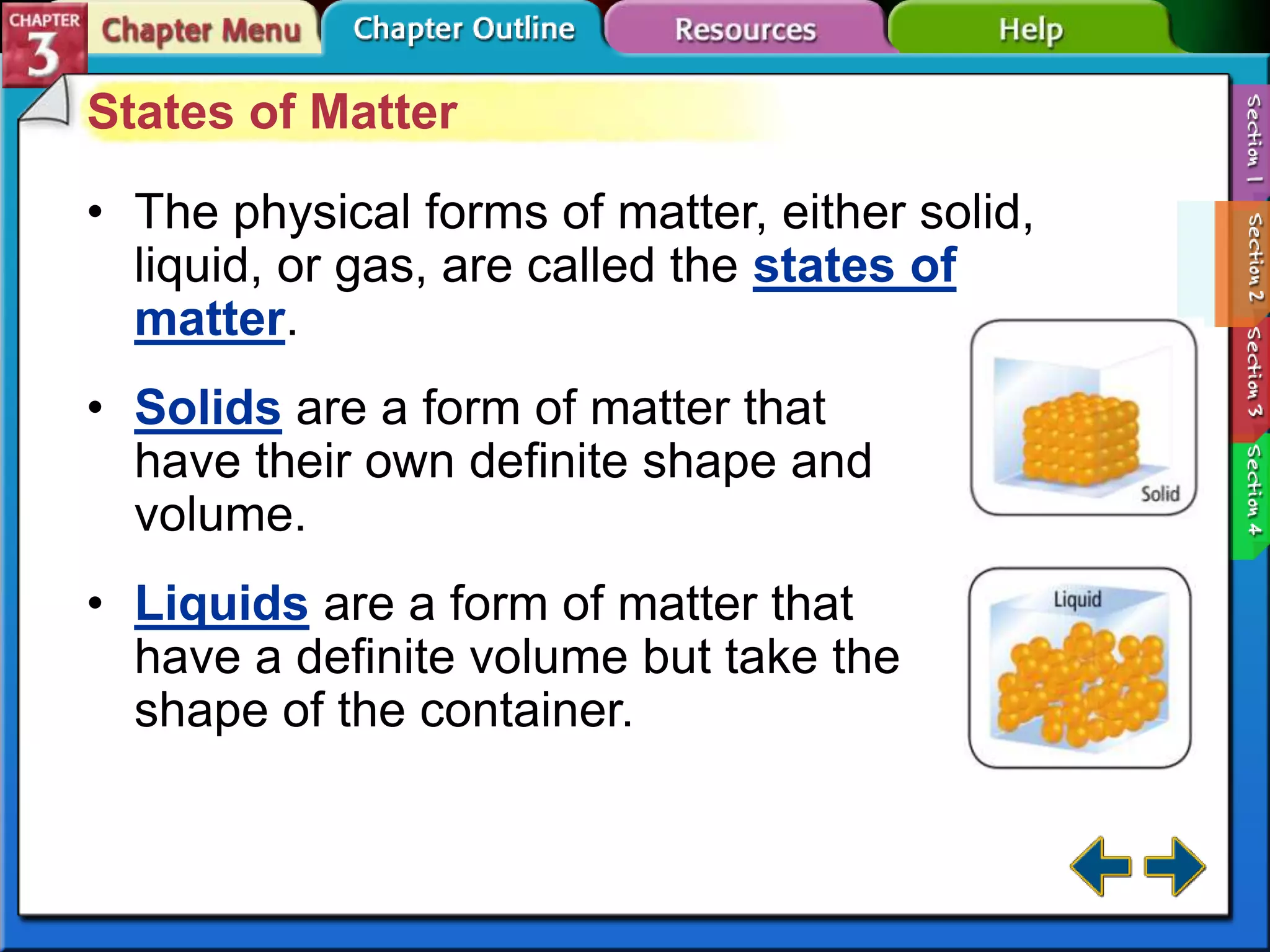
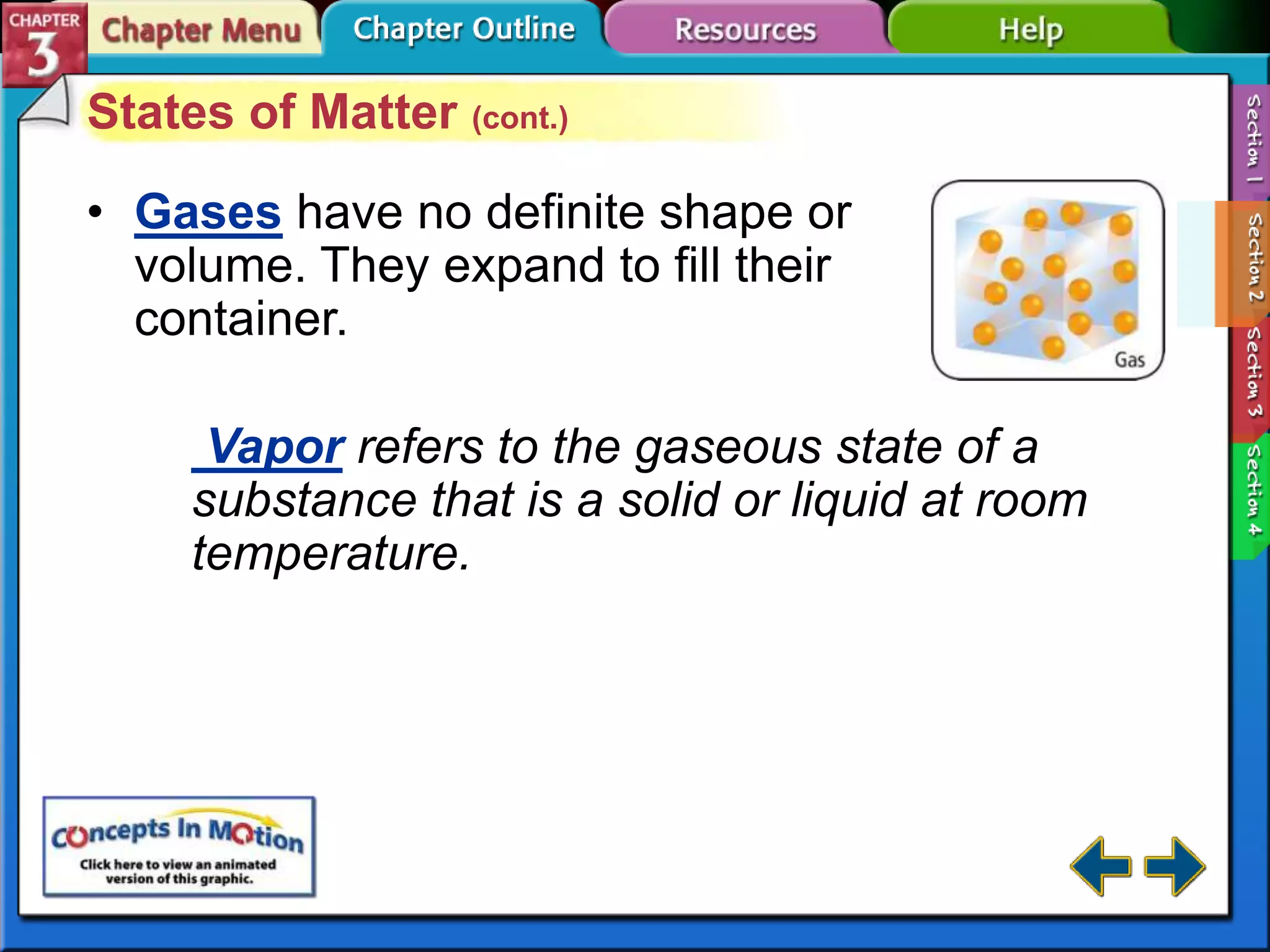
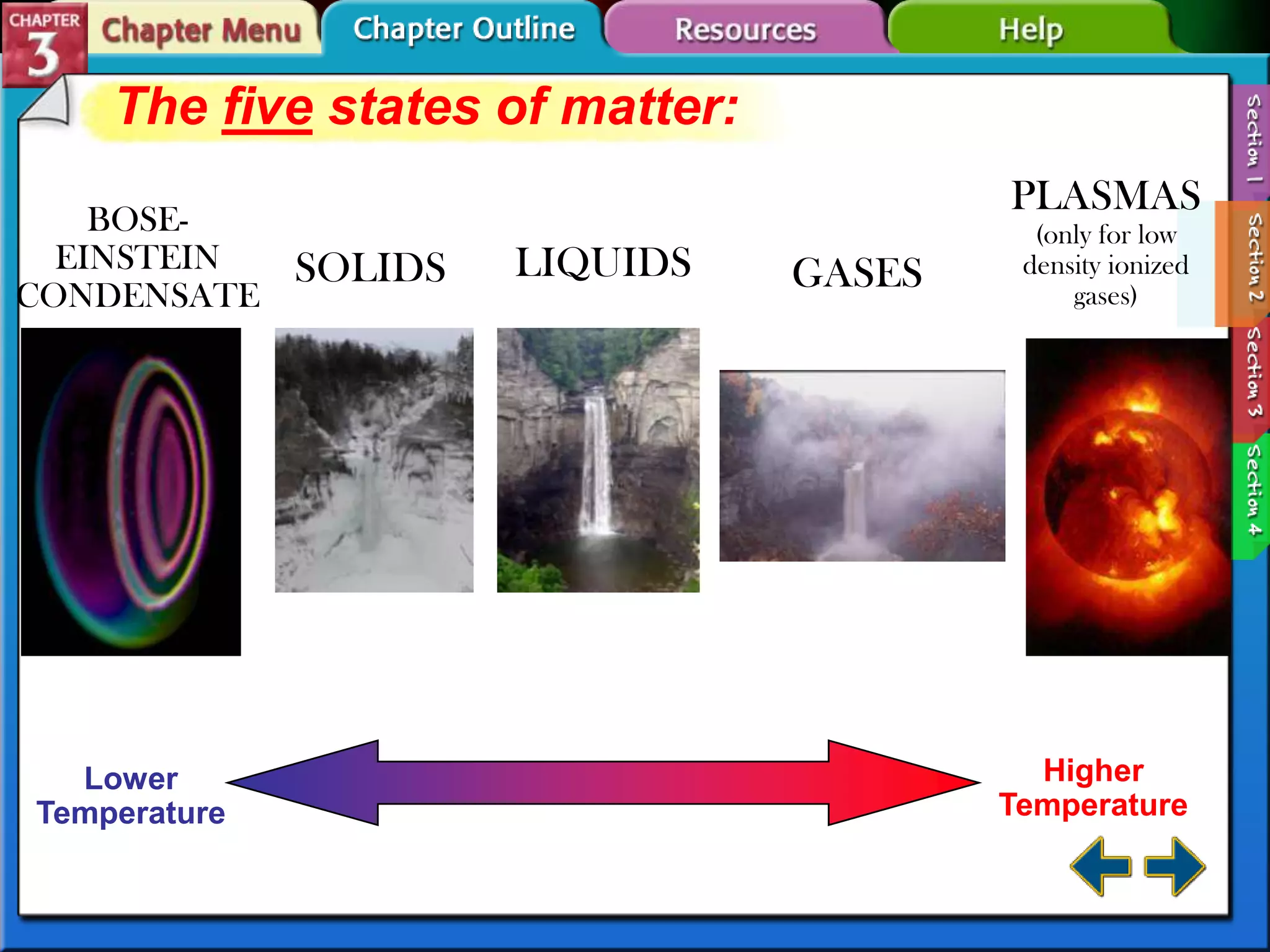
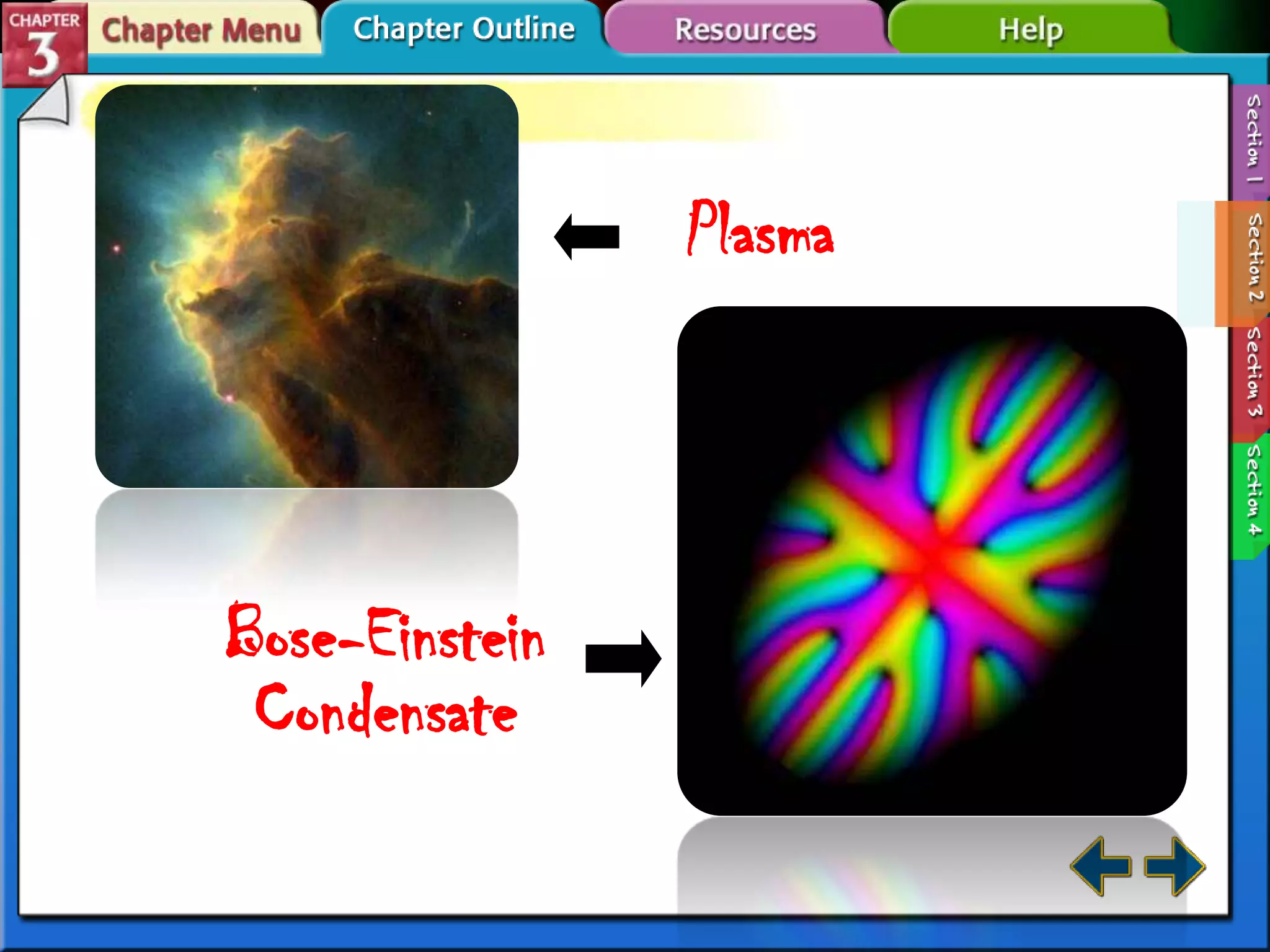
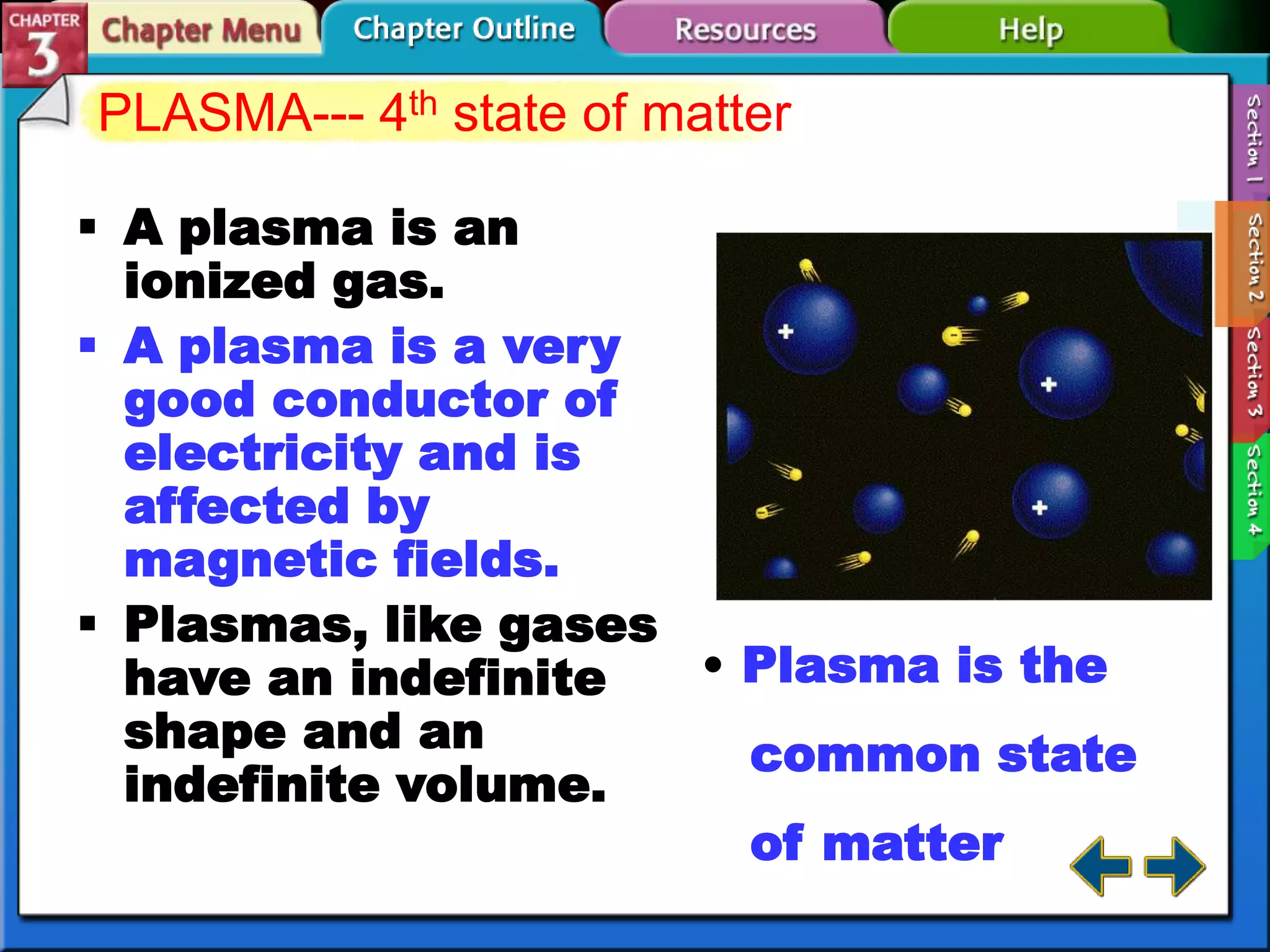
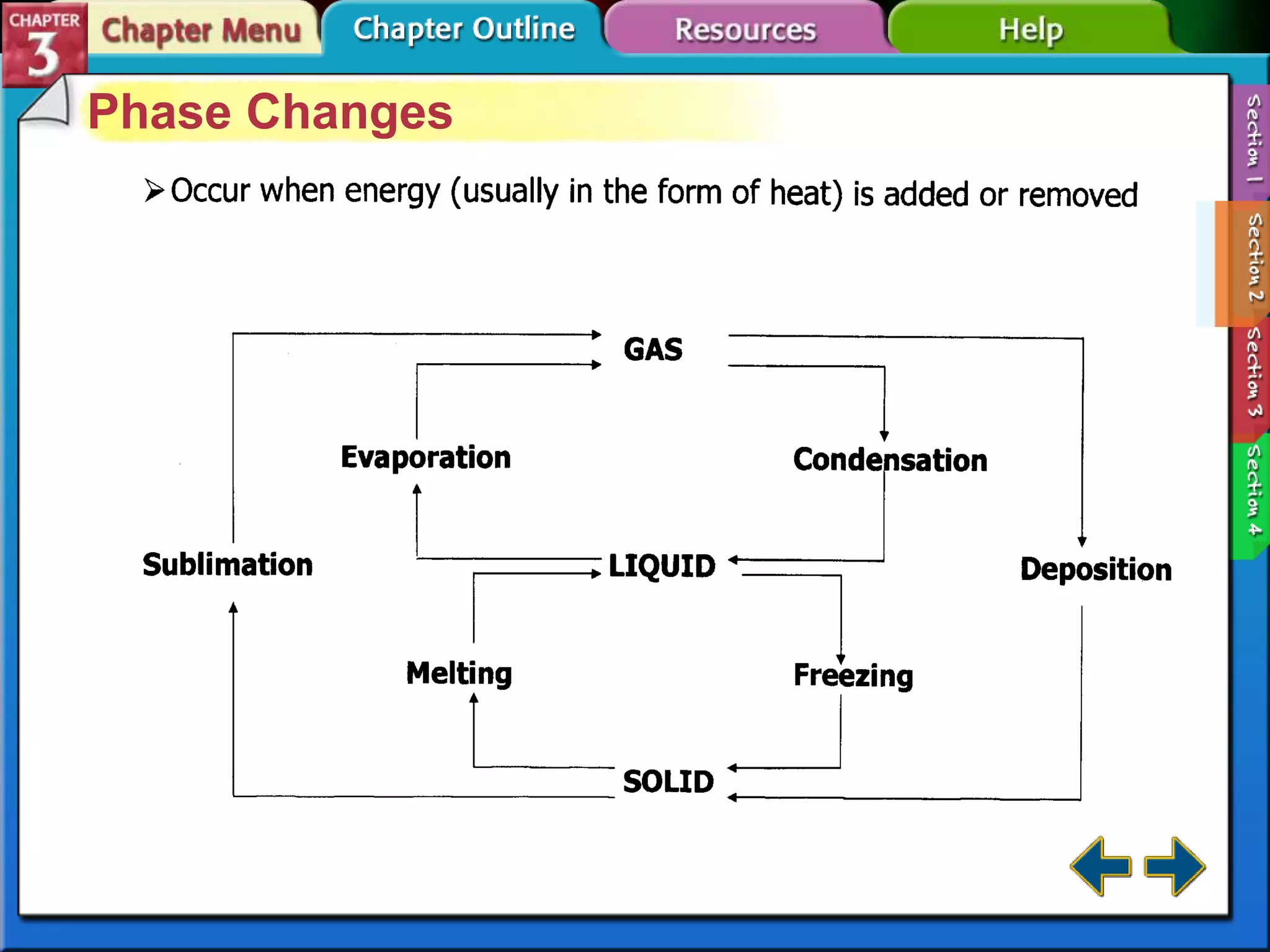
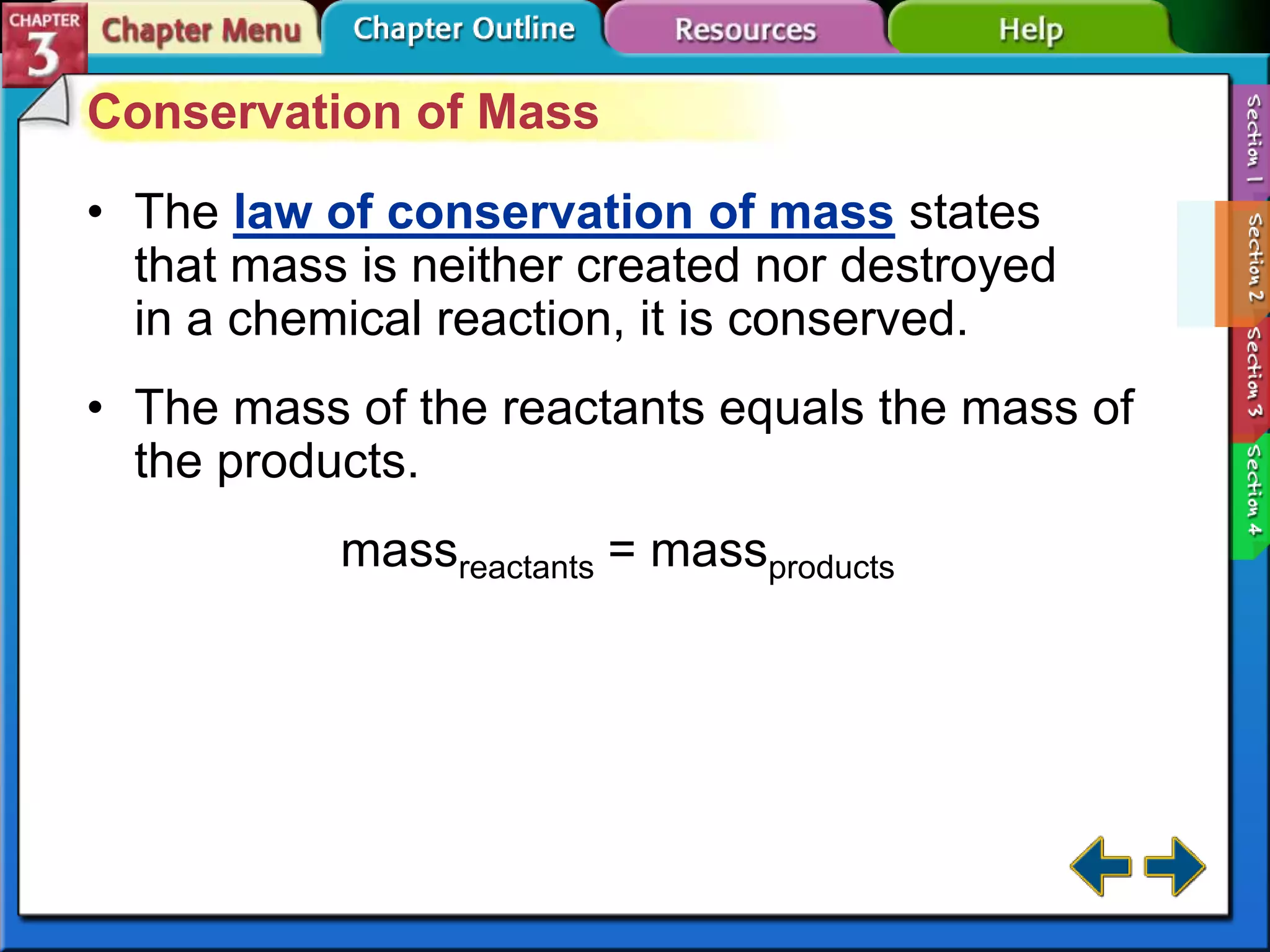
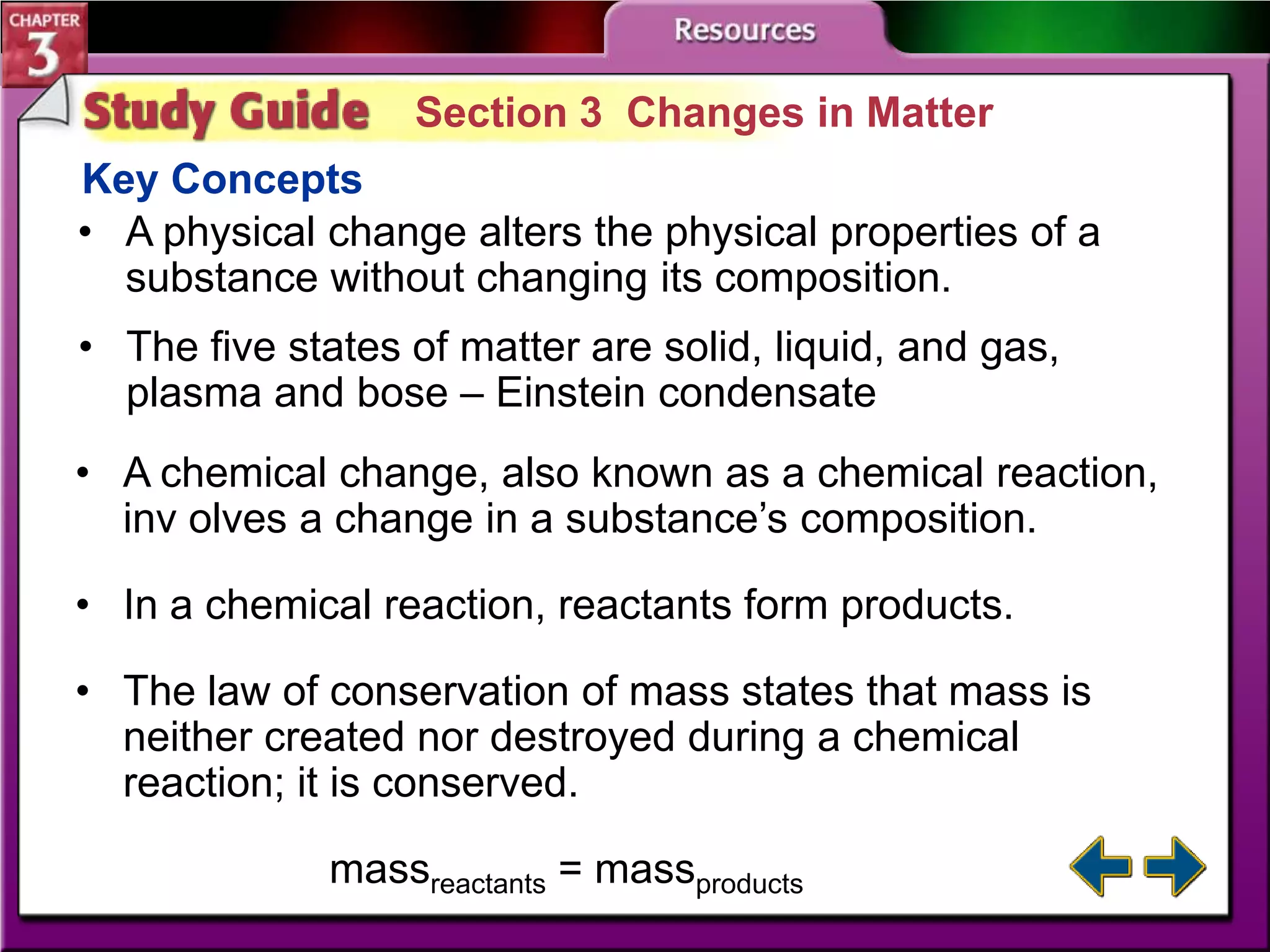
This document discusses different types of changes in matter. It defines physical changes as changes that alter a substance's properties without changing its composition, giving examples like breaking, cutting, dissolving, and changes in state. Chemical changes are defined as changes involving one or more substances turning into new substances. The five states of matter are identified as solid, liquid, gas, plasma and Bose-Einstein condensate. Phase changes between these states are explained. The law of conservation of mass is also defined as mass being neither created nor destroyed in chemical reactions.