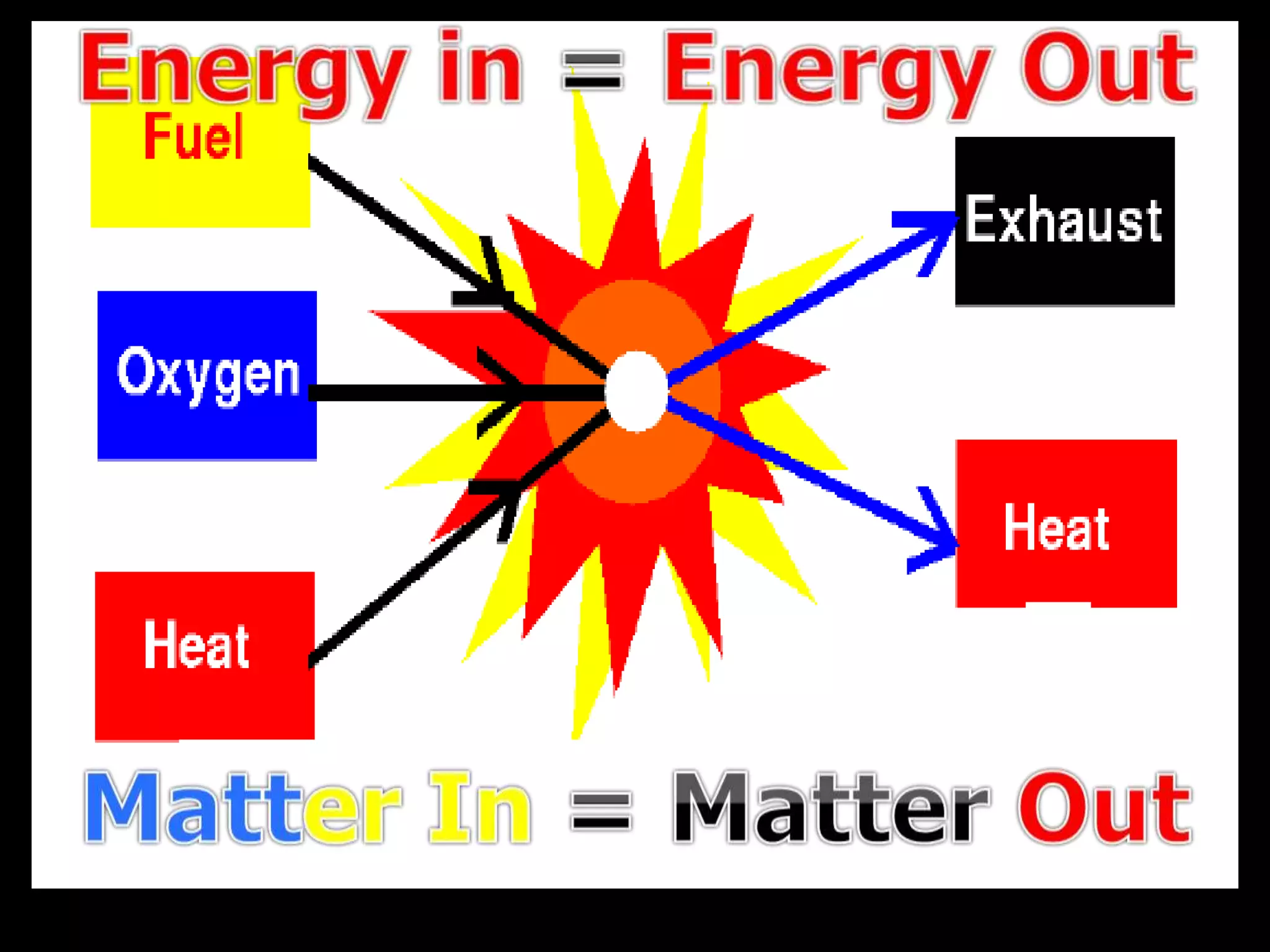
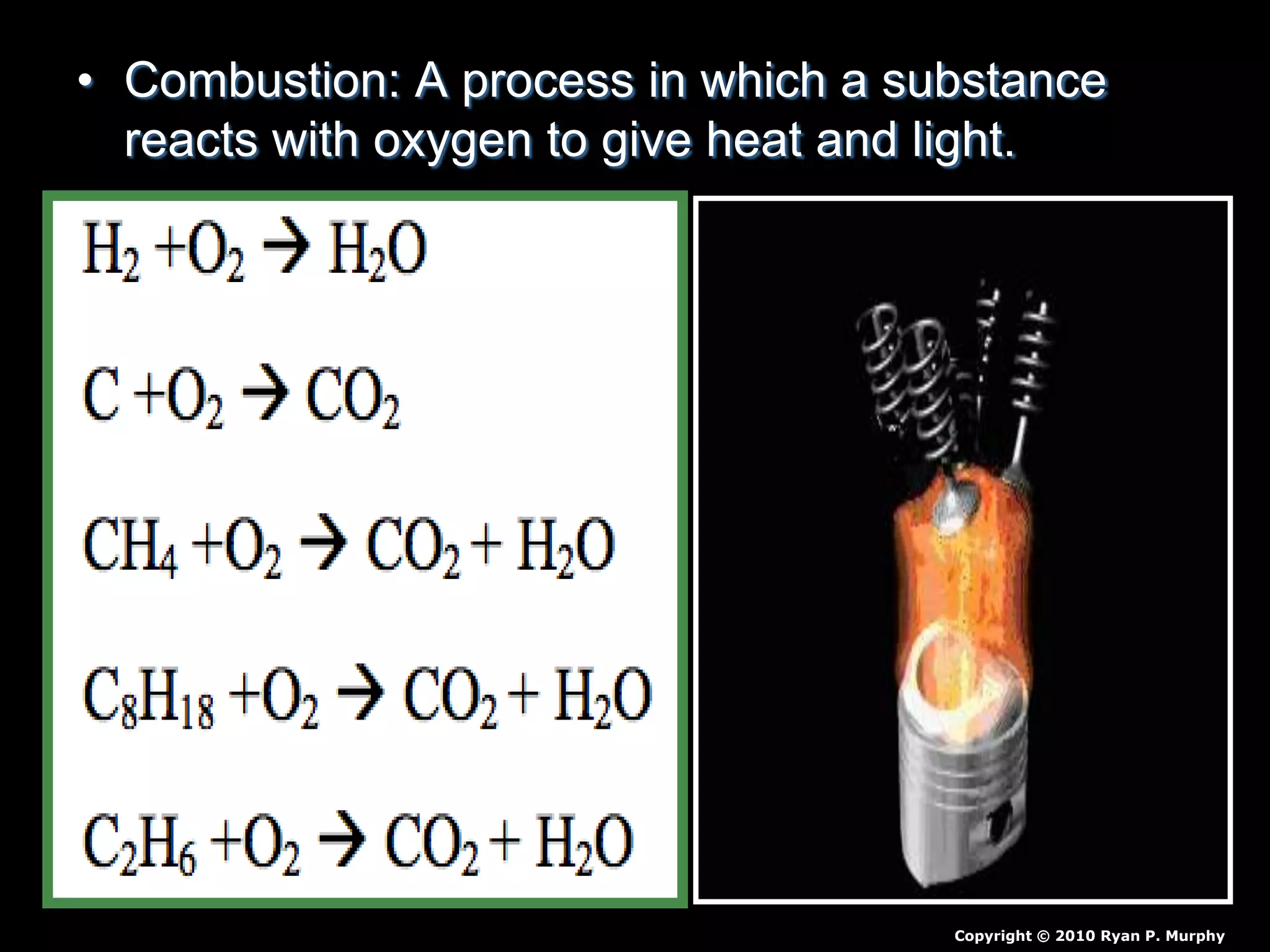
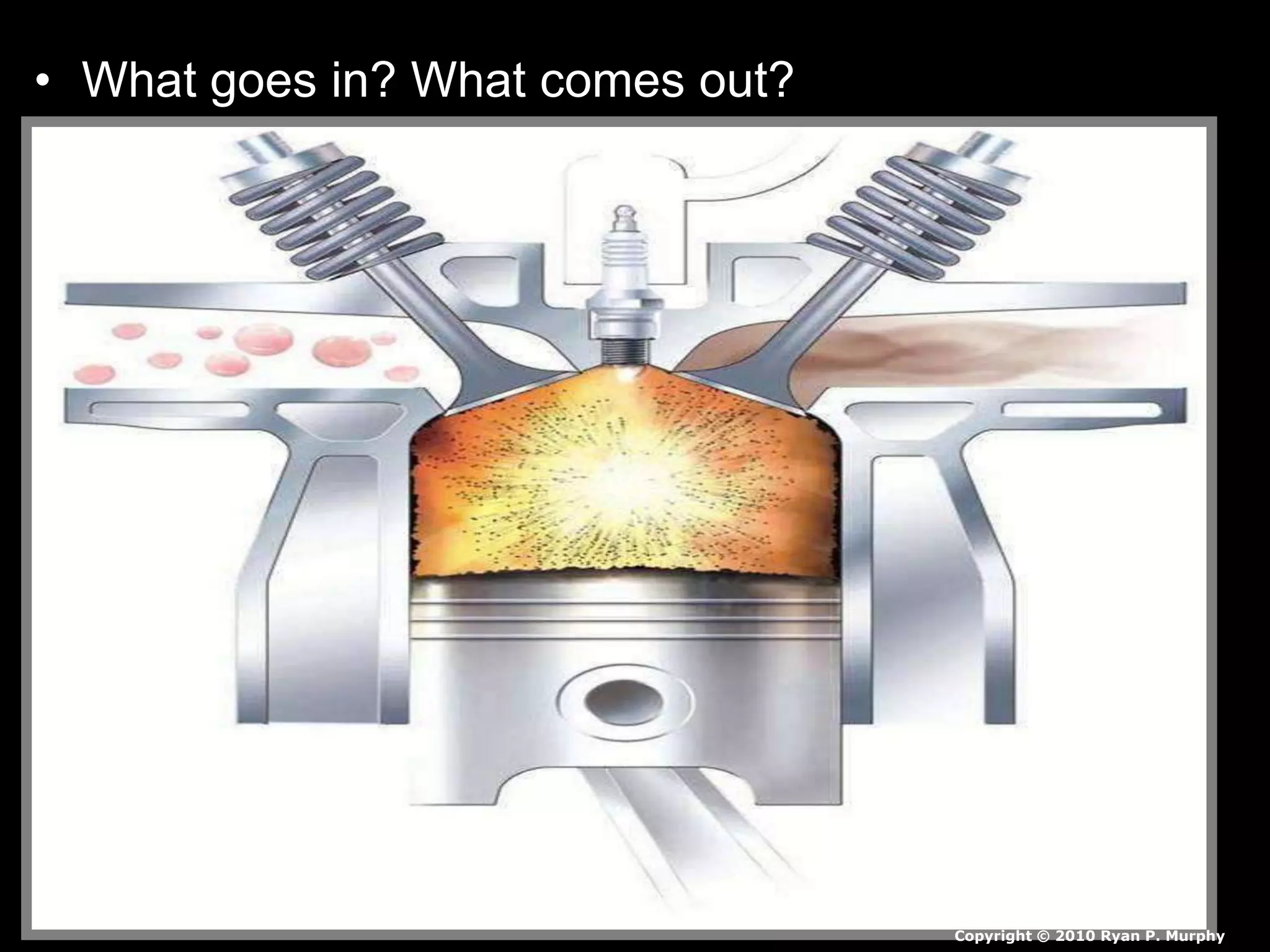
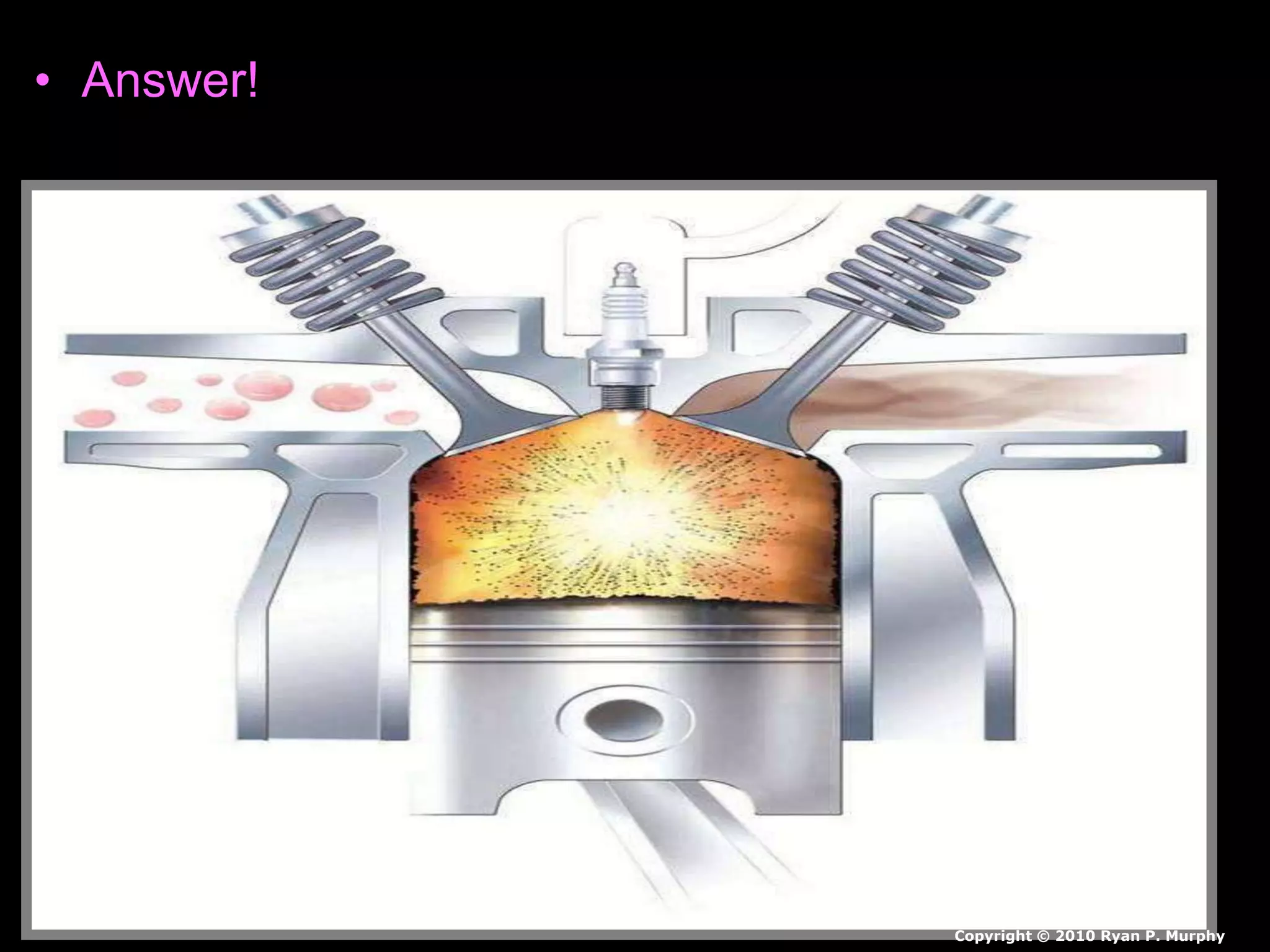
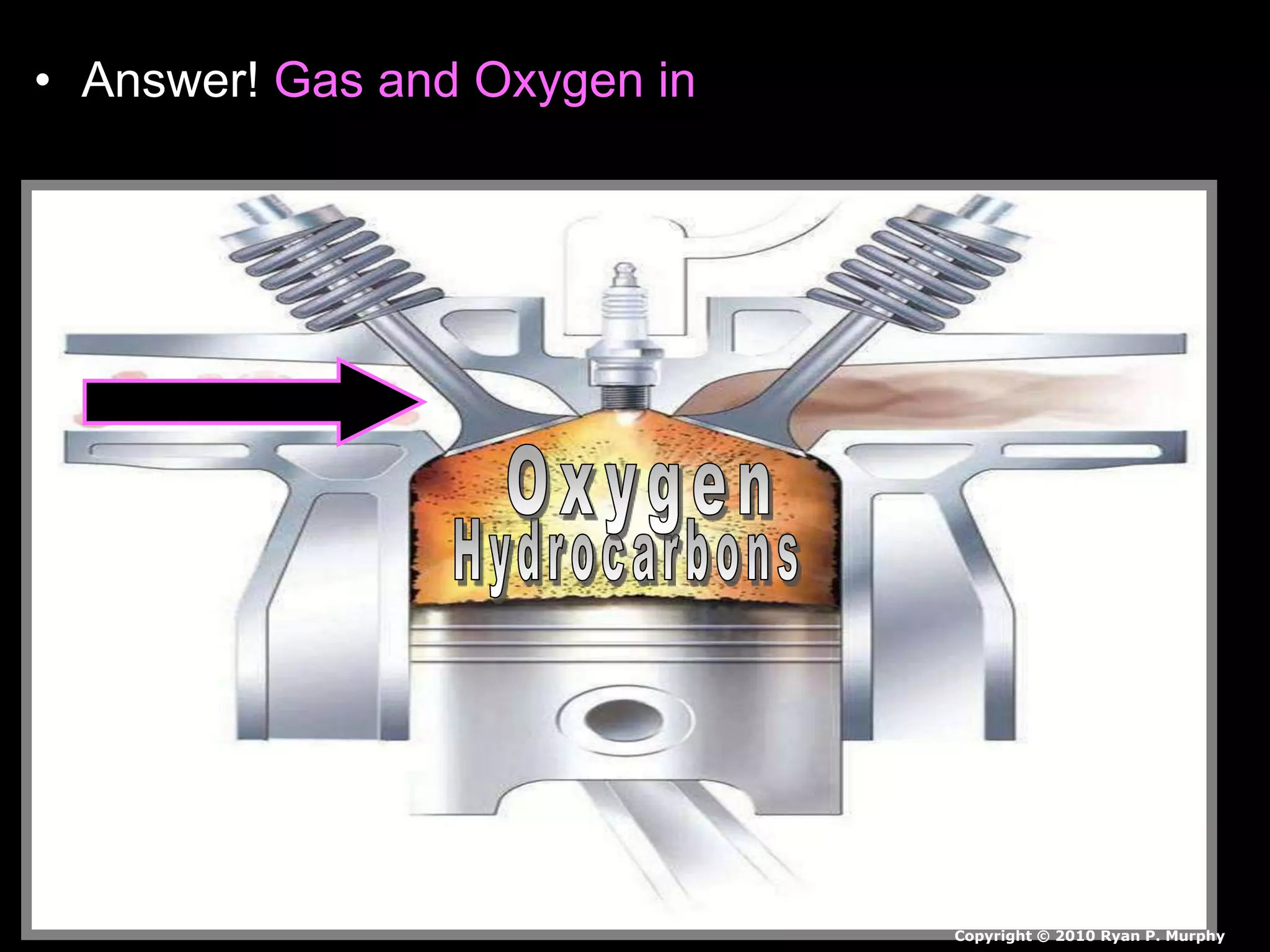
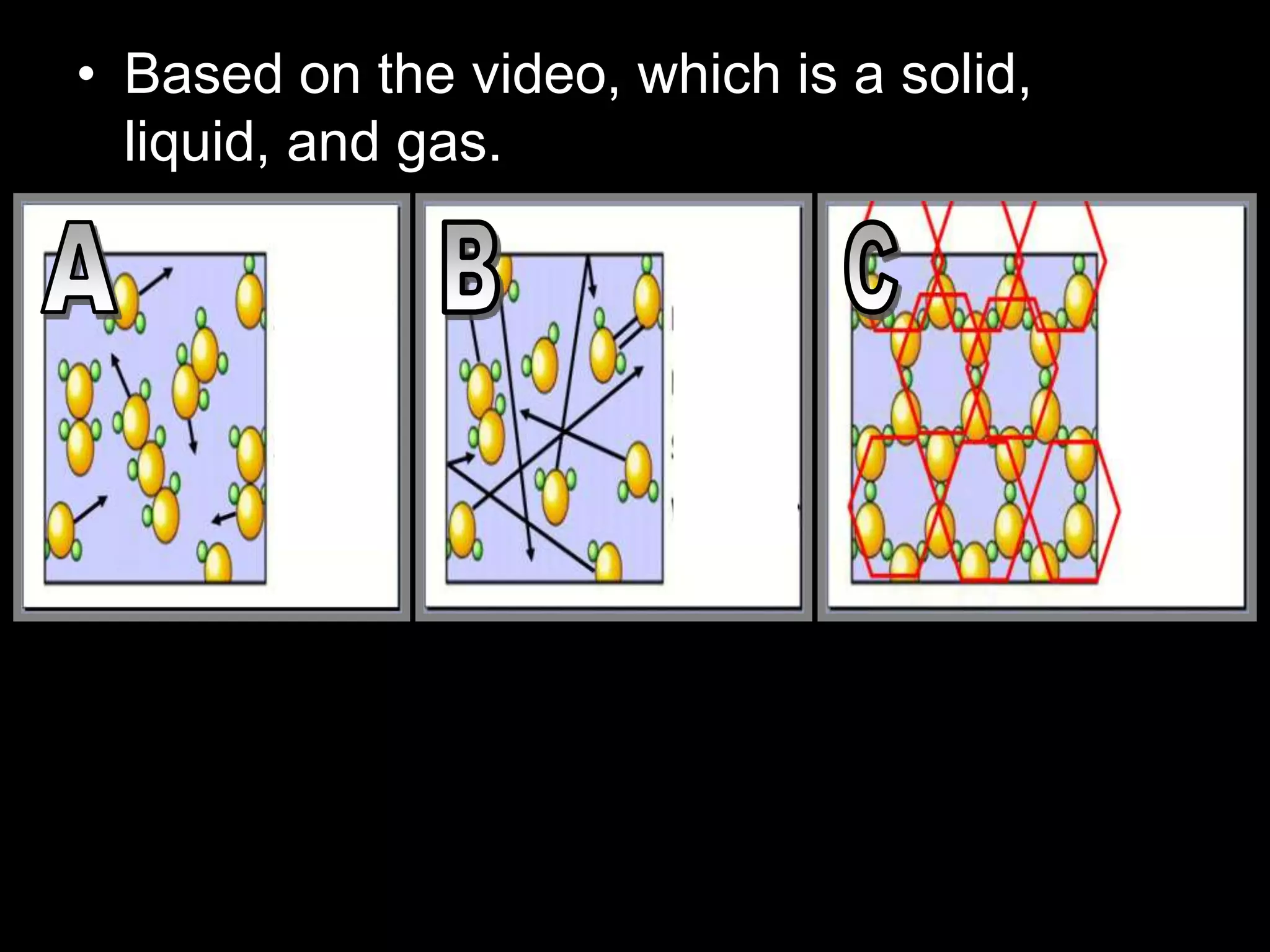
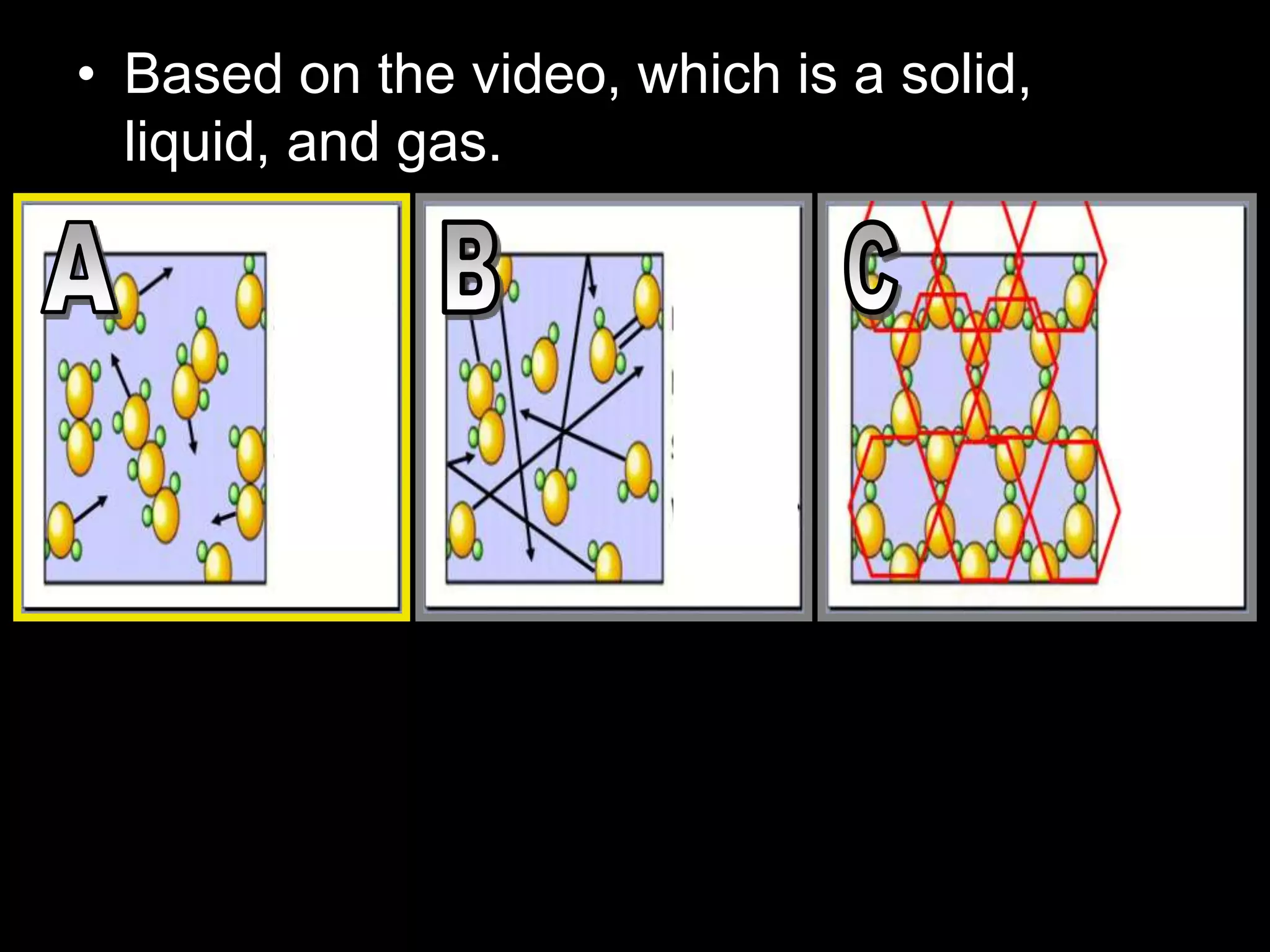
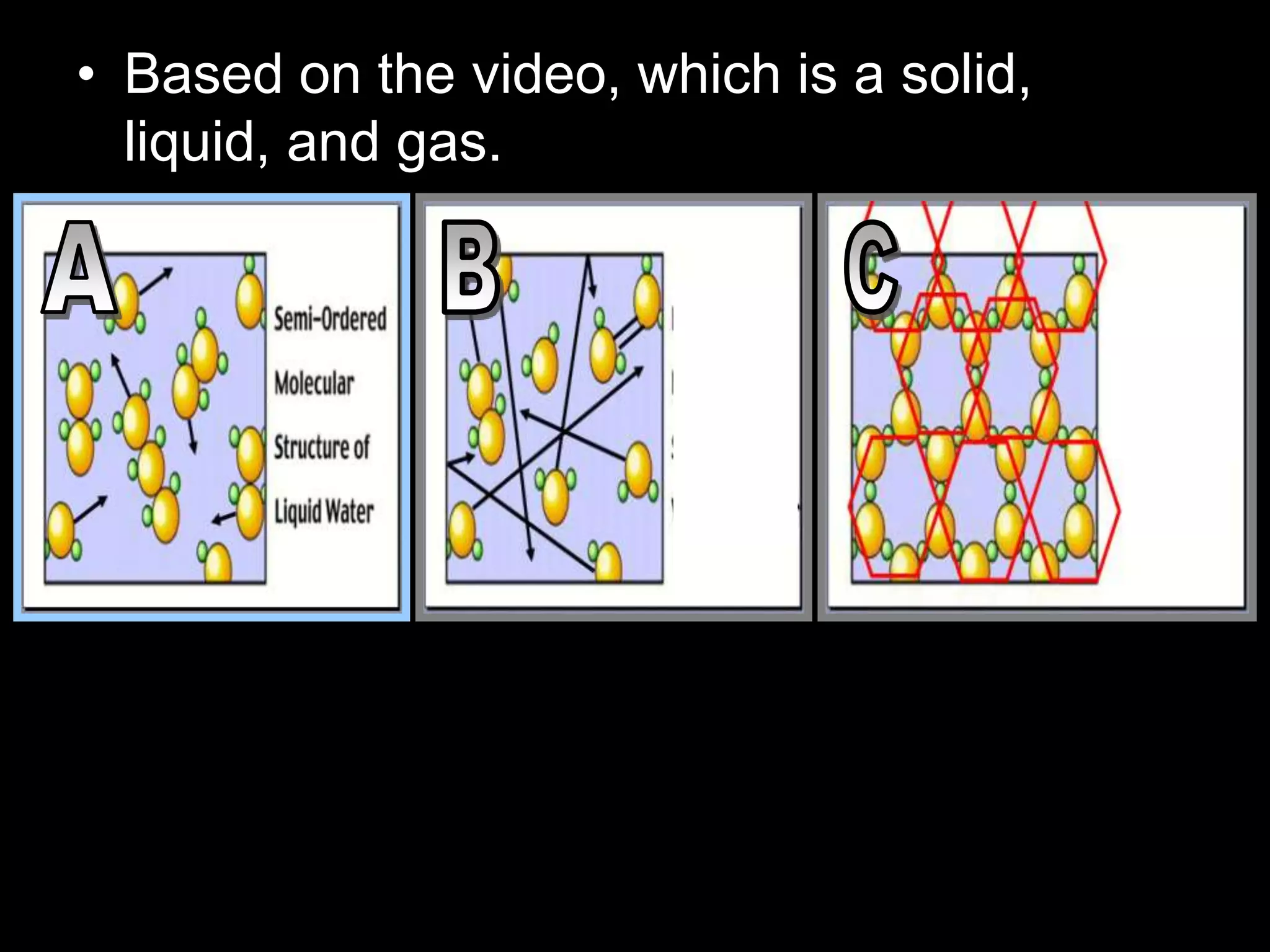
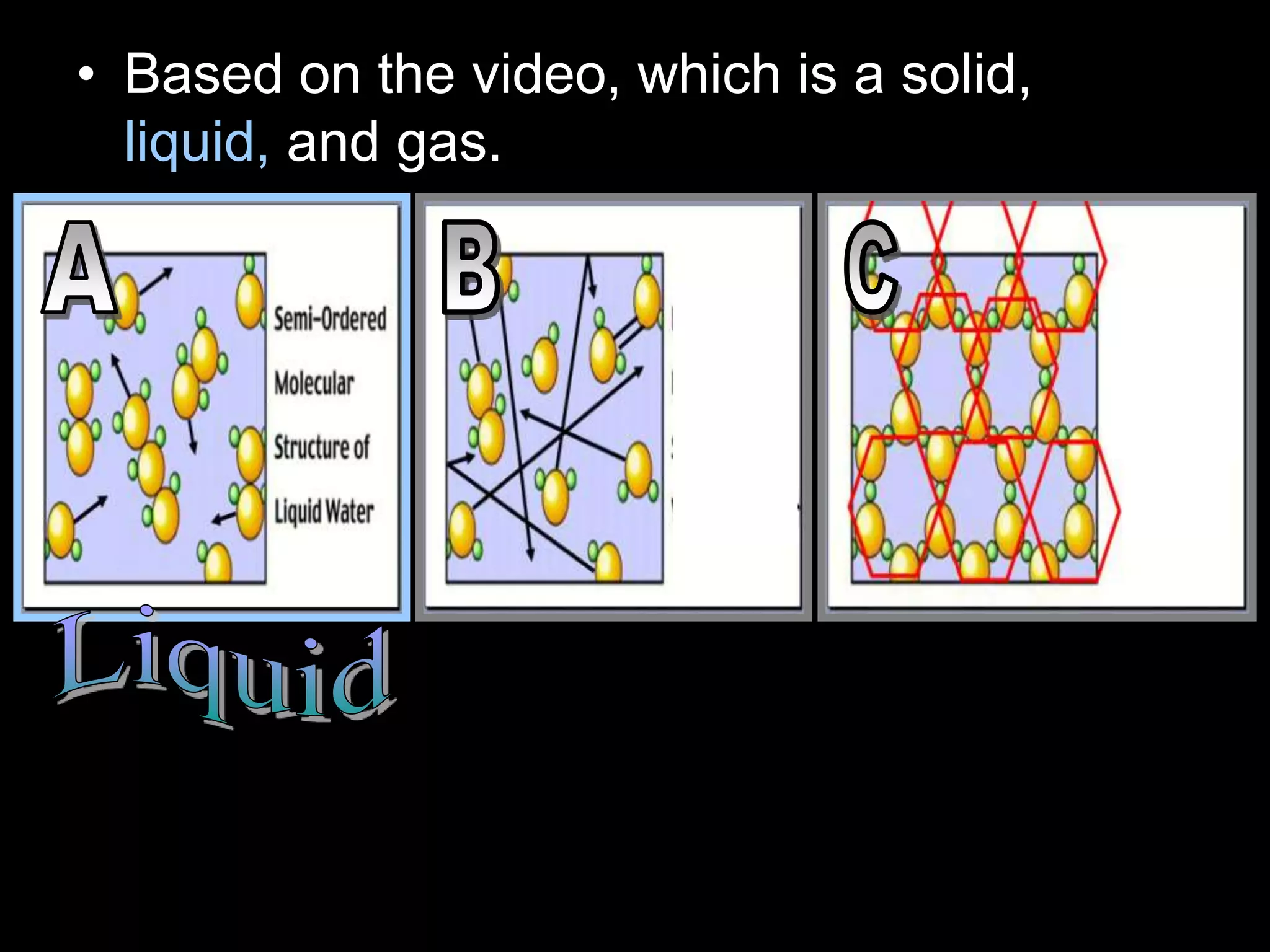
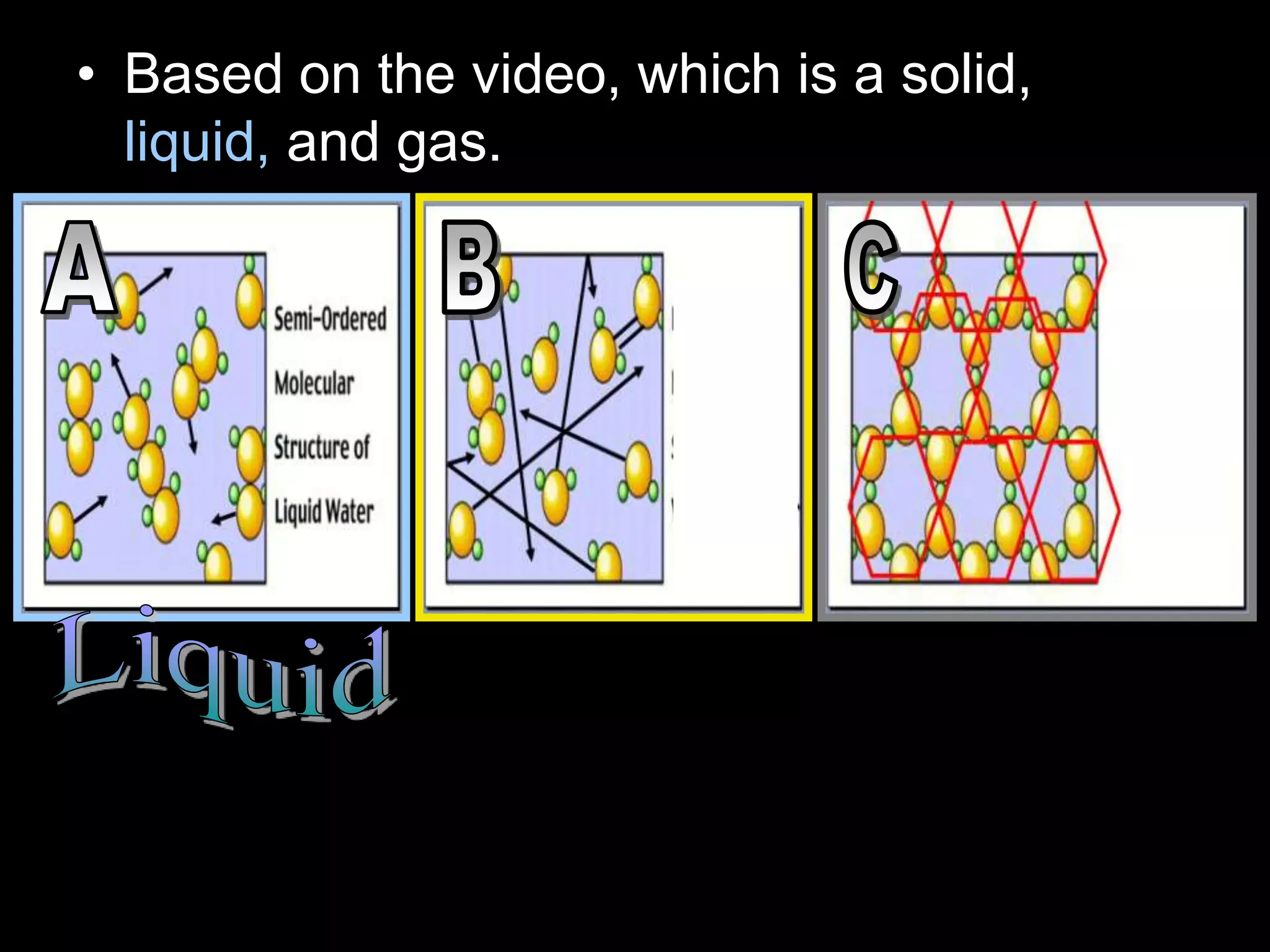
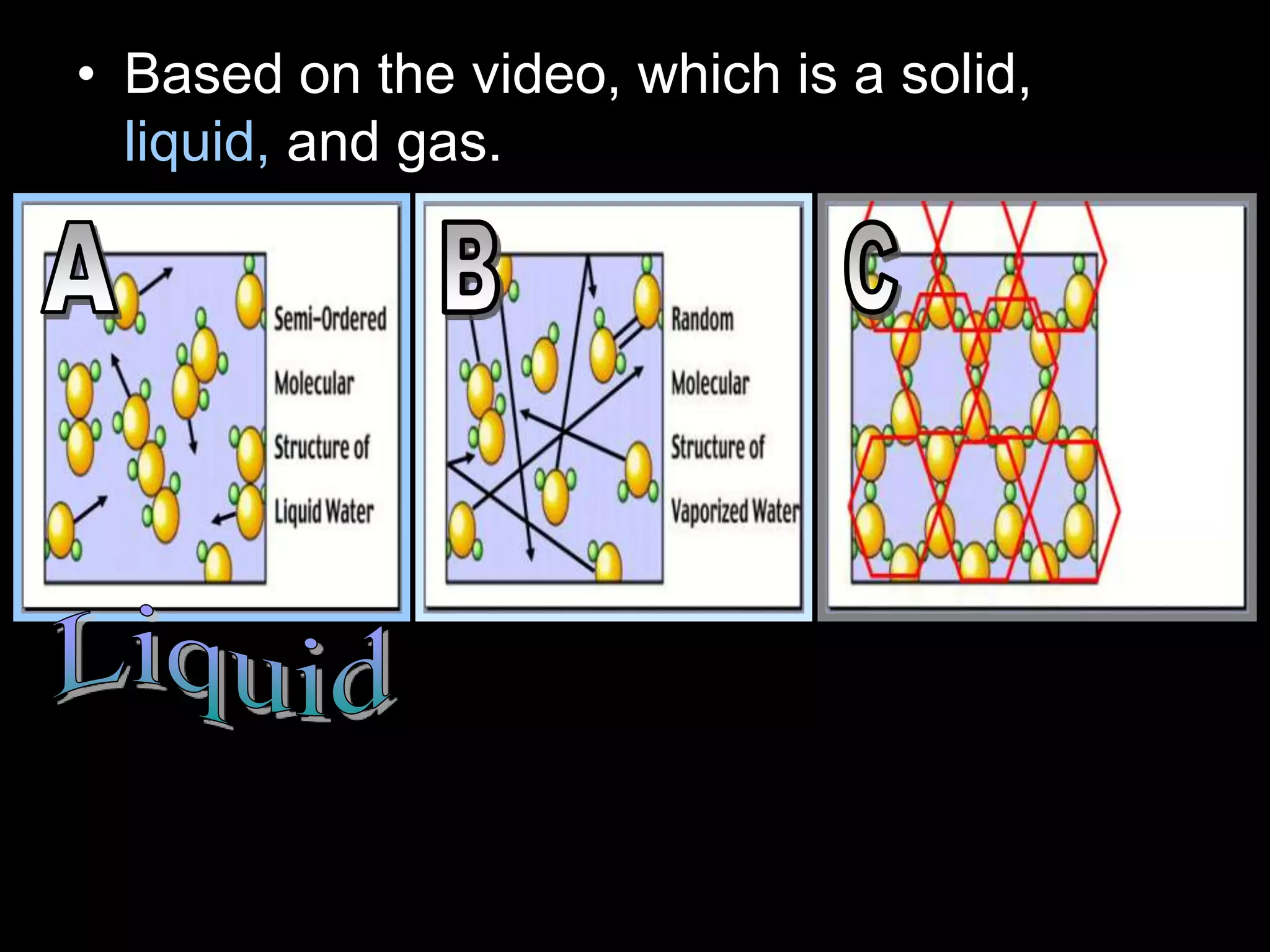
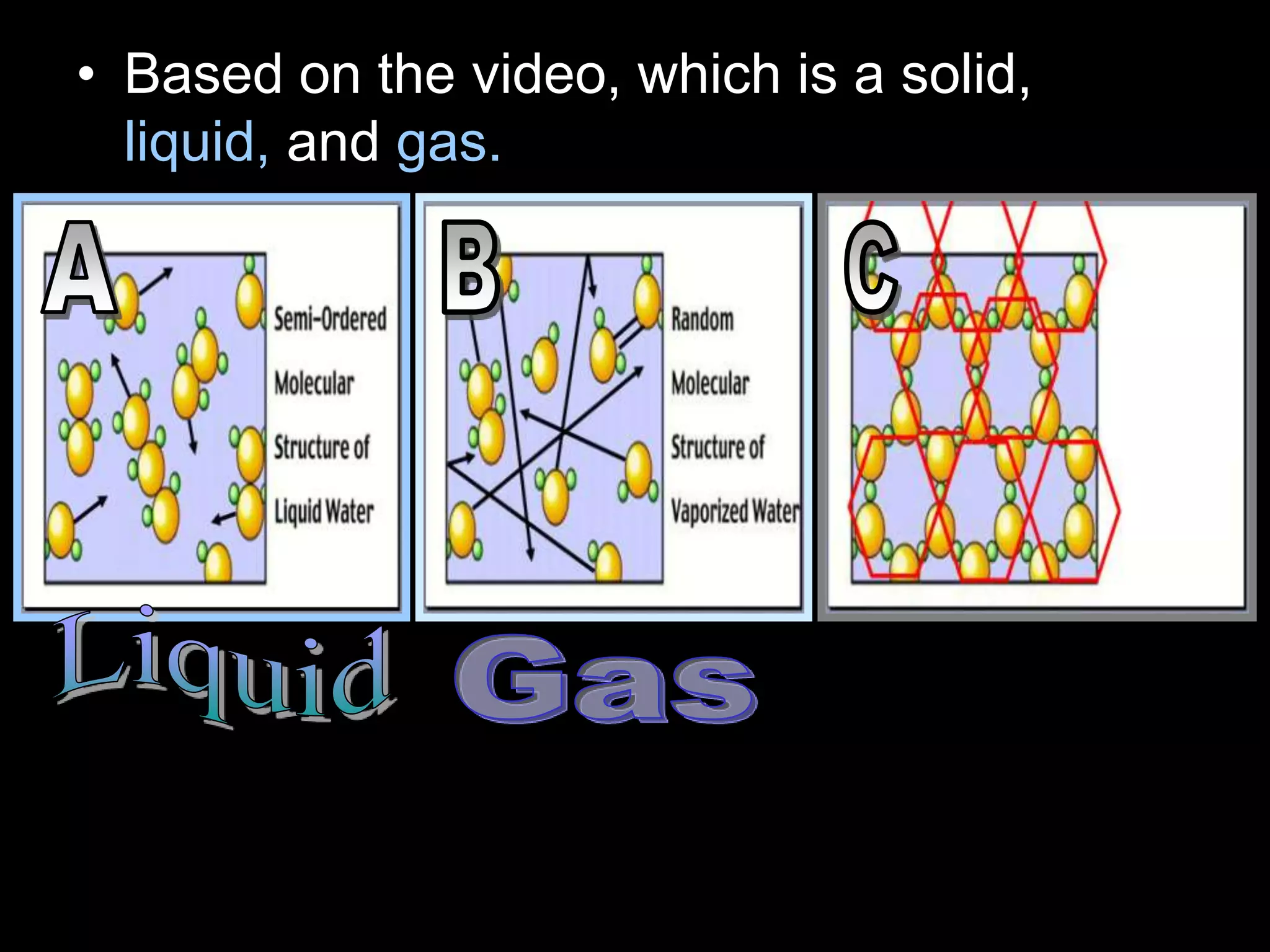
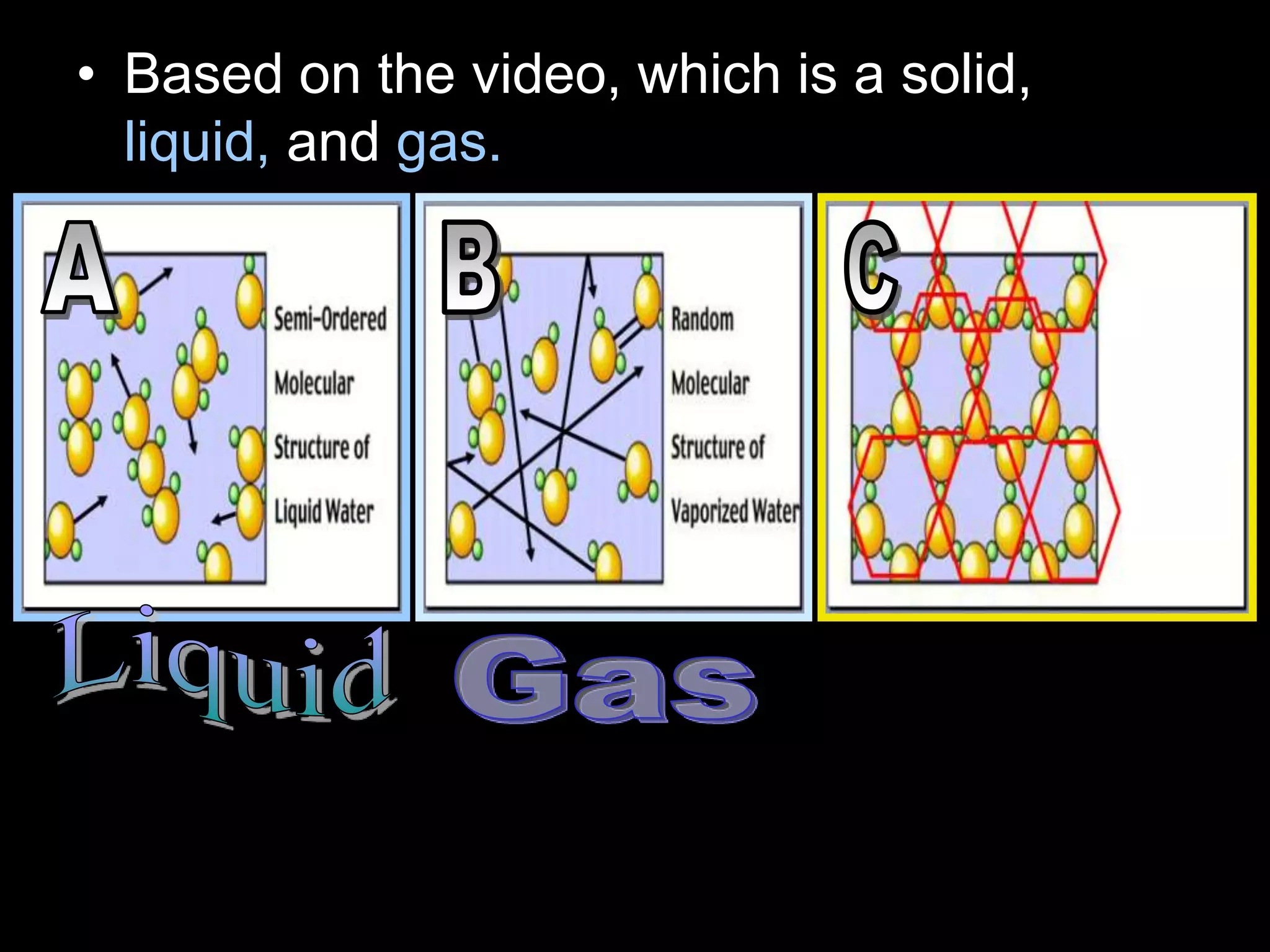
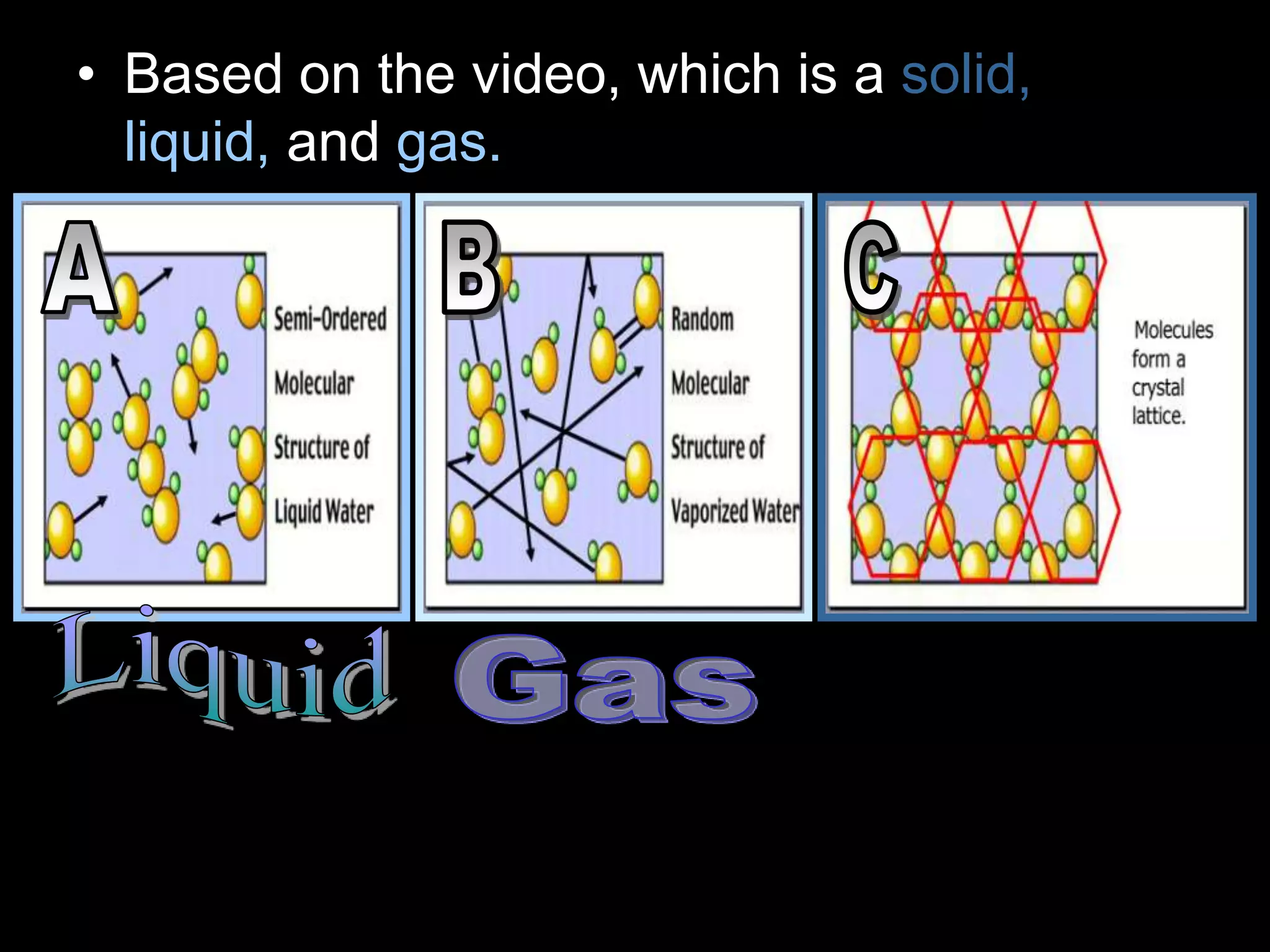
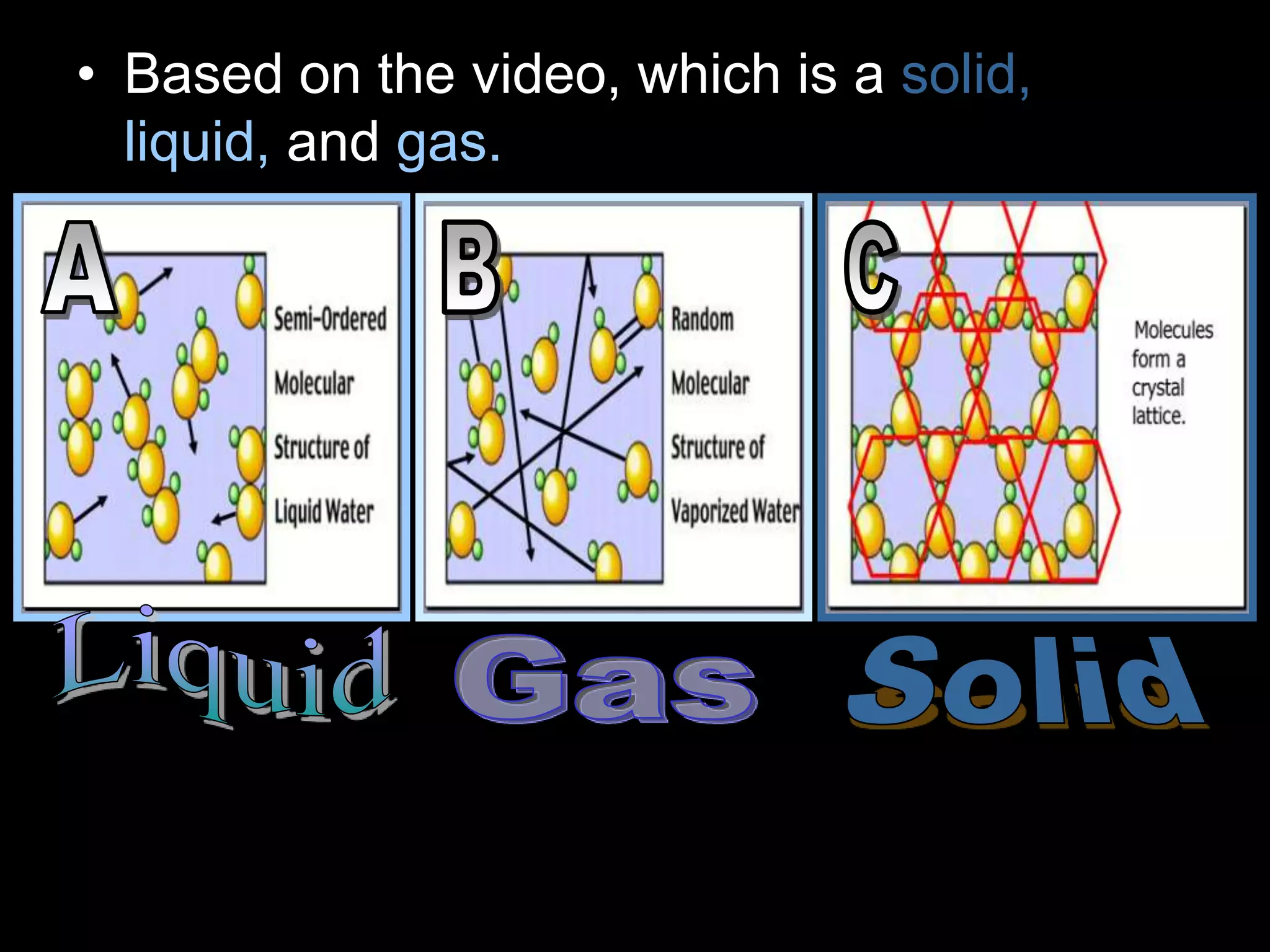
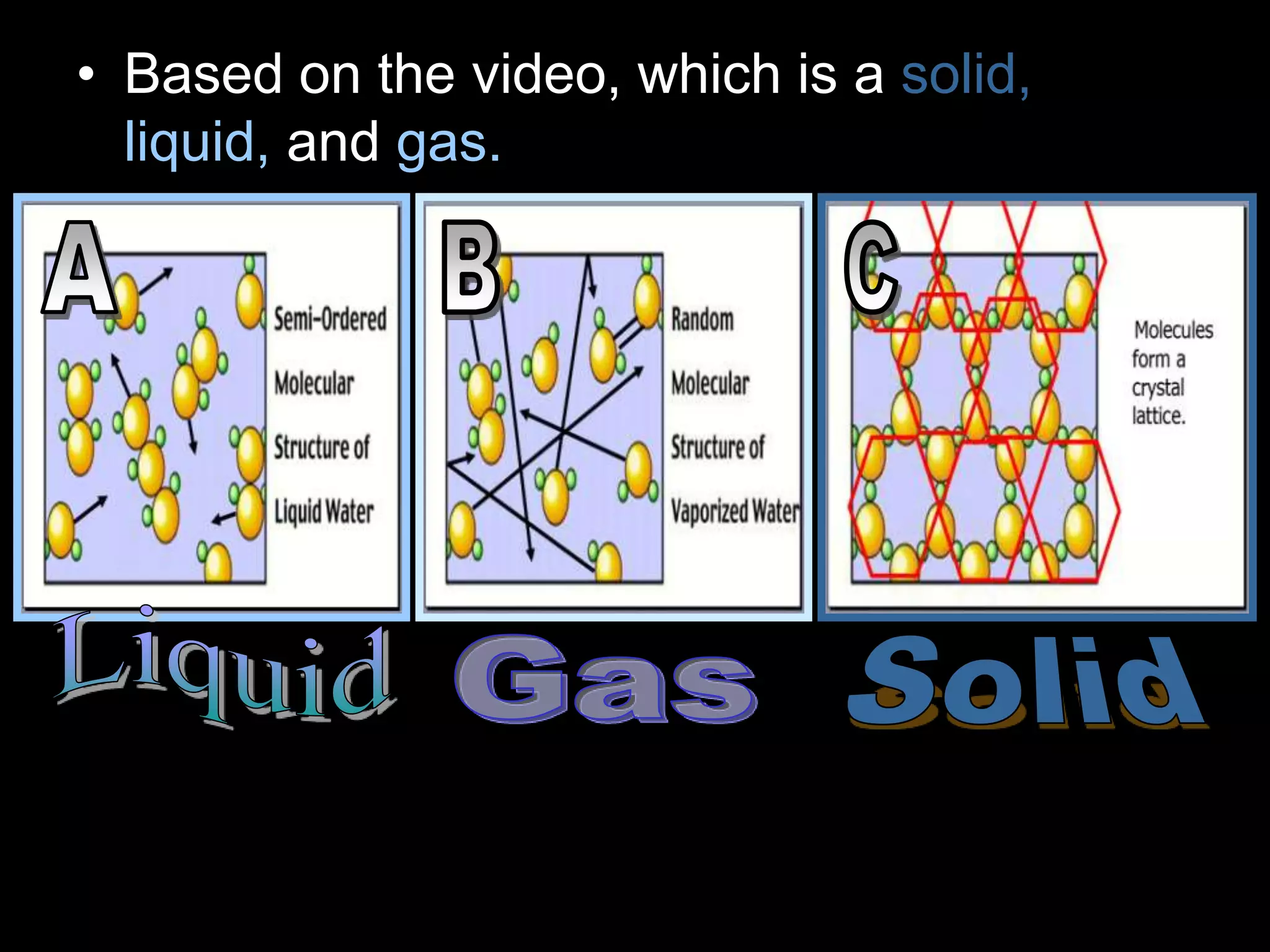
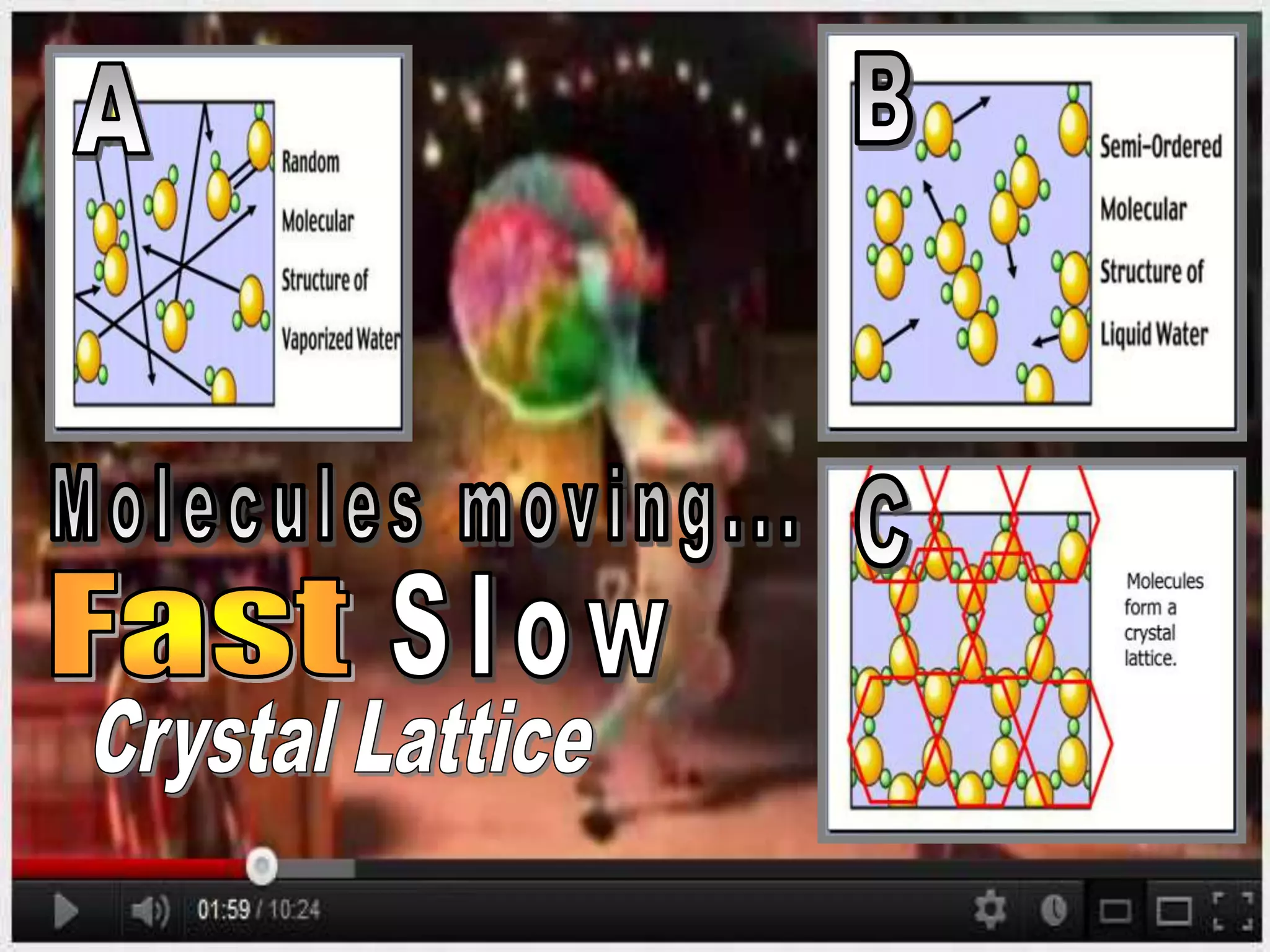
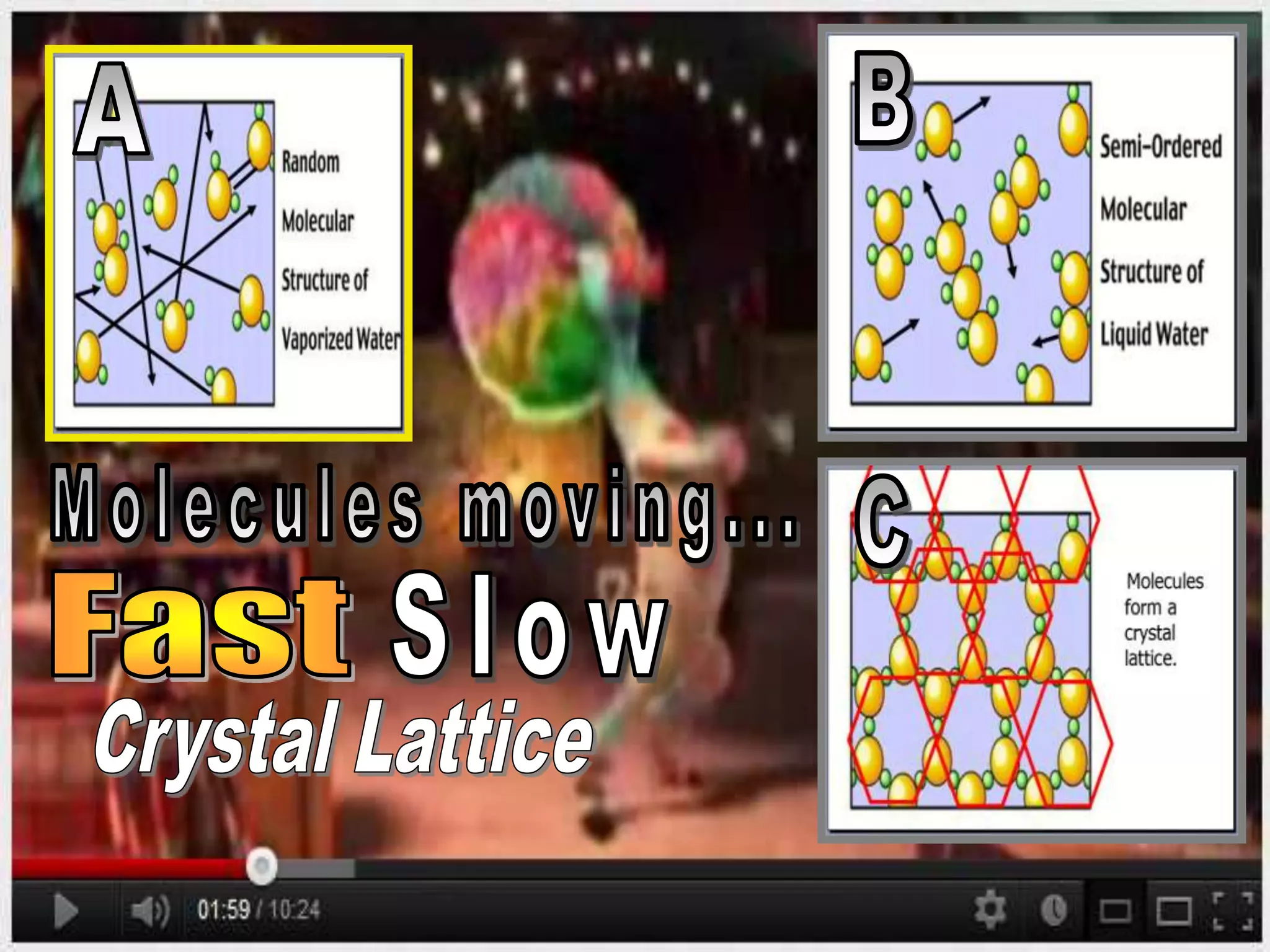
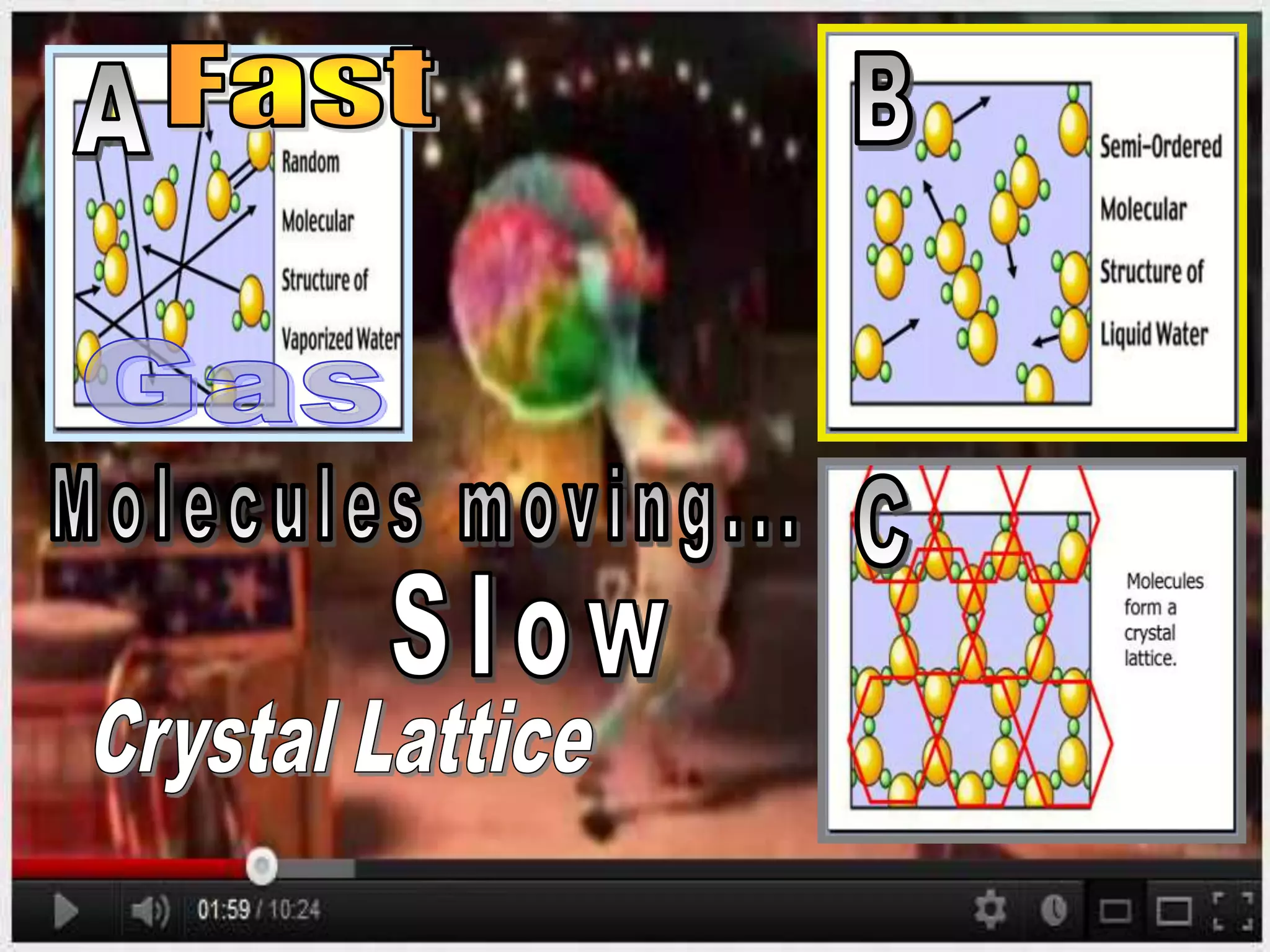
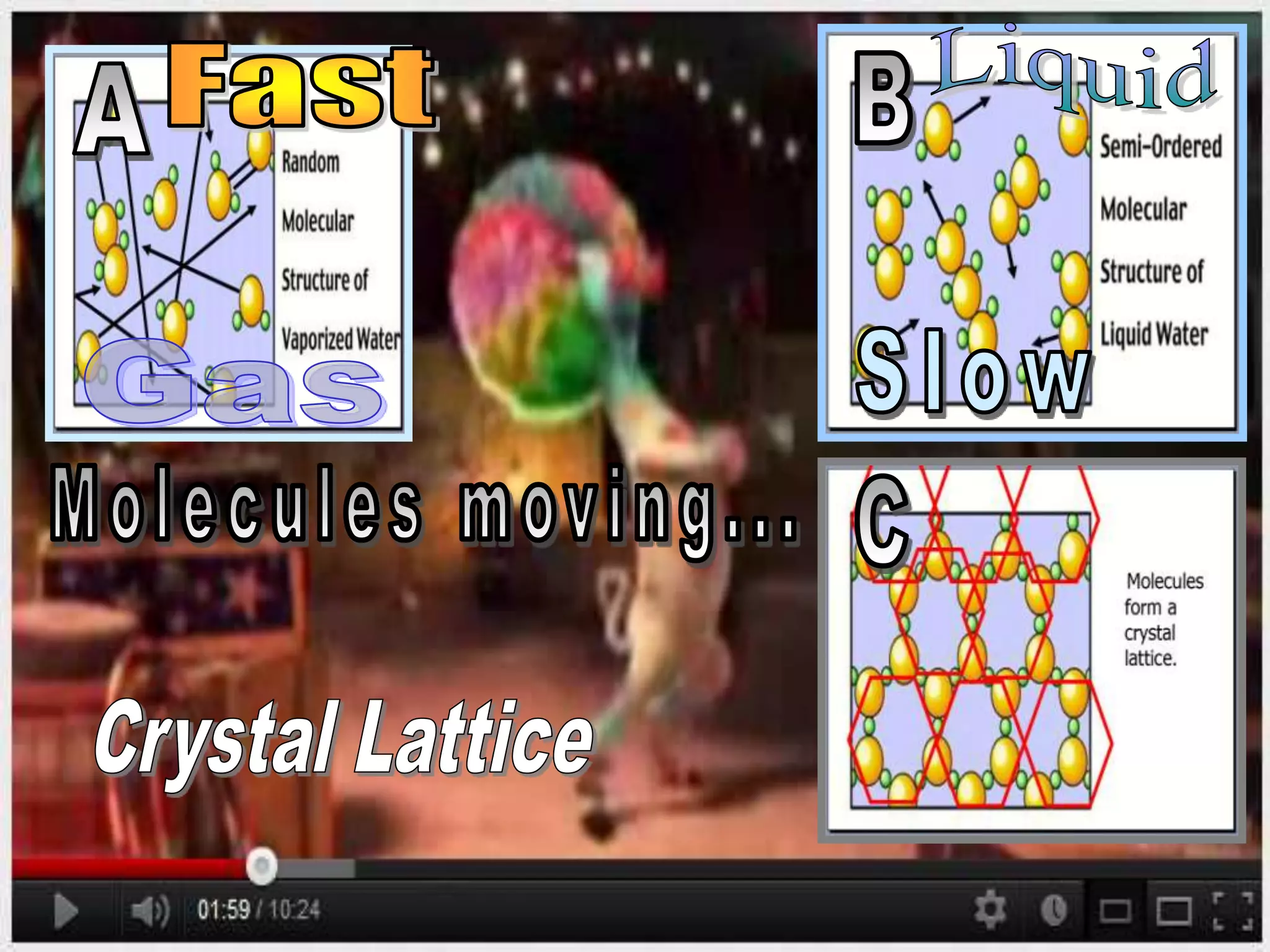
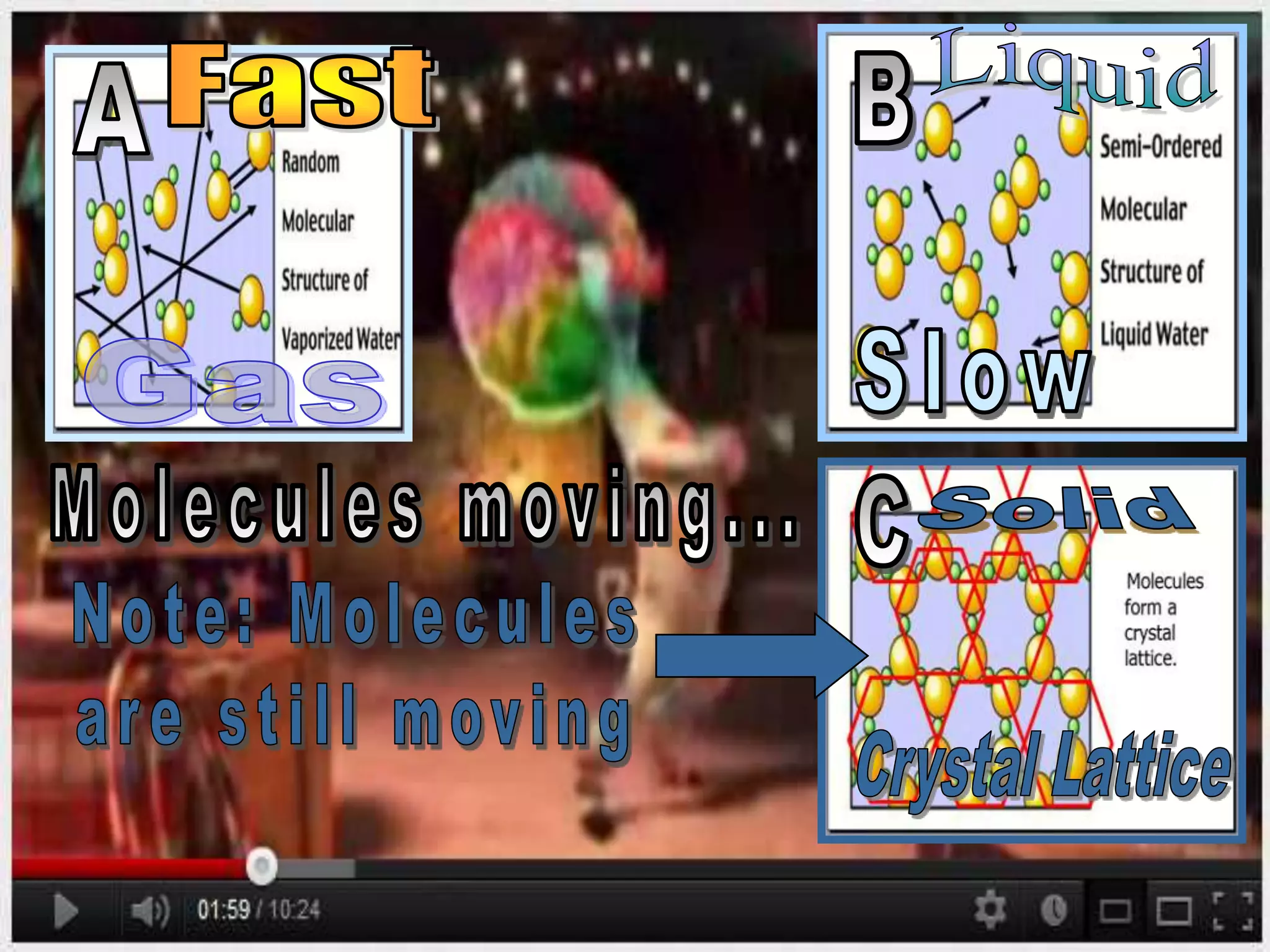
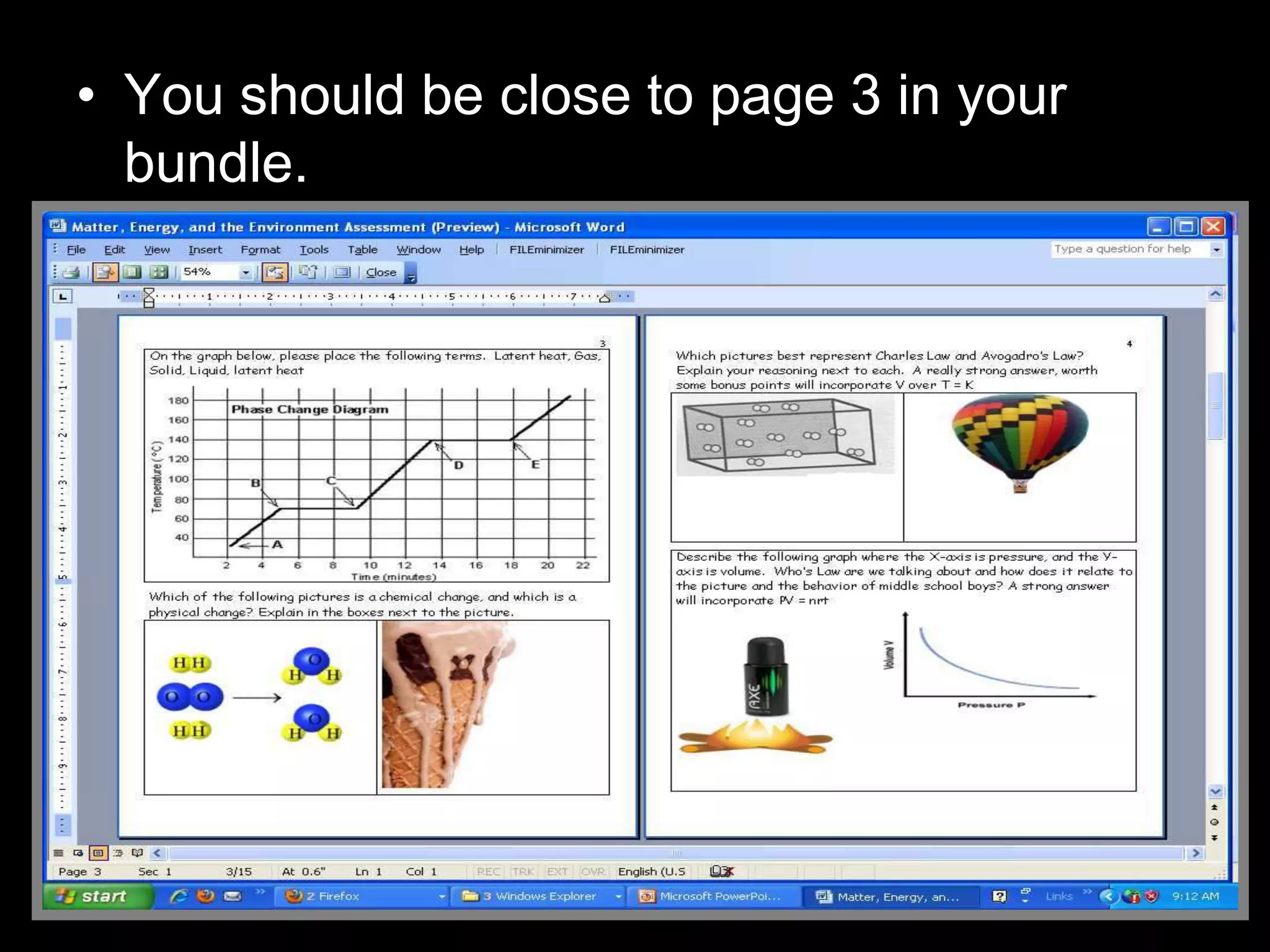
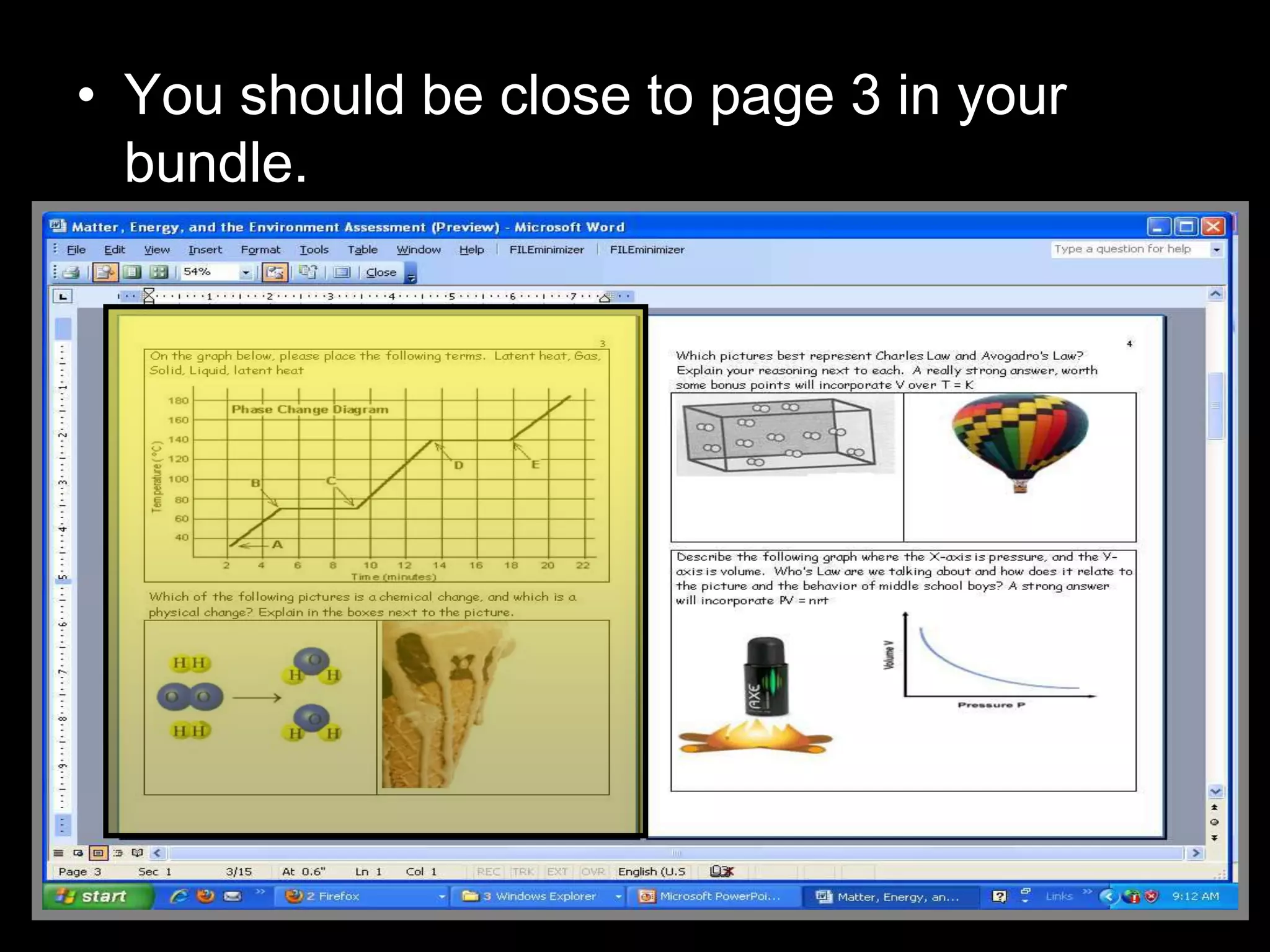
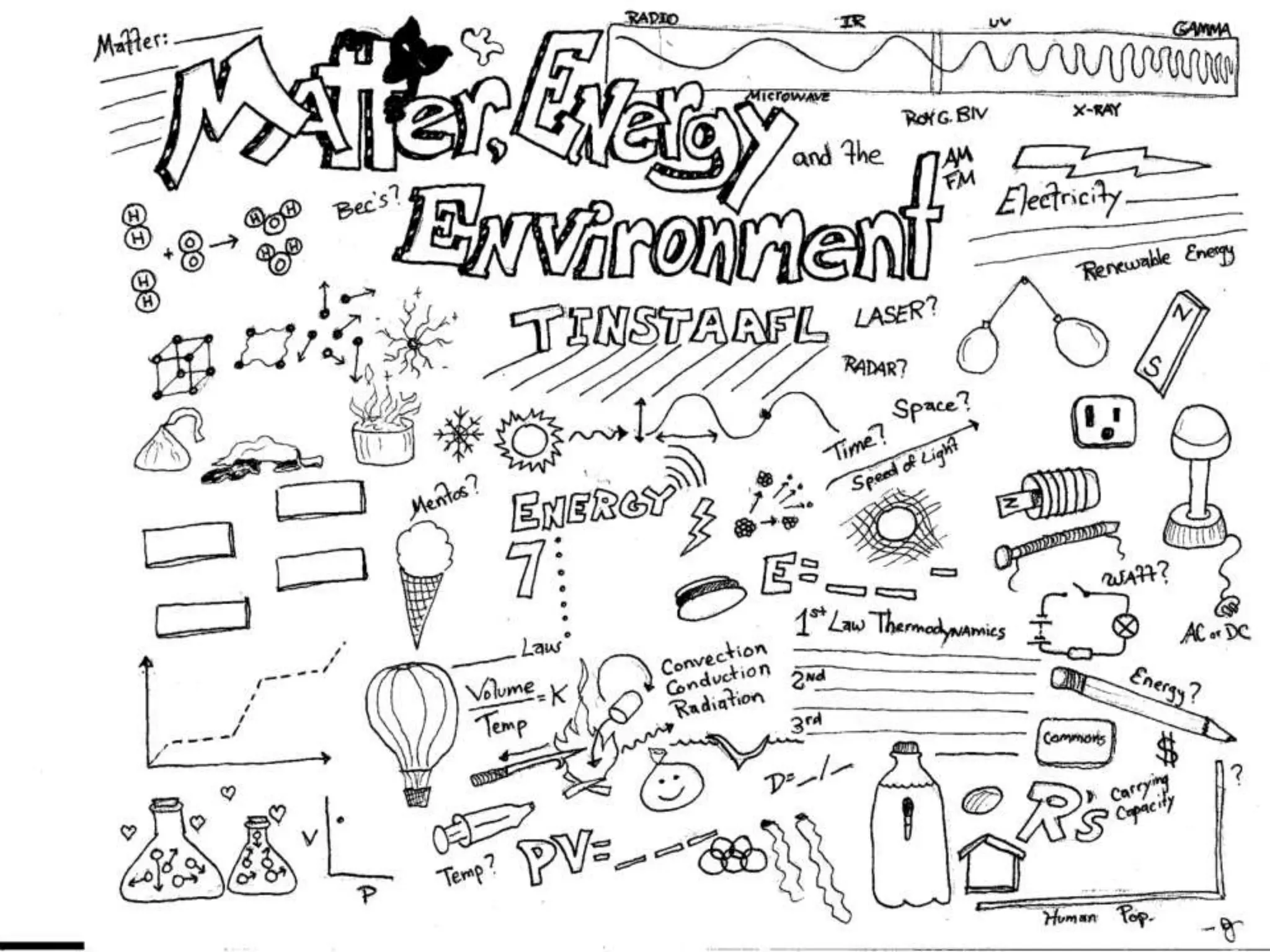
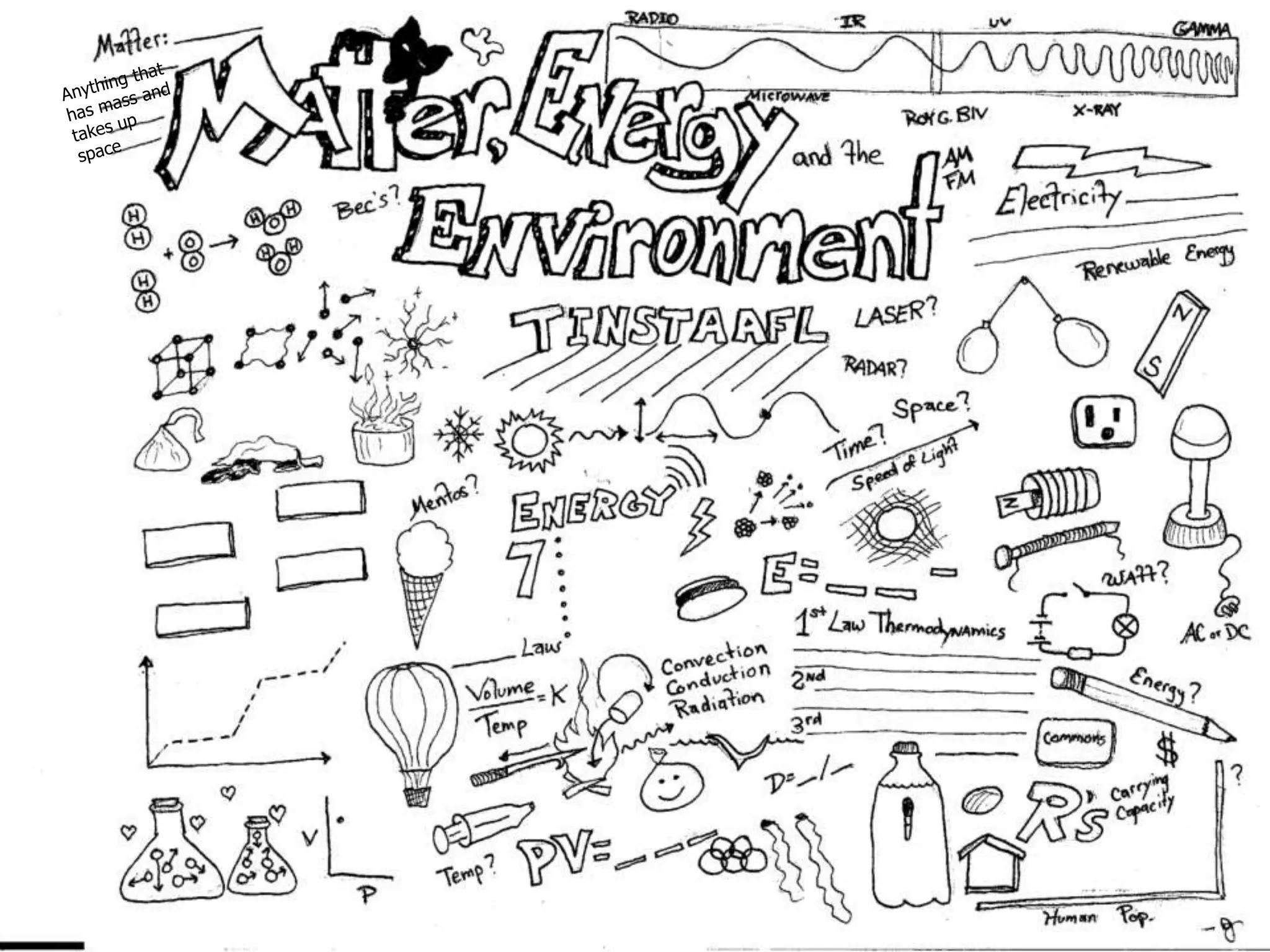
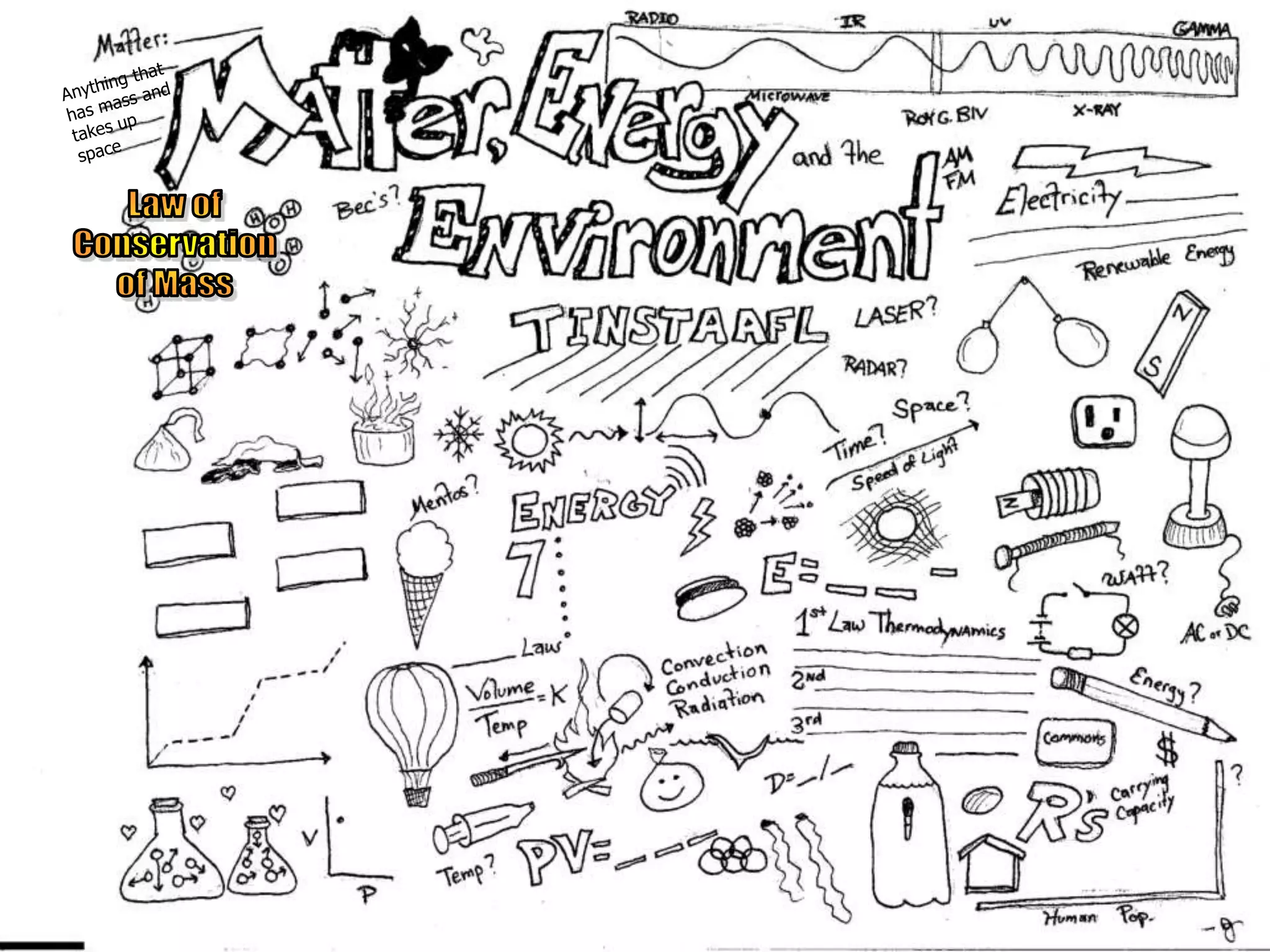
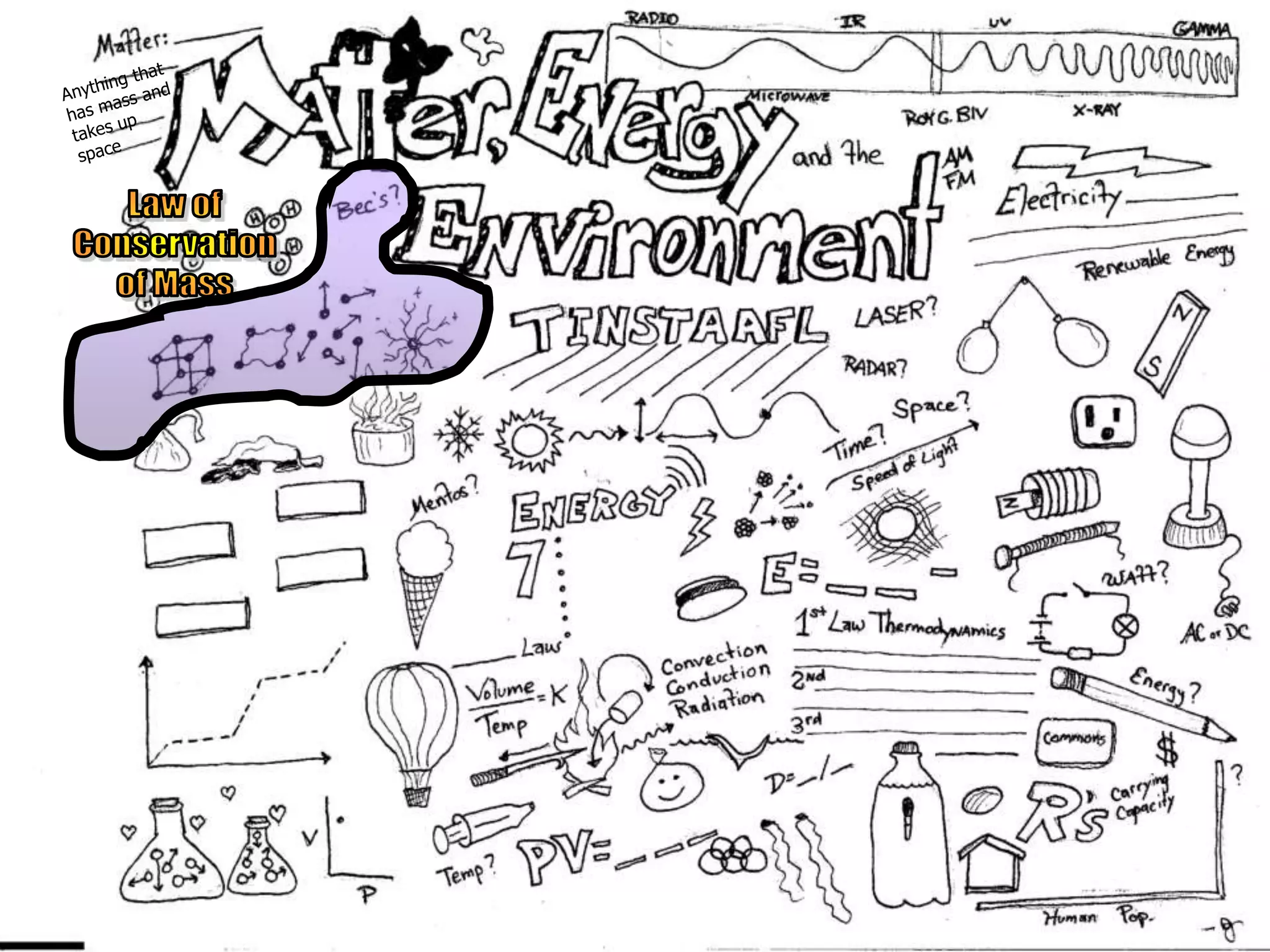
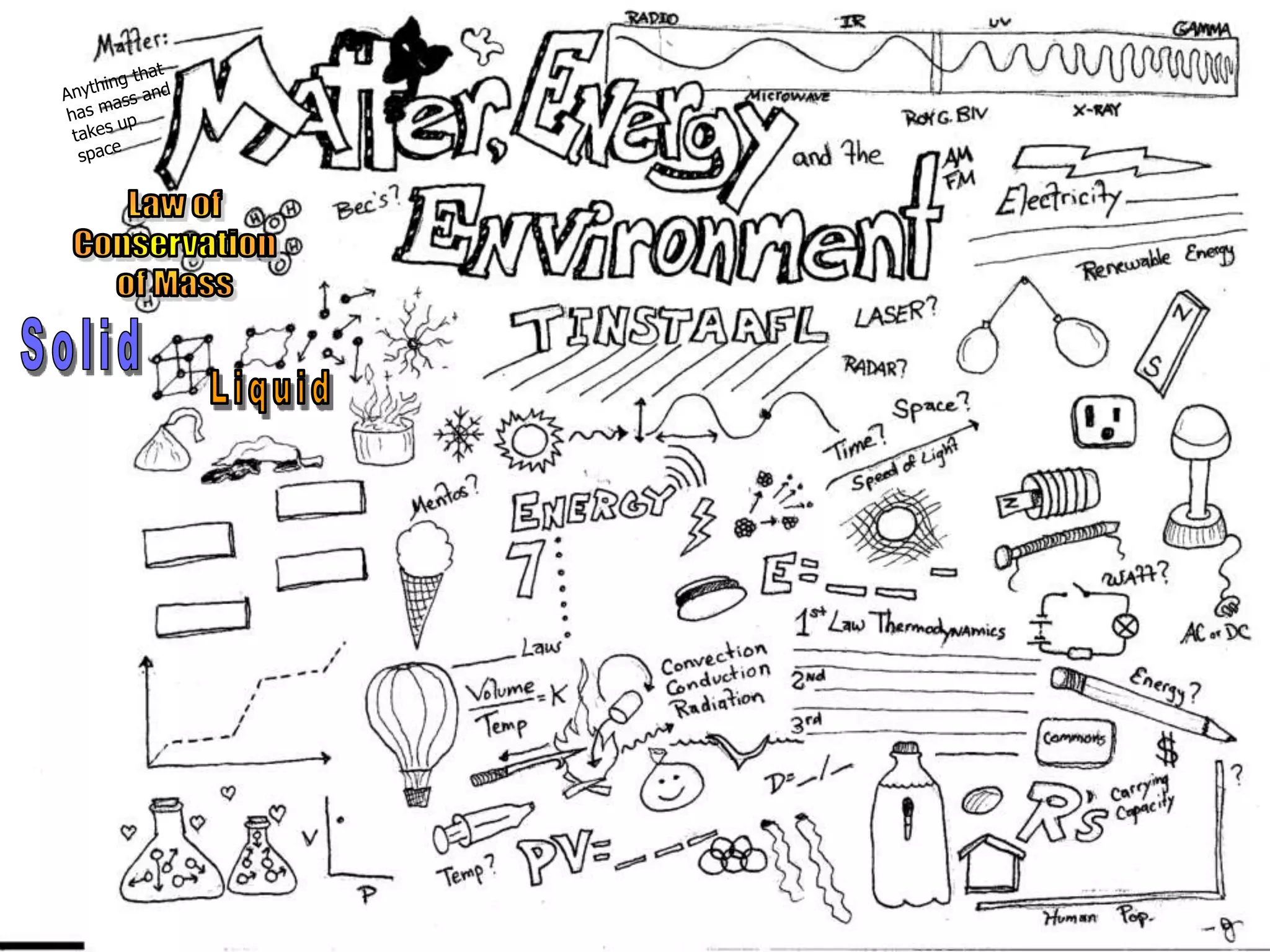
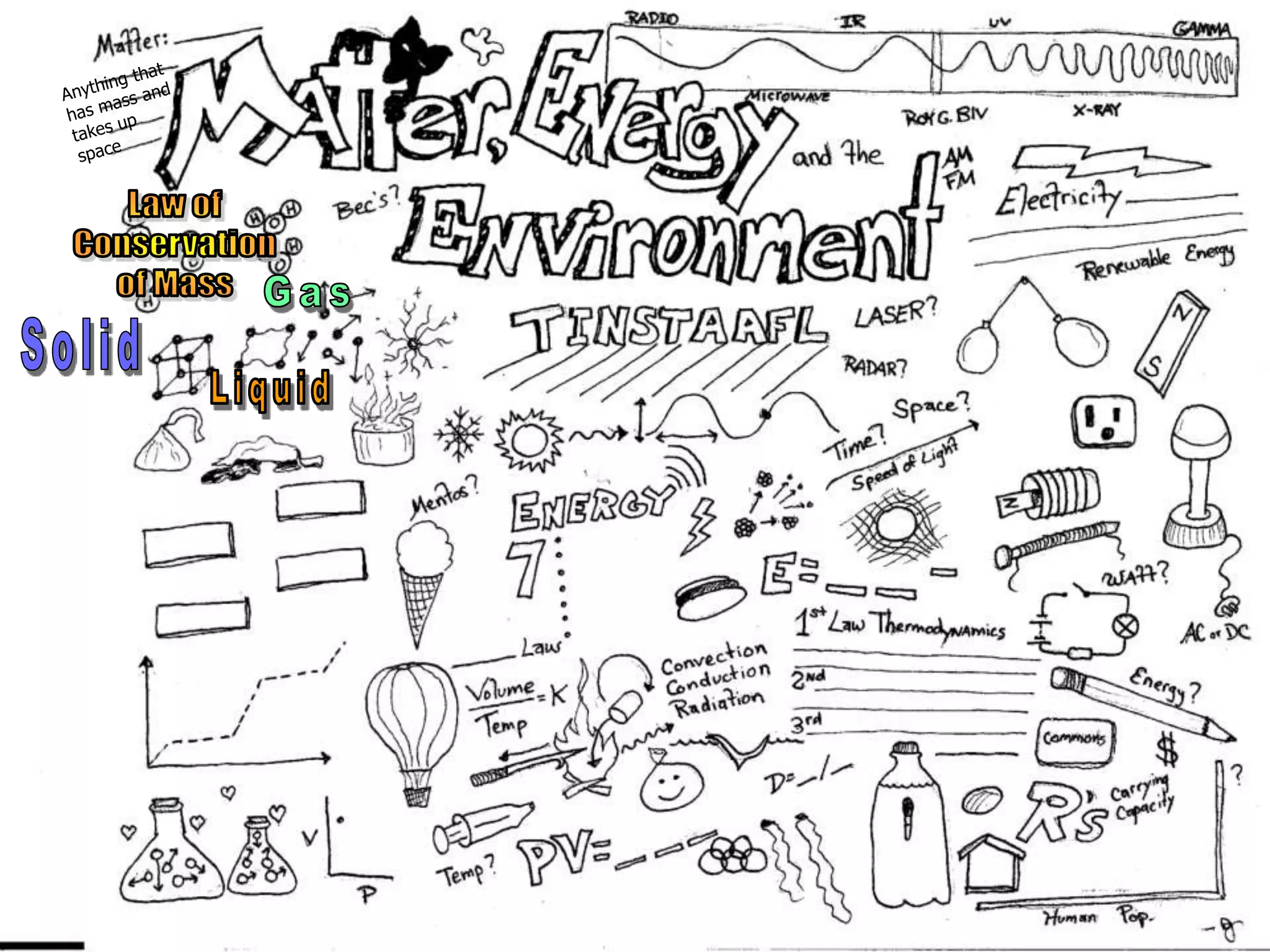
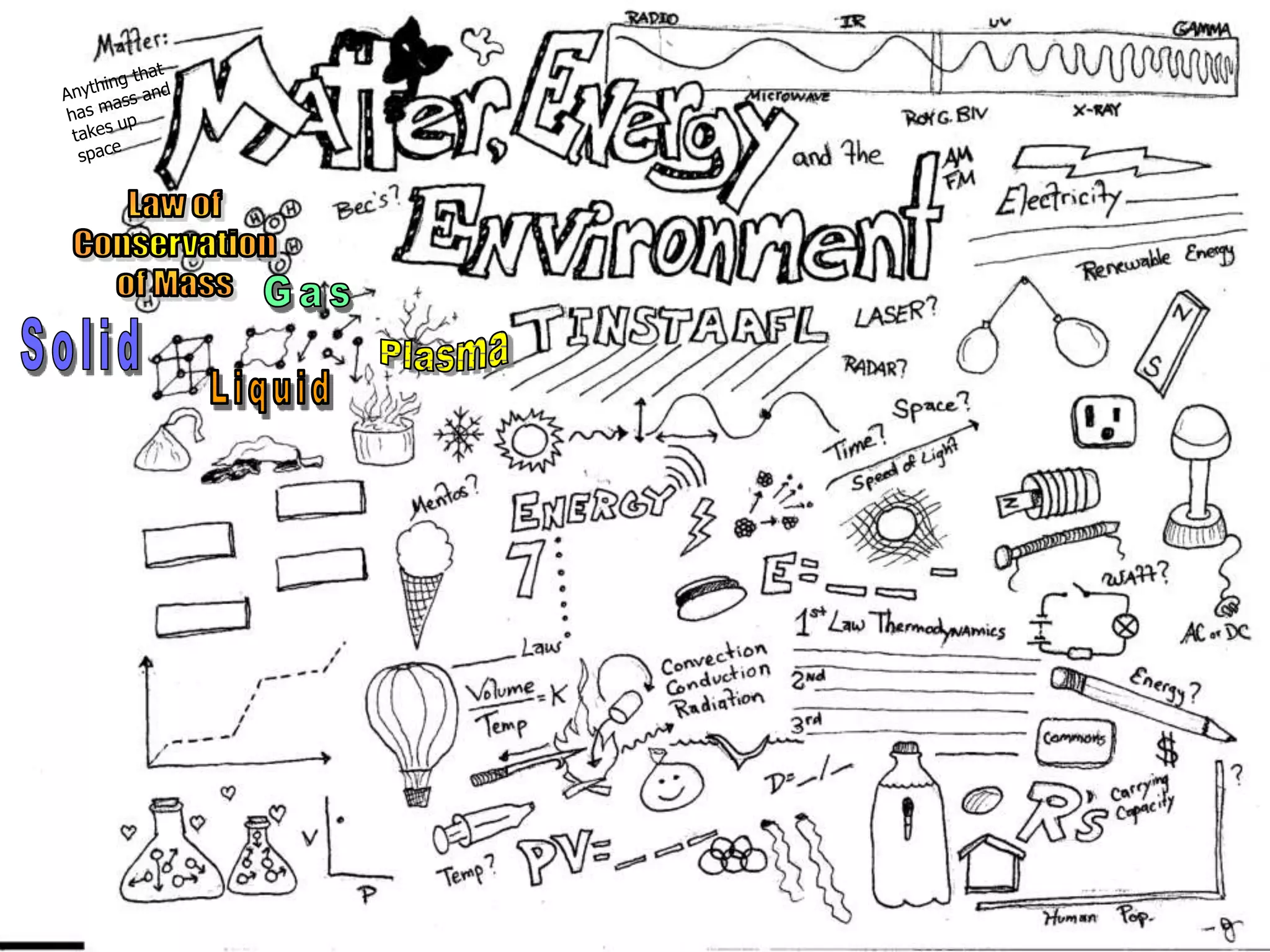
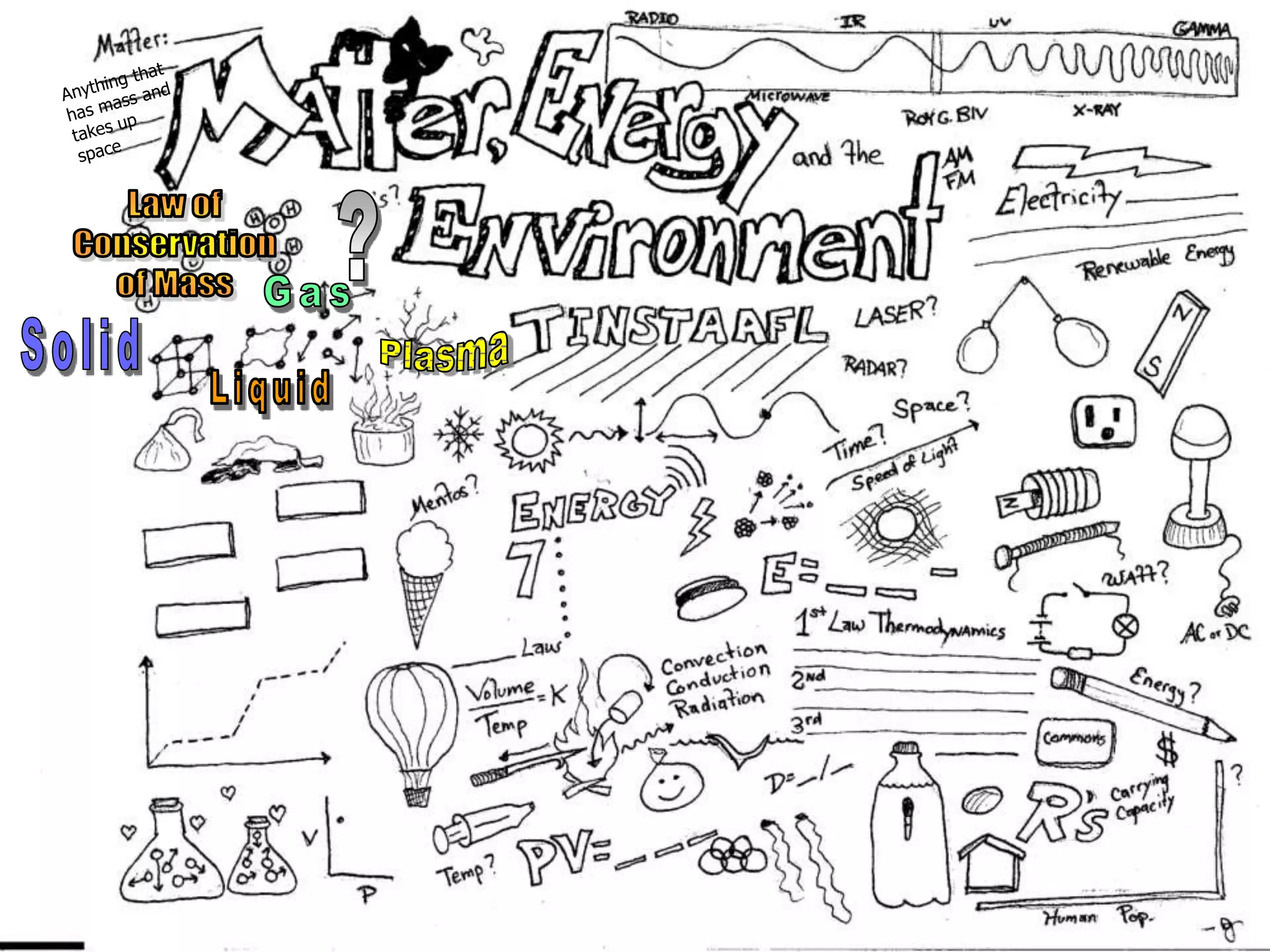
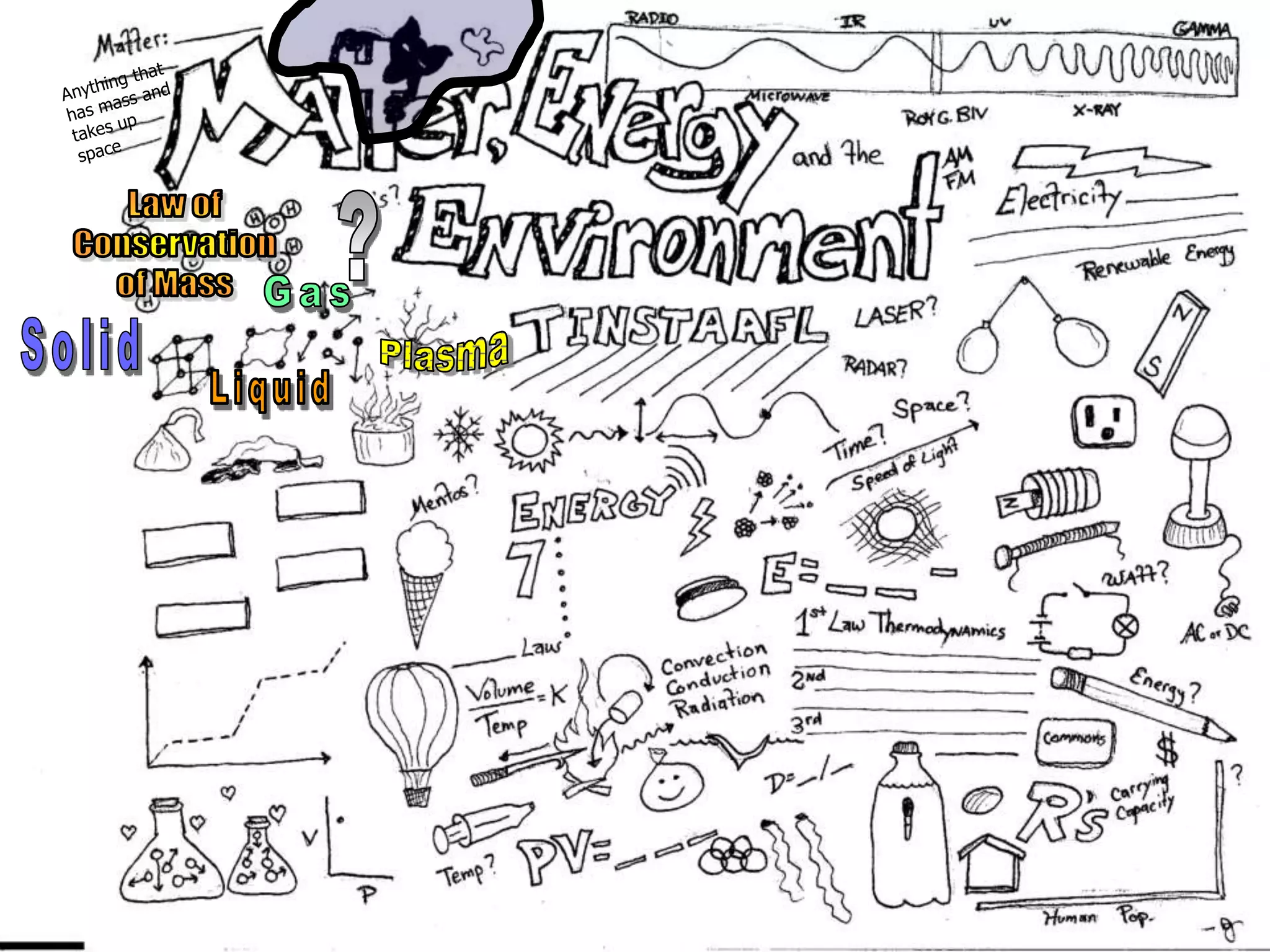
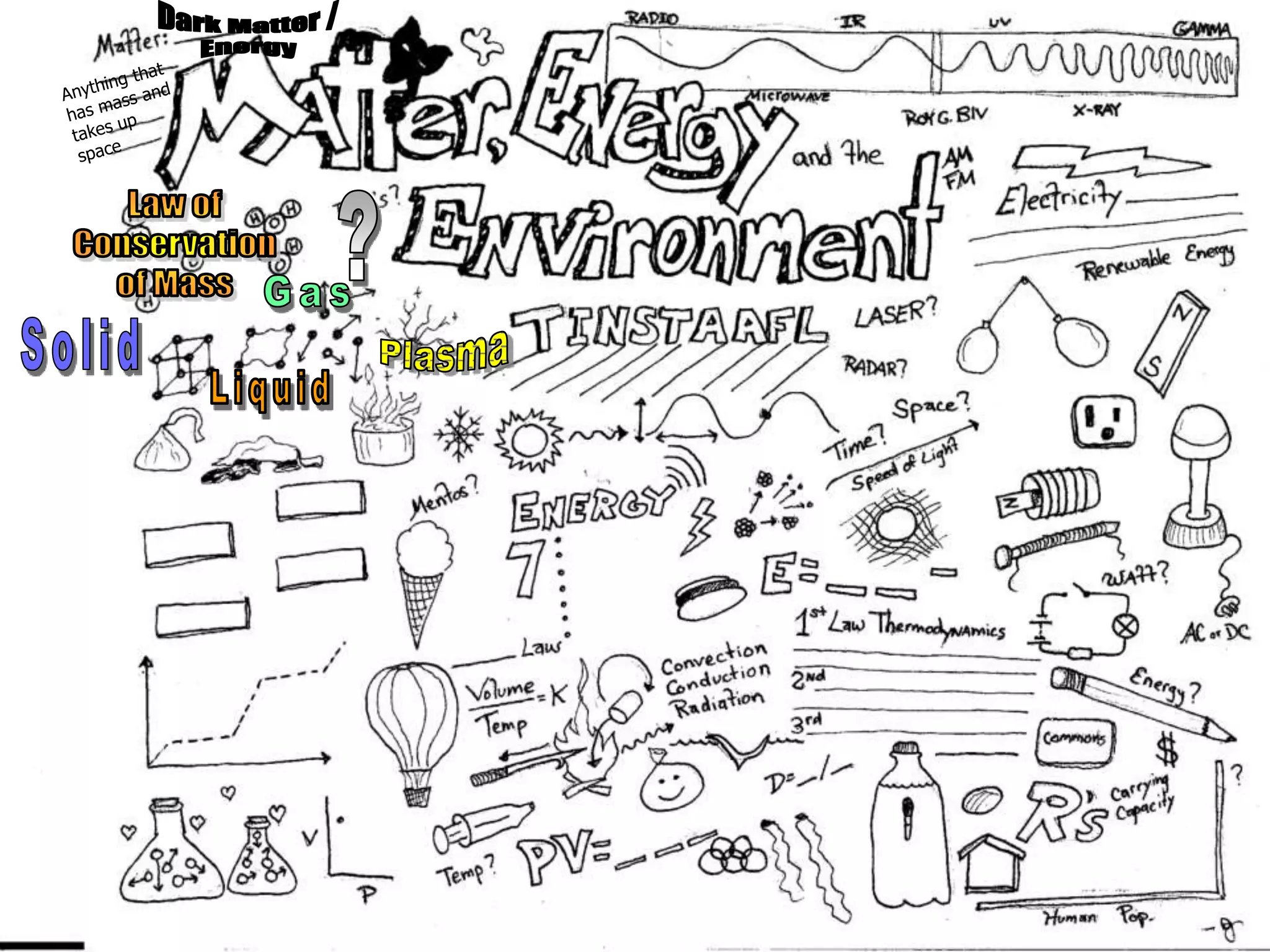
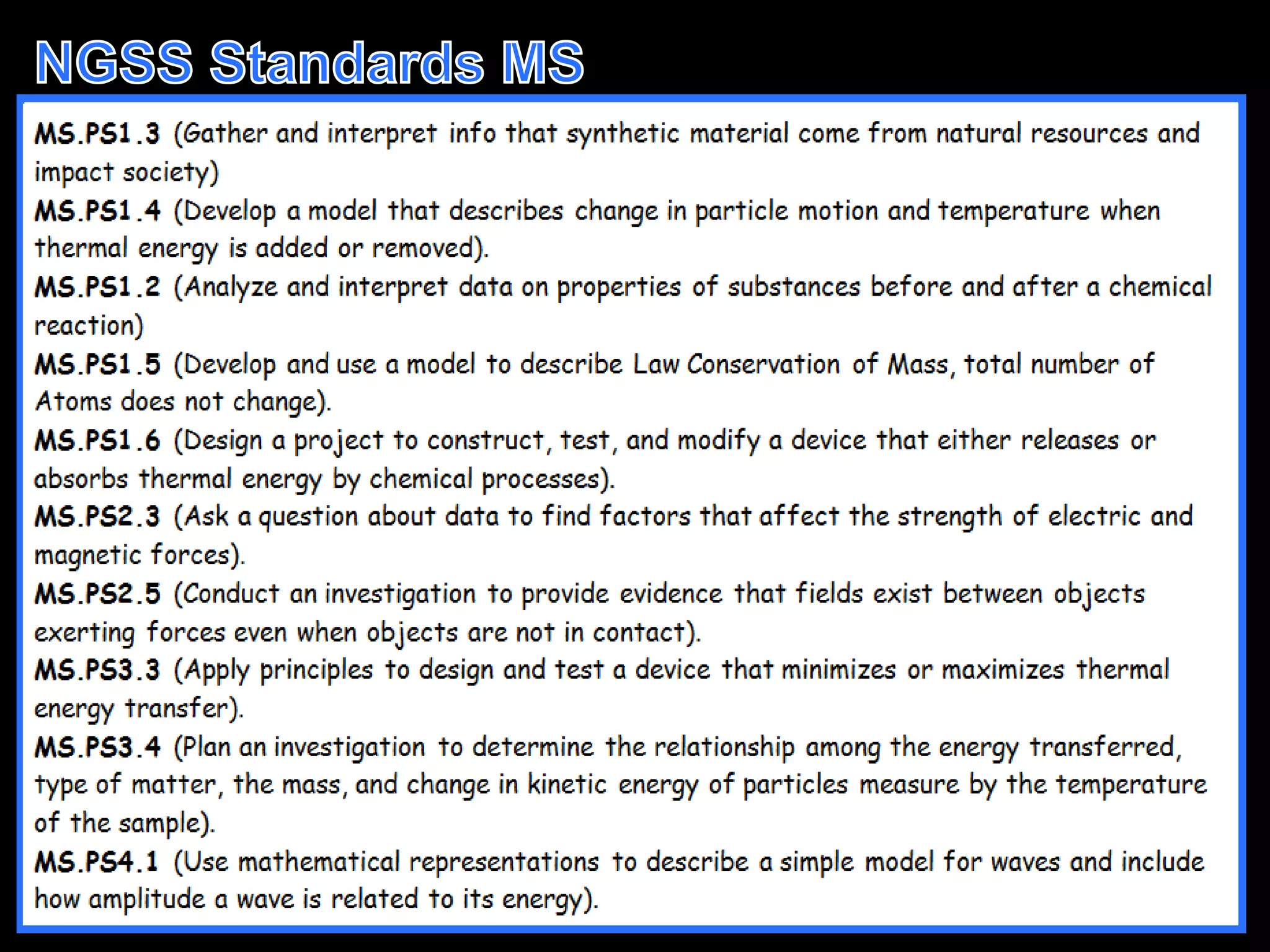
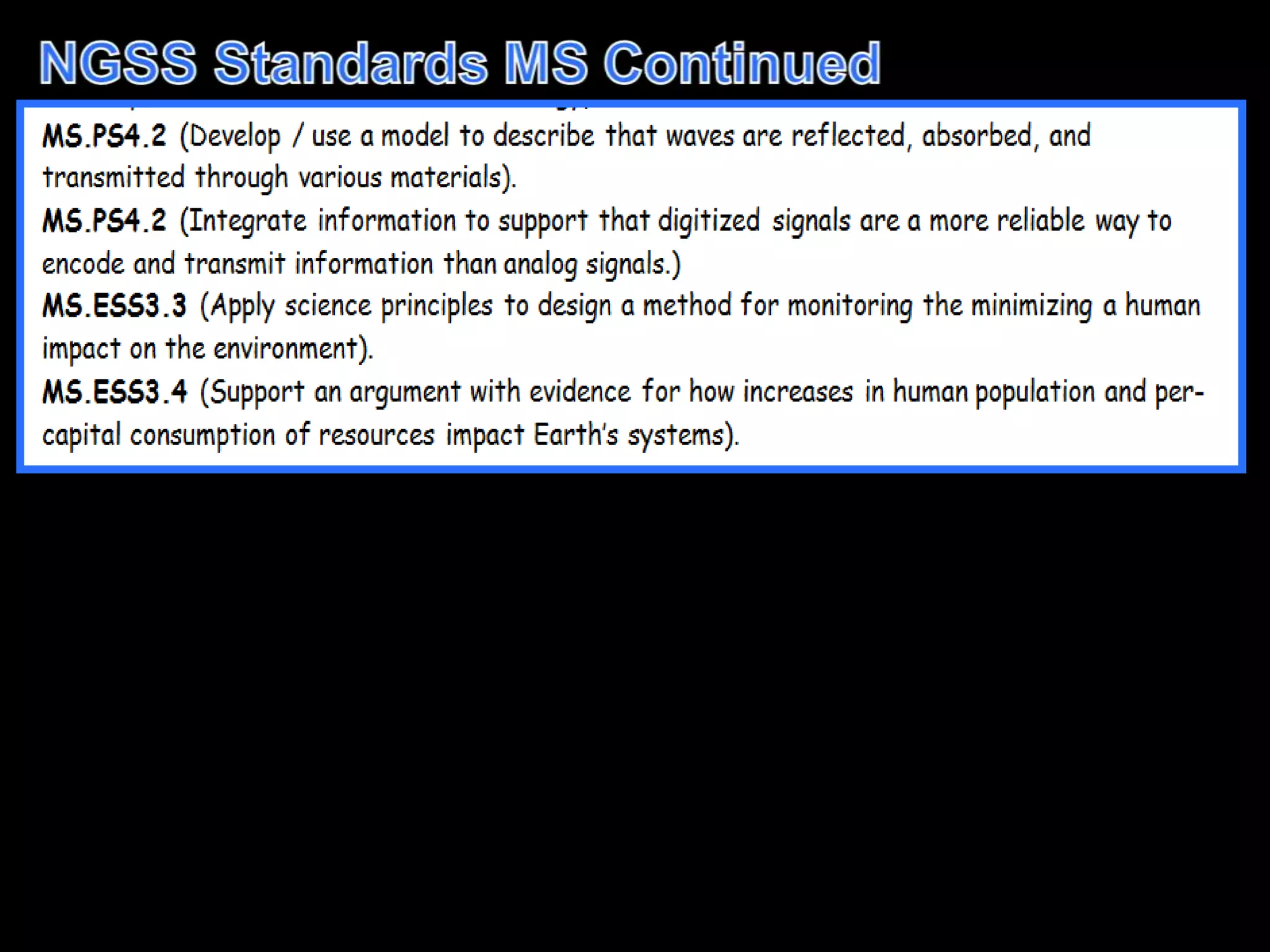
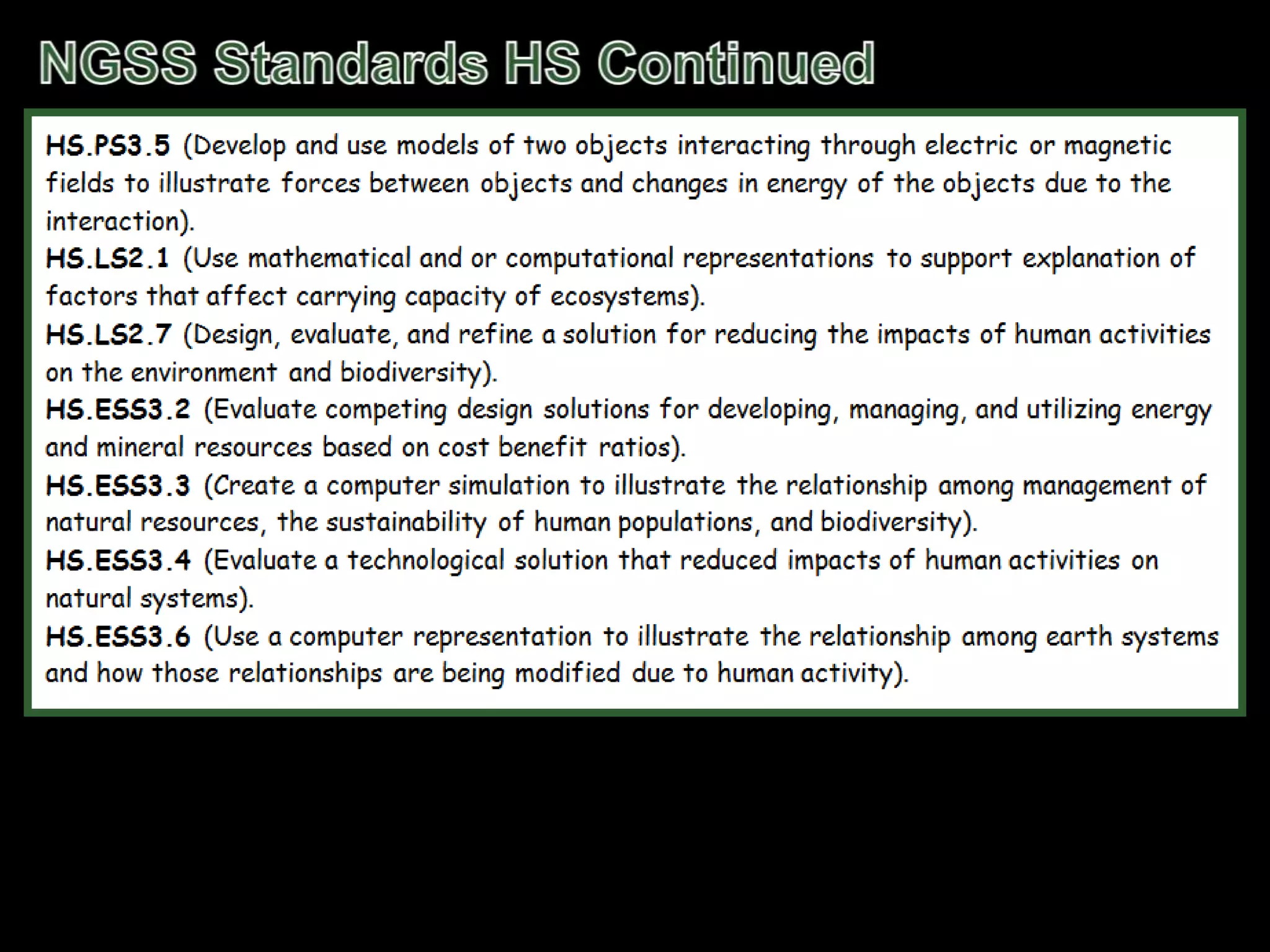
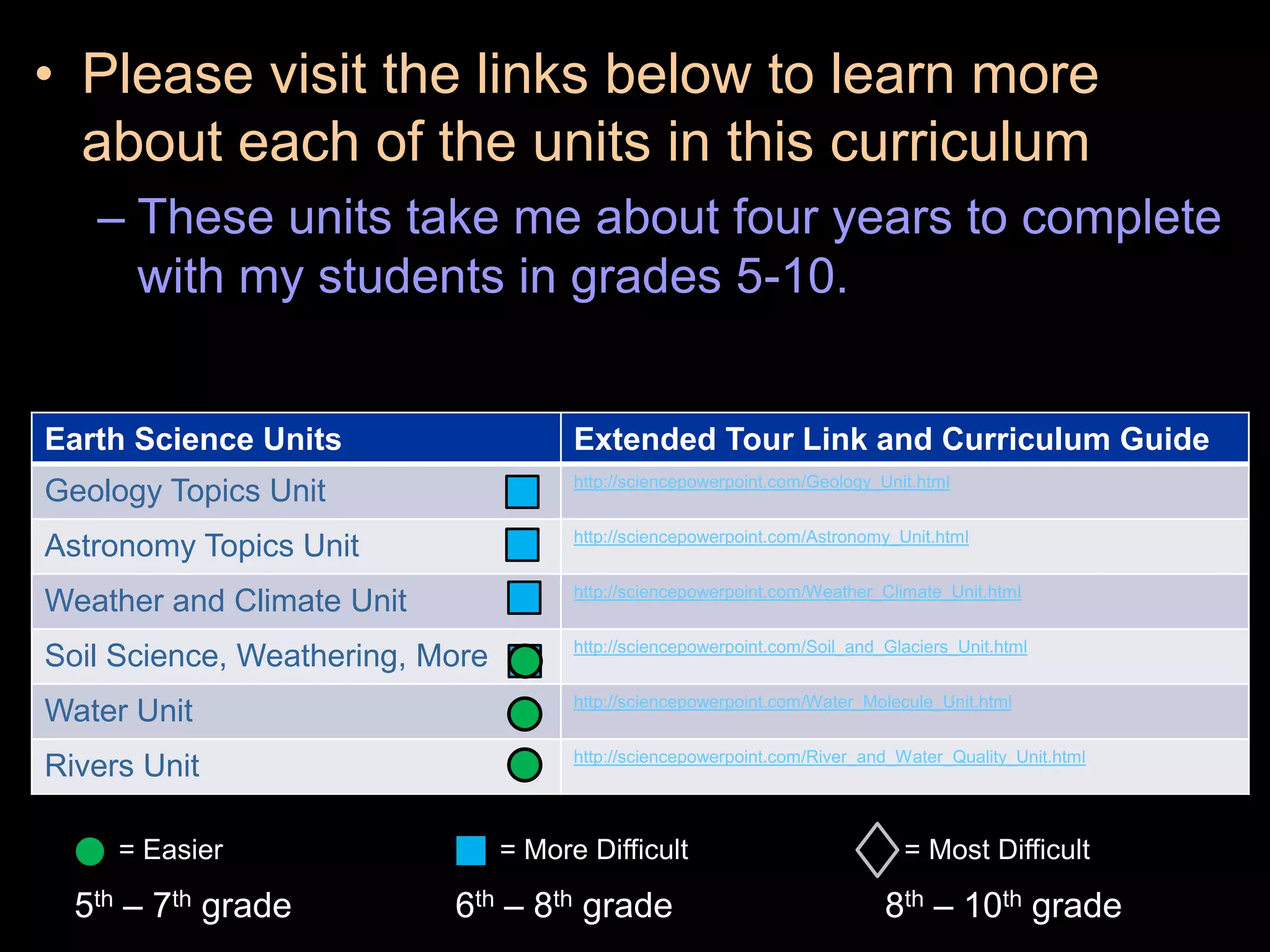
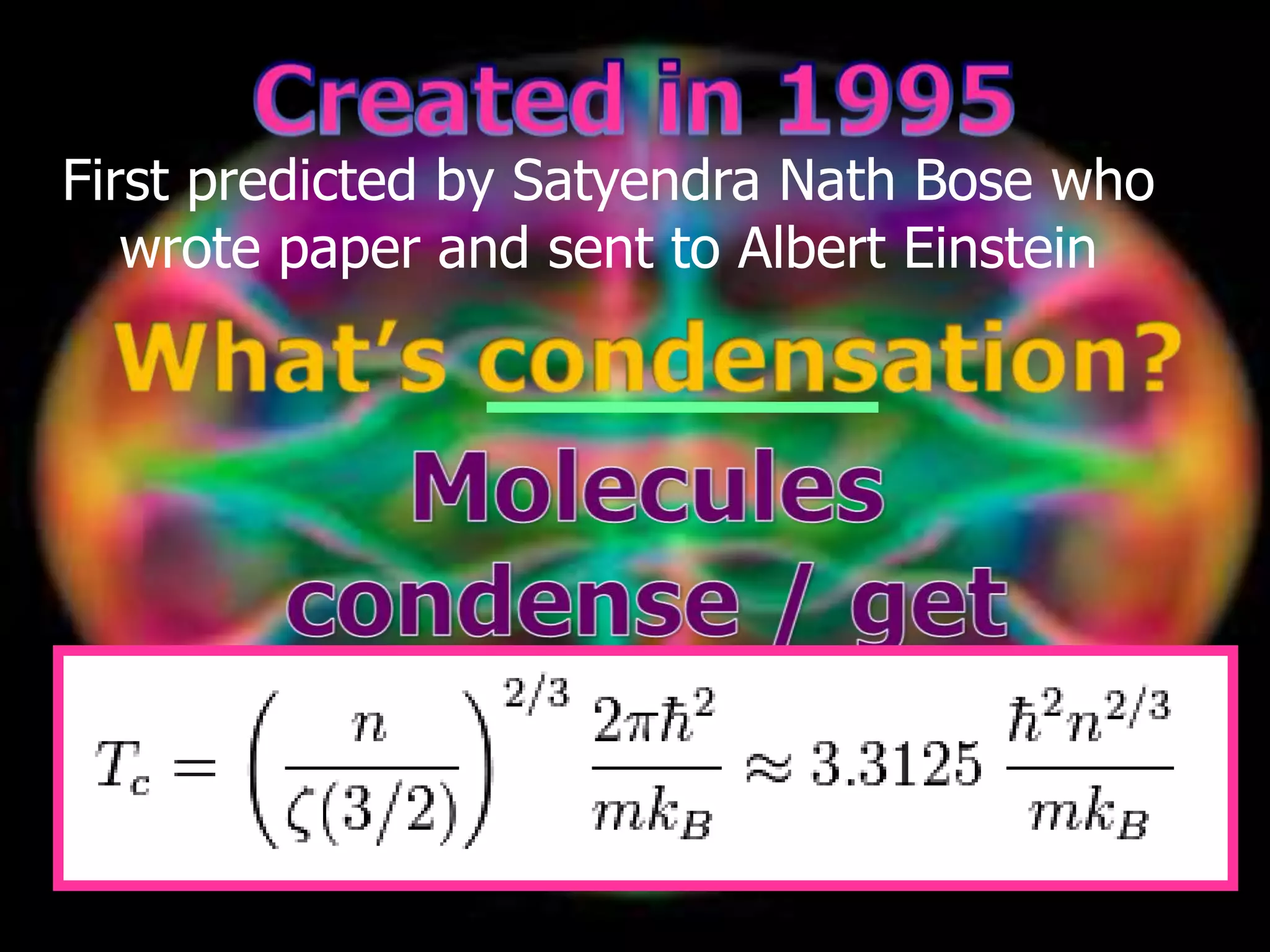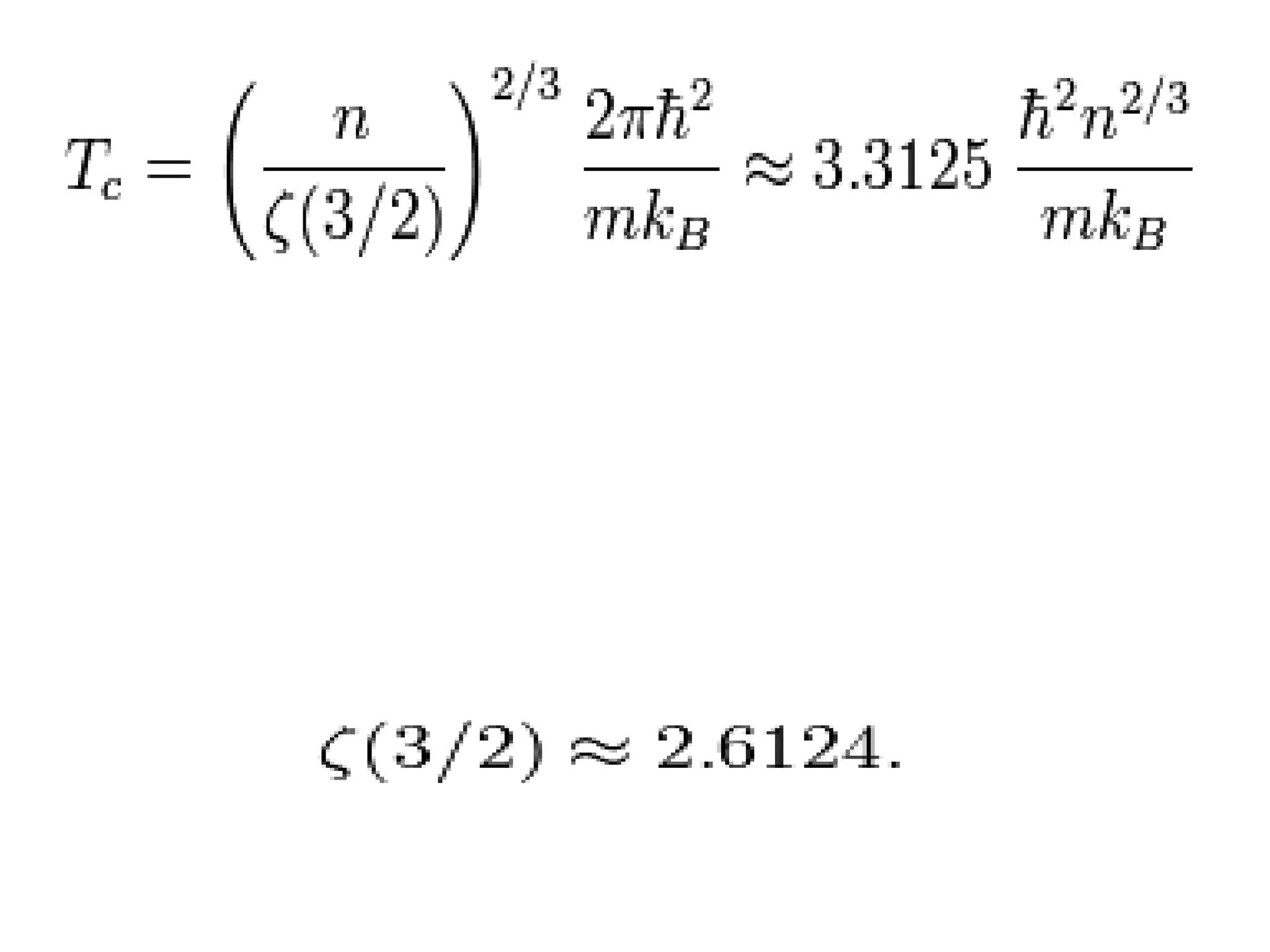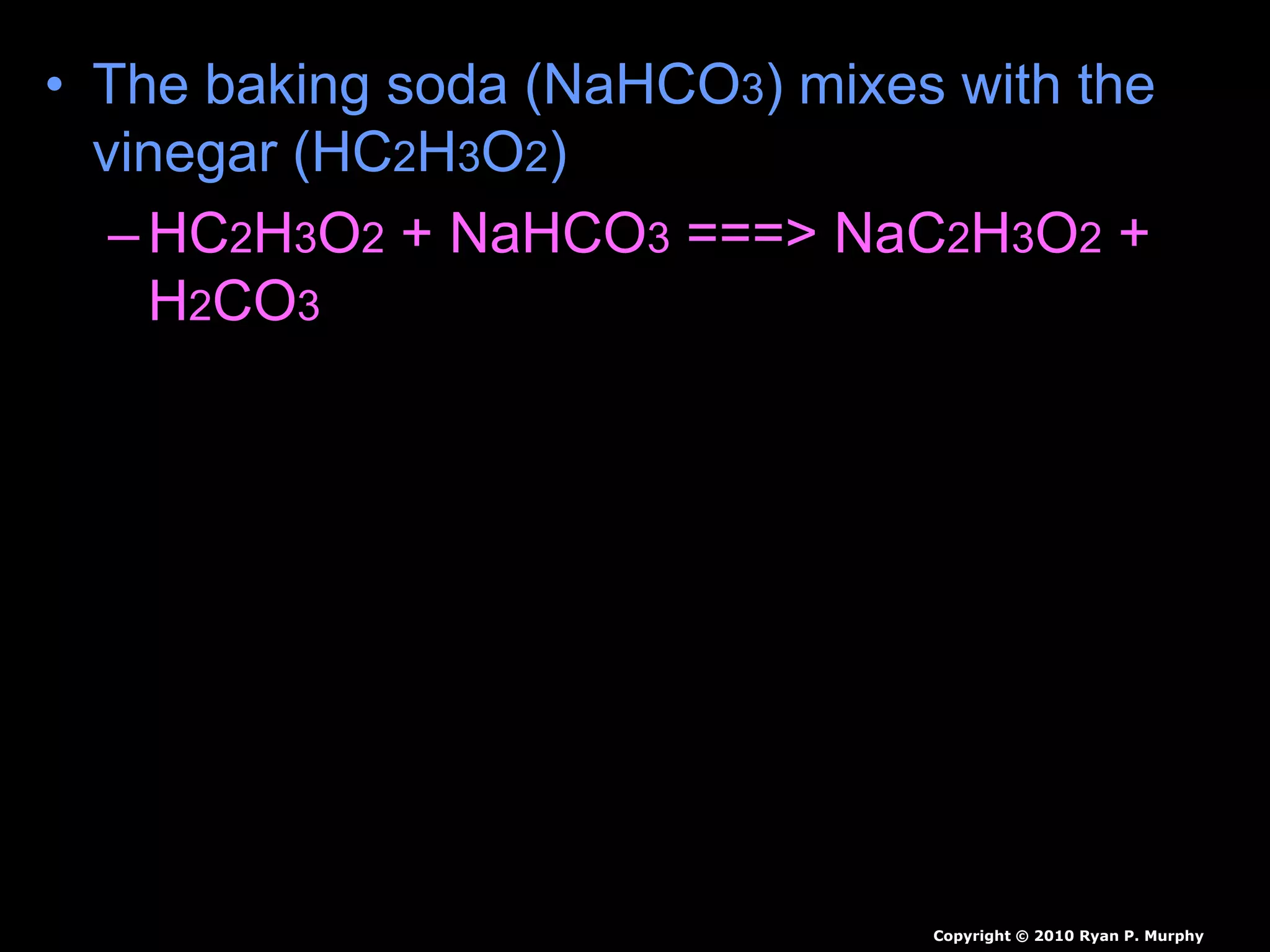The document outlines key concepts related to matter, including definitions of matter, elements, and mixtures, along with the law of conservation of matter, which states that matter is neither created nor destroyed during physical or chemical changes. It describes various experiments to demonstrate these principles, including candle combustion and the interaction of Alka-Seltzer with water. Additionally, it touches on the kinetic molecular theory, emphasizing that molecules are in constant motion and this behavior varies among the states of matter.