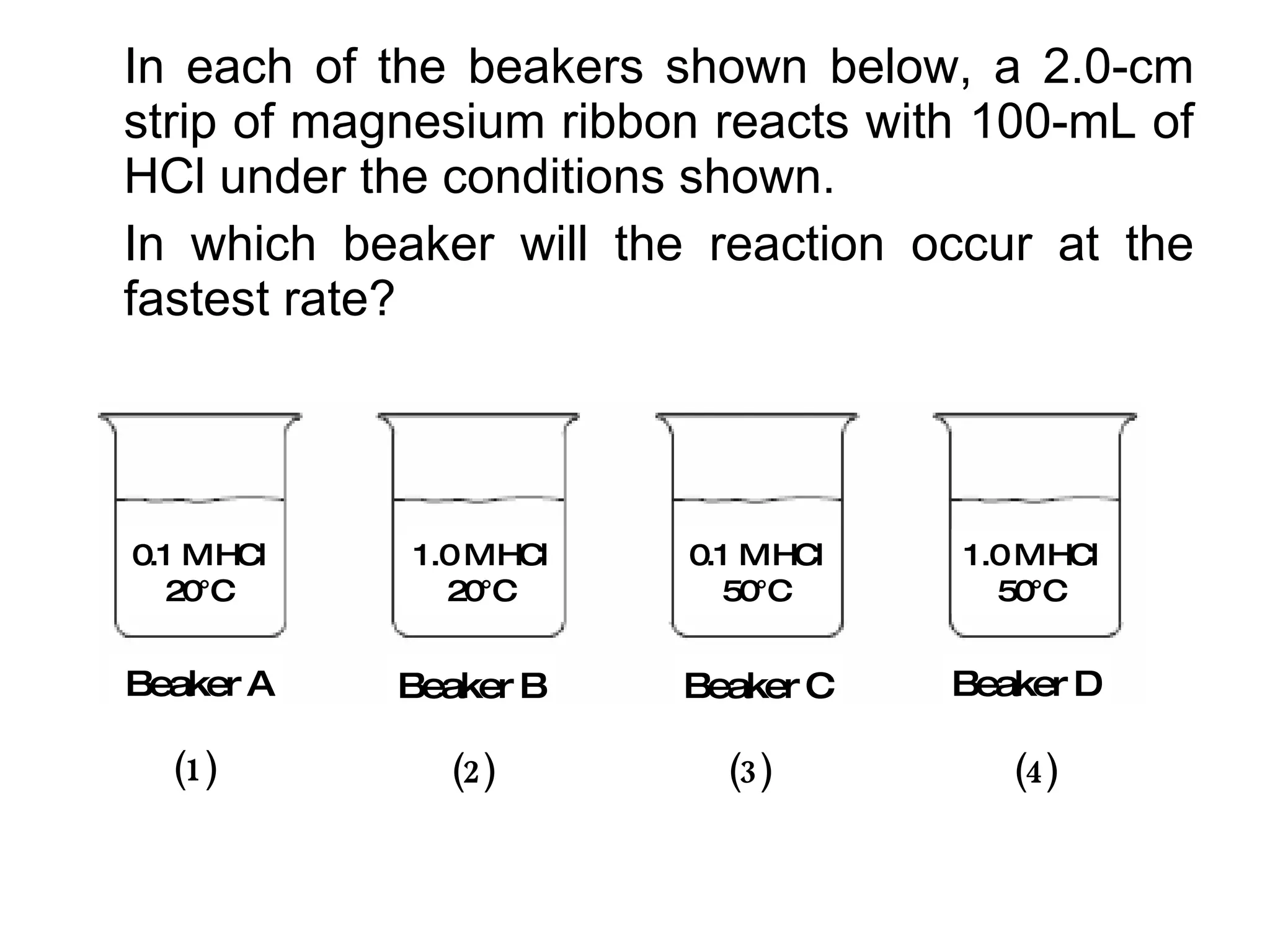
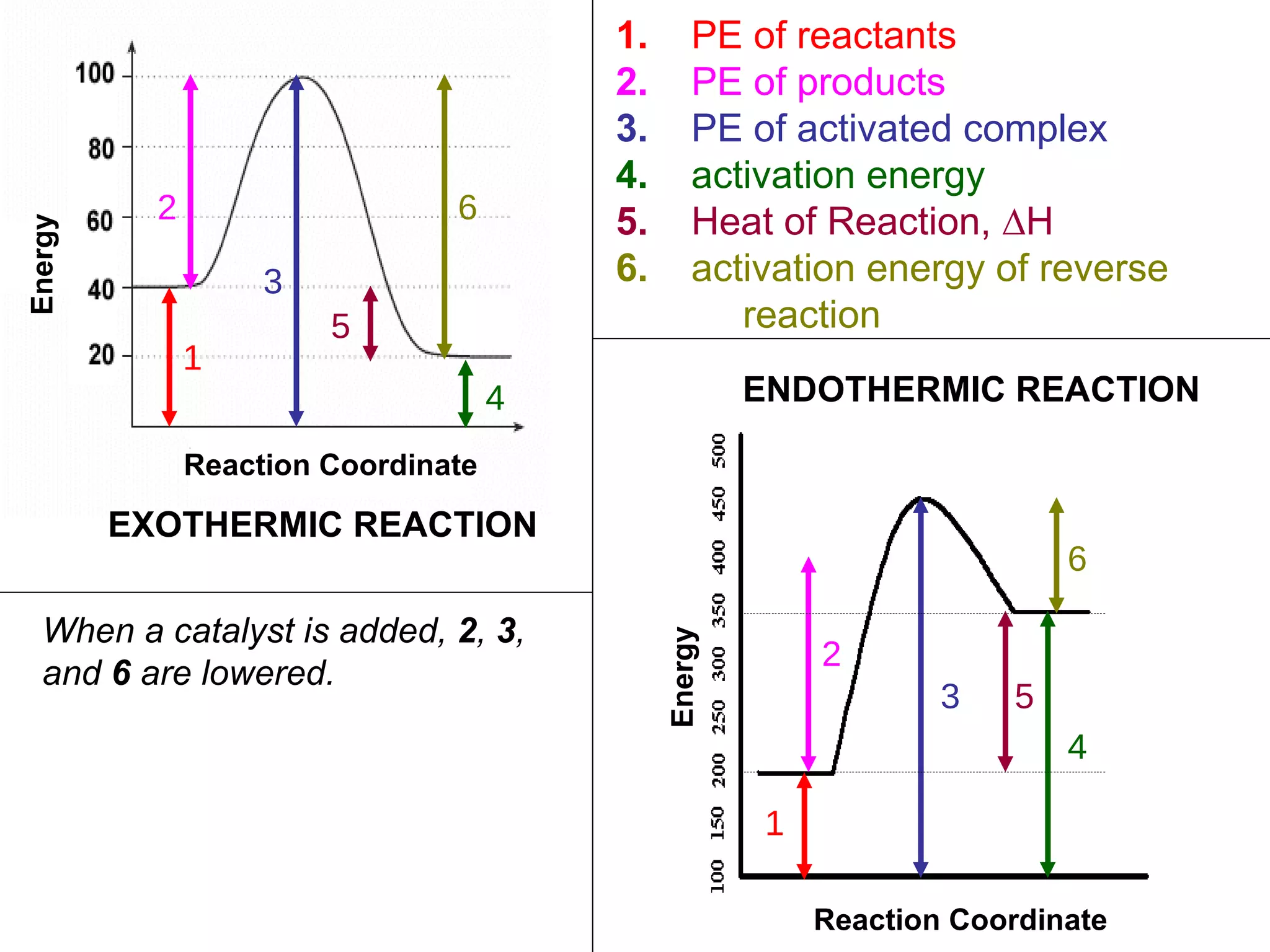
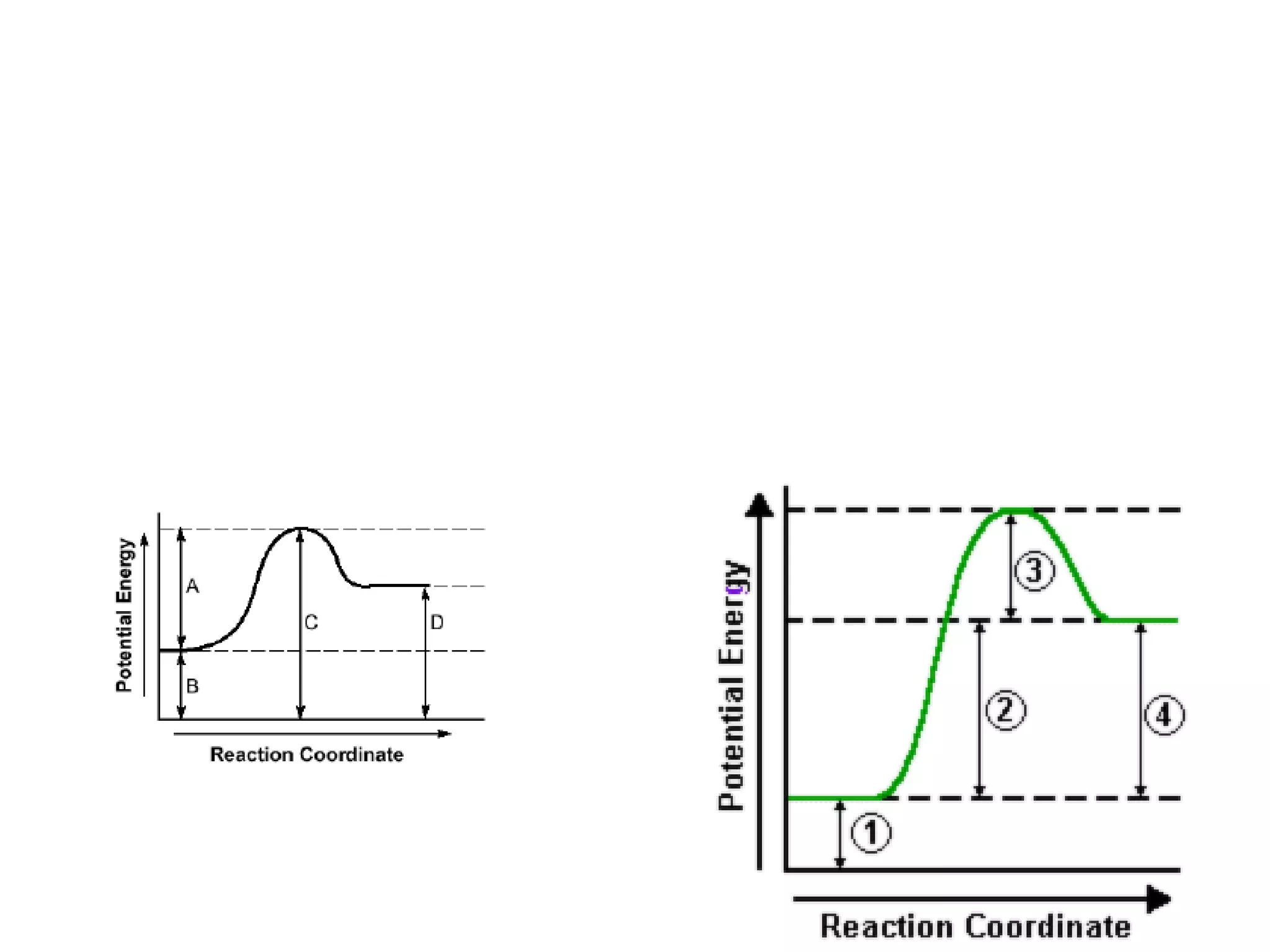
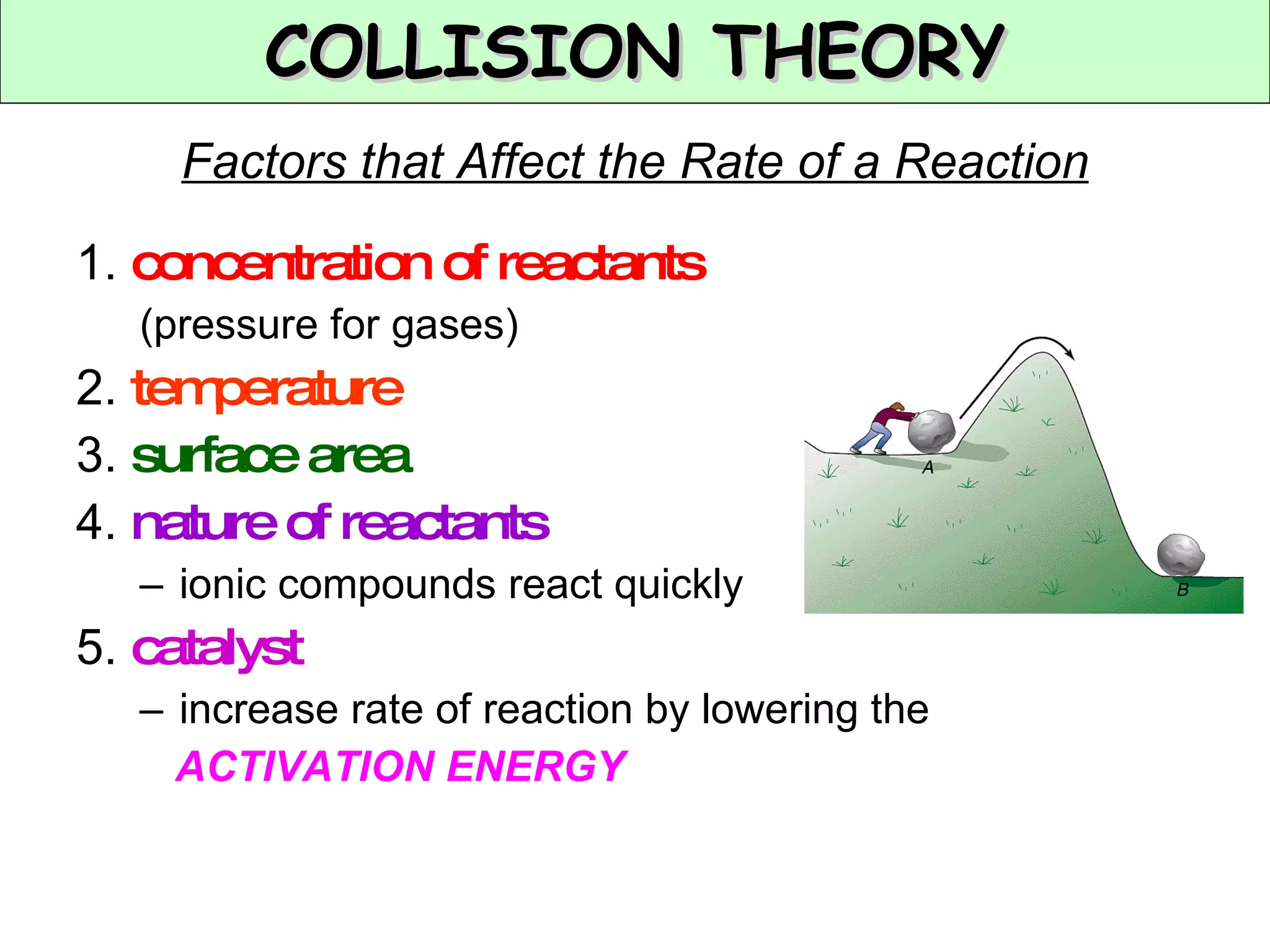
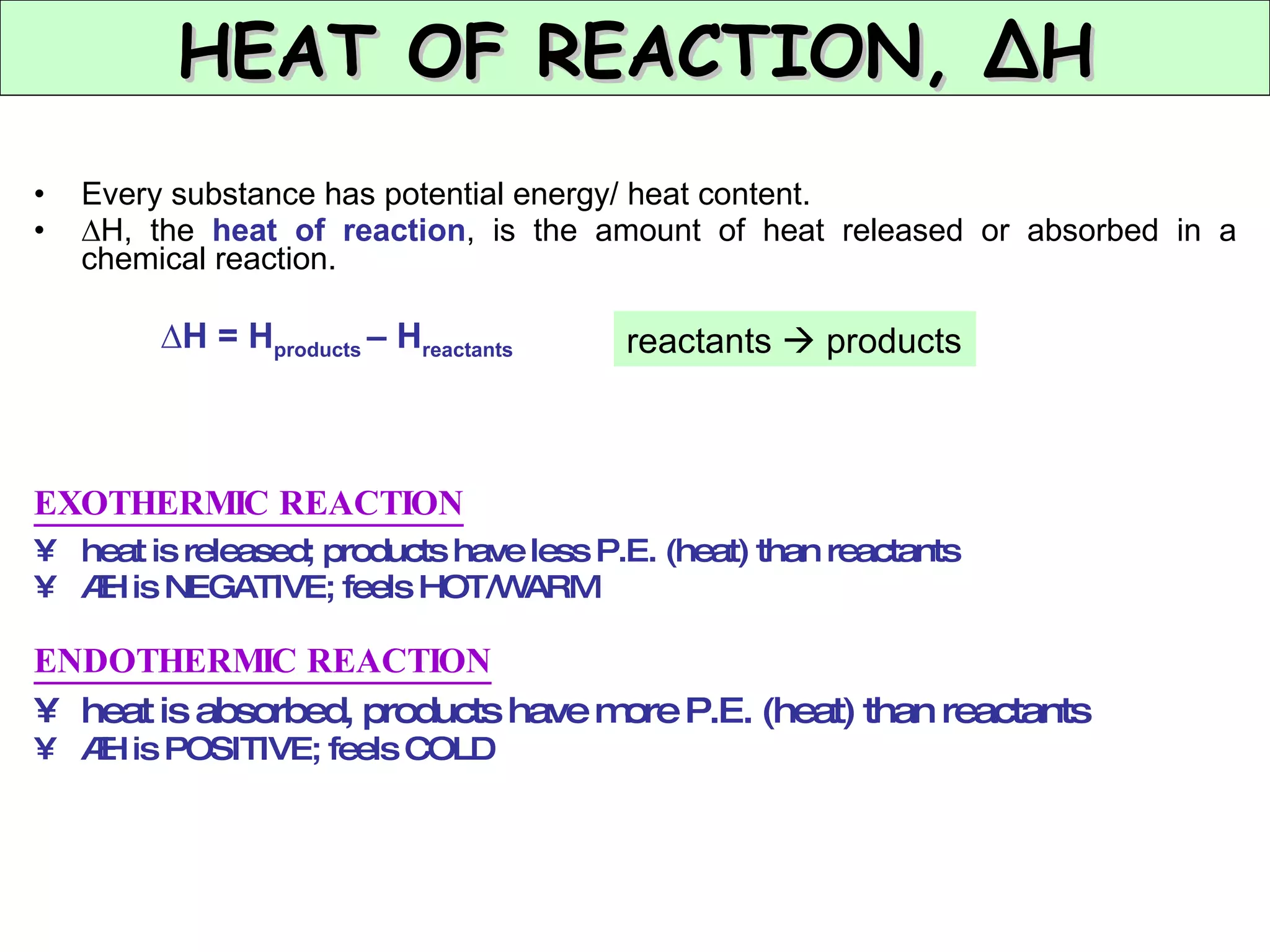
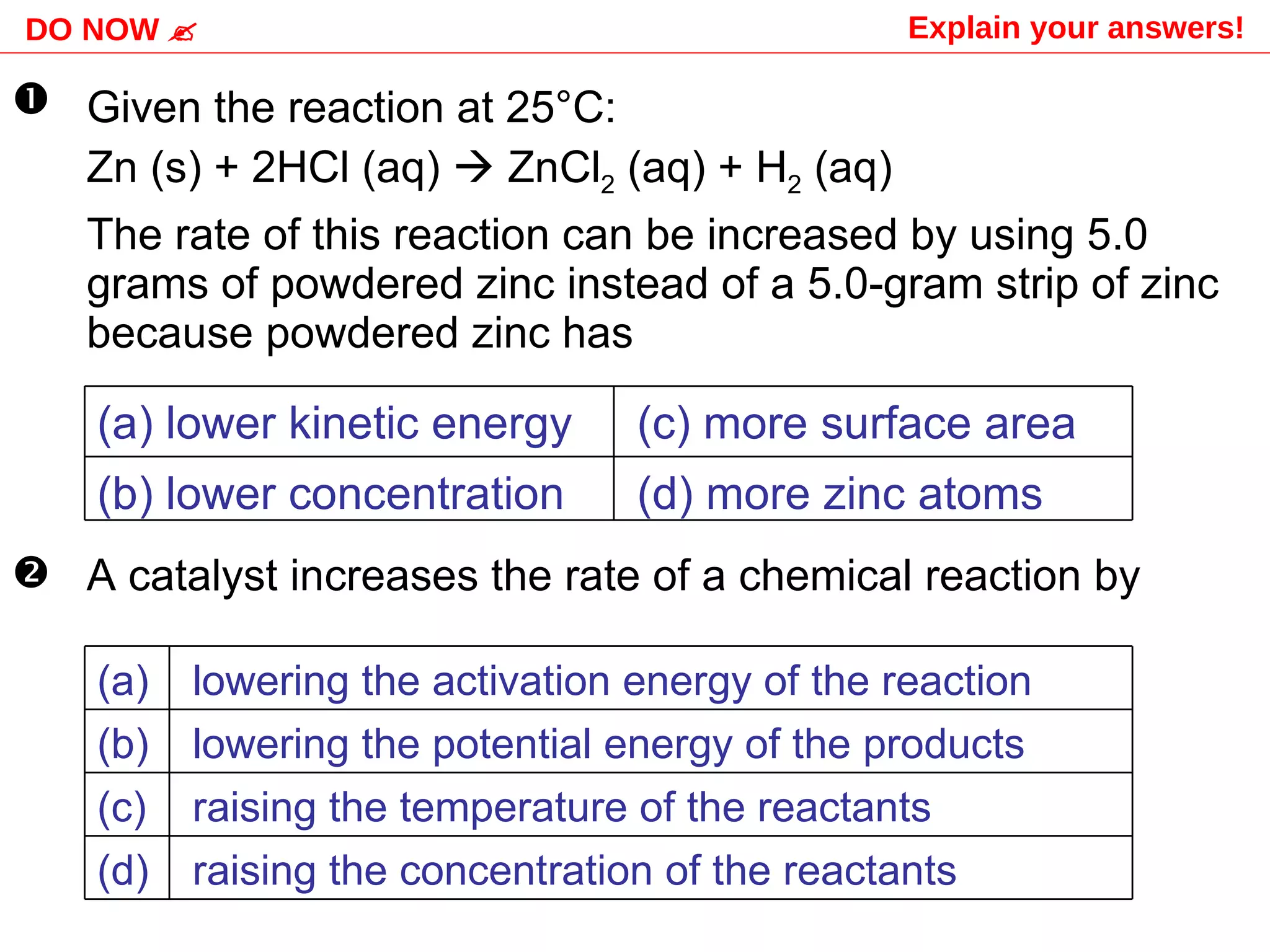
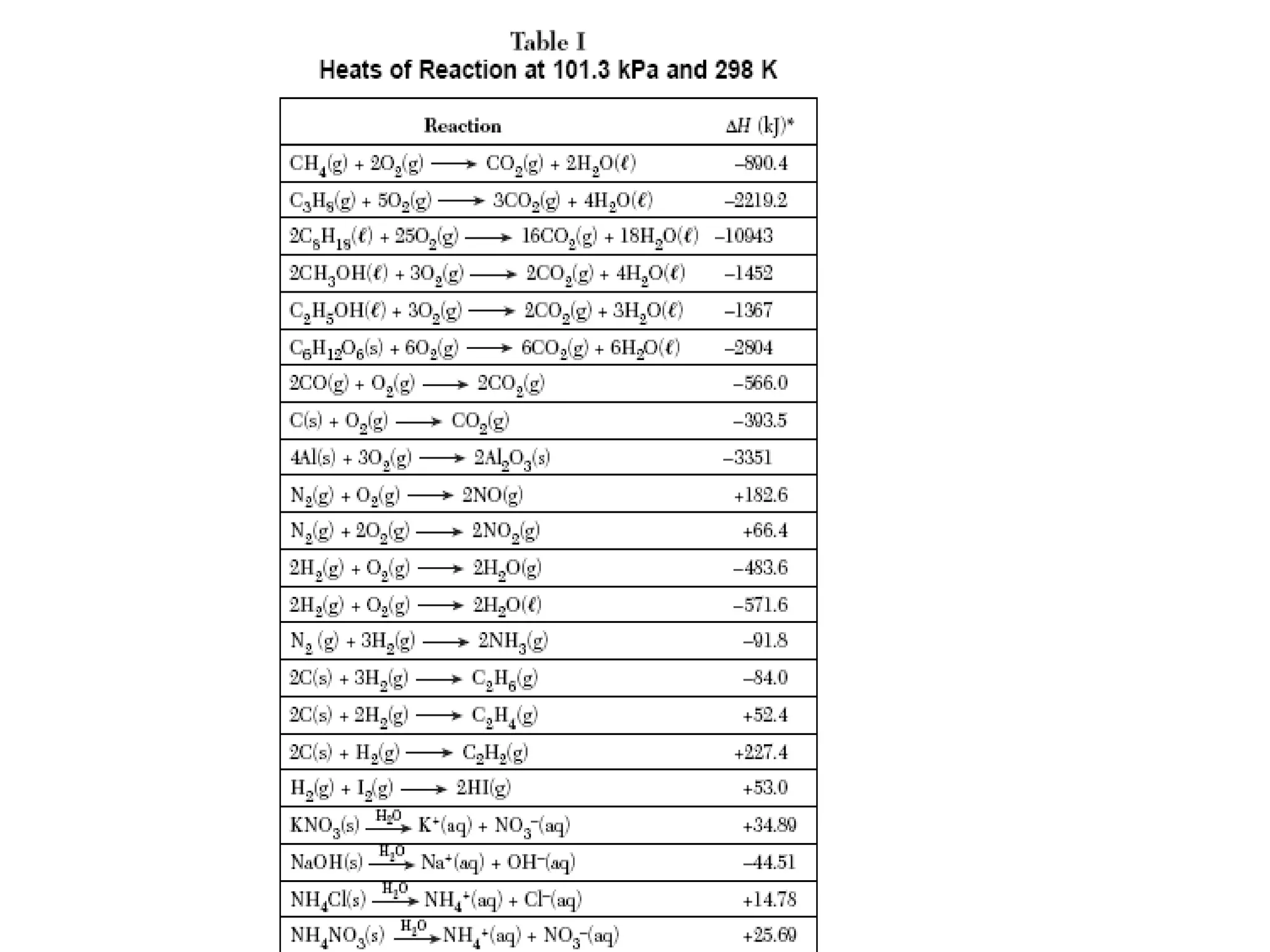
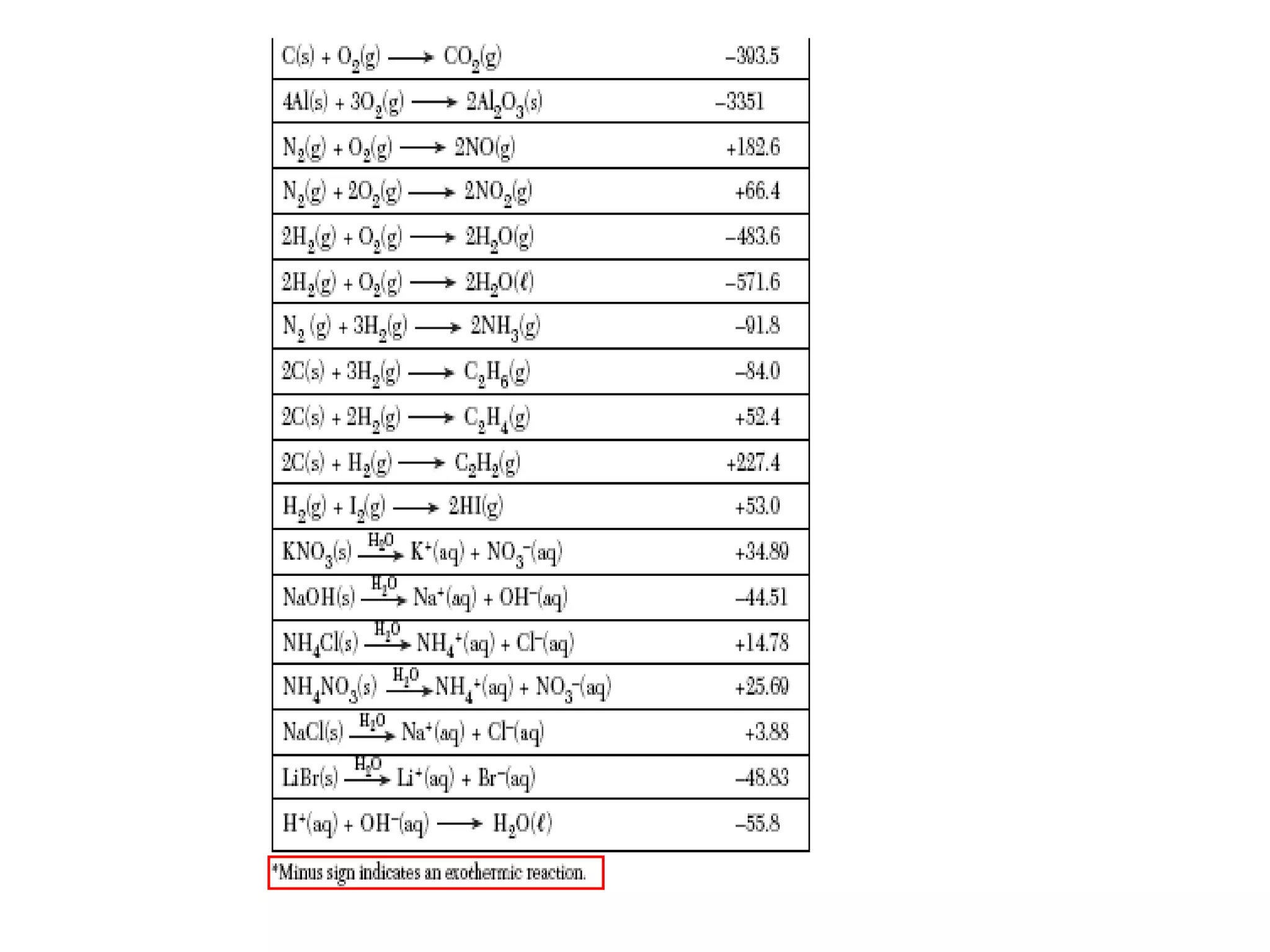
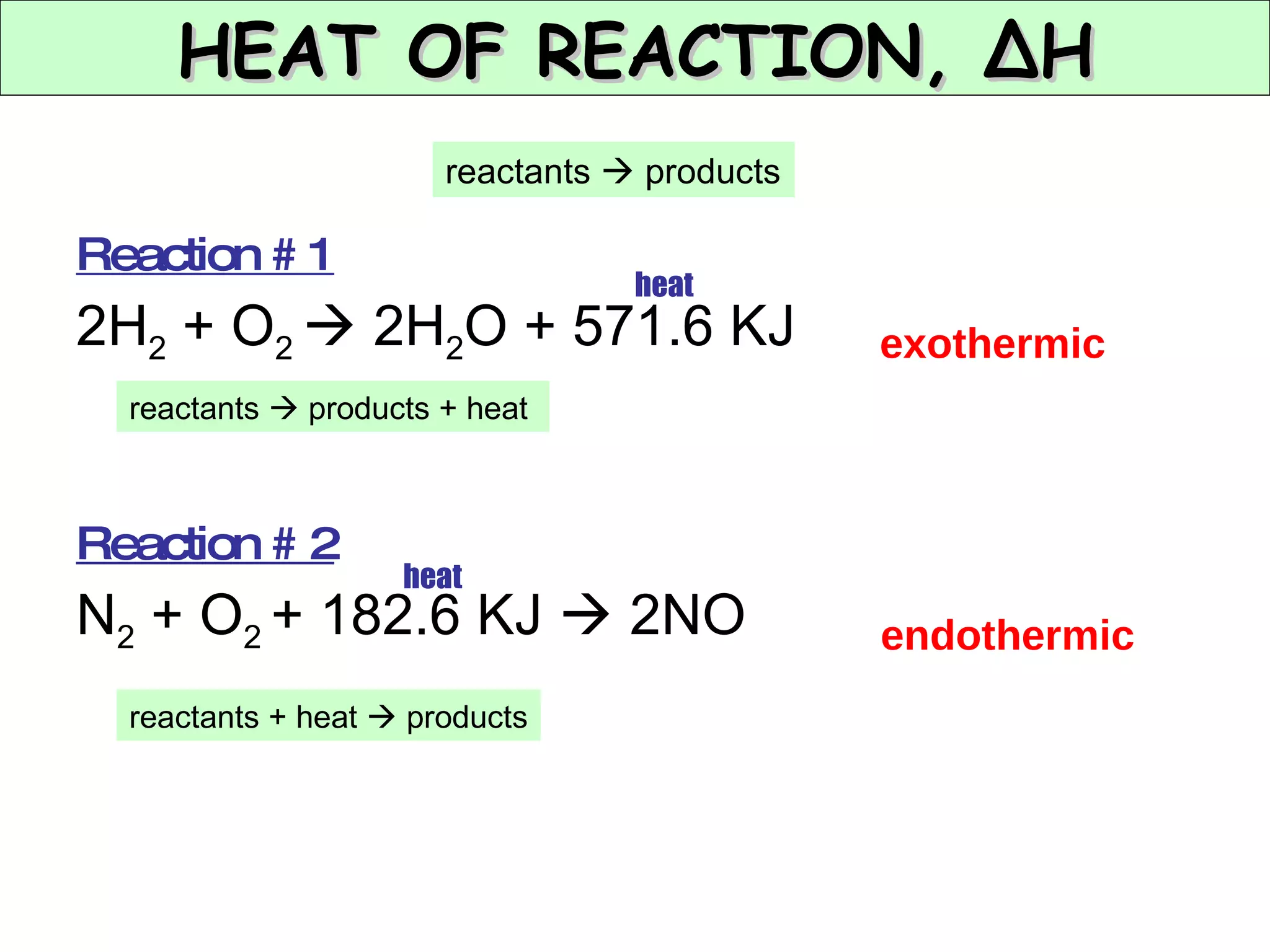
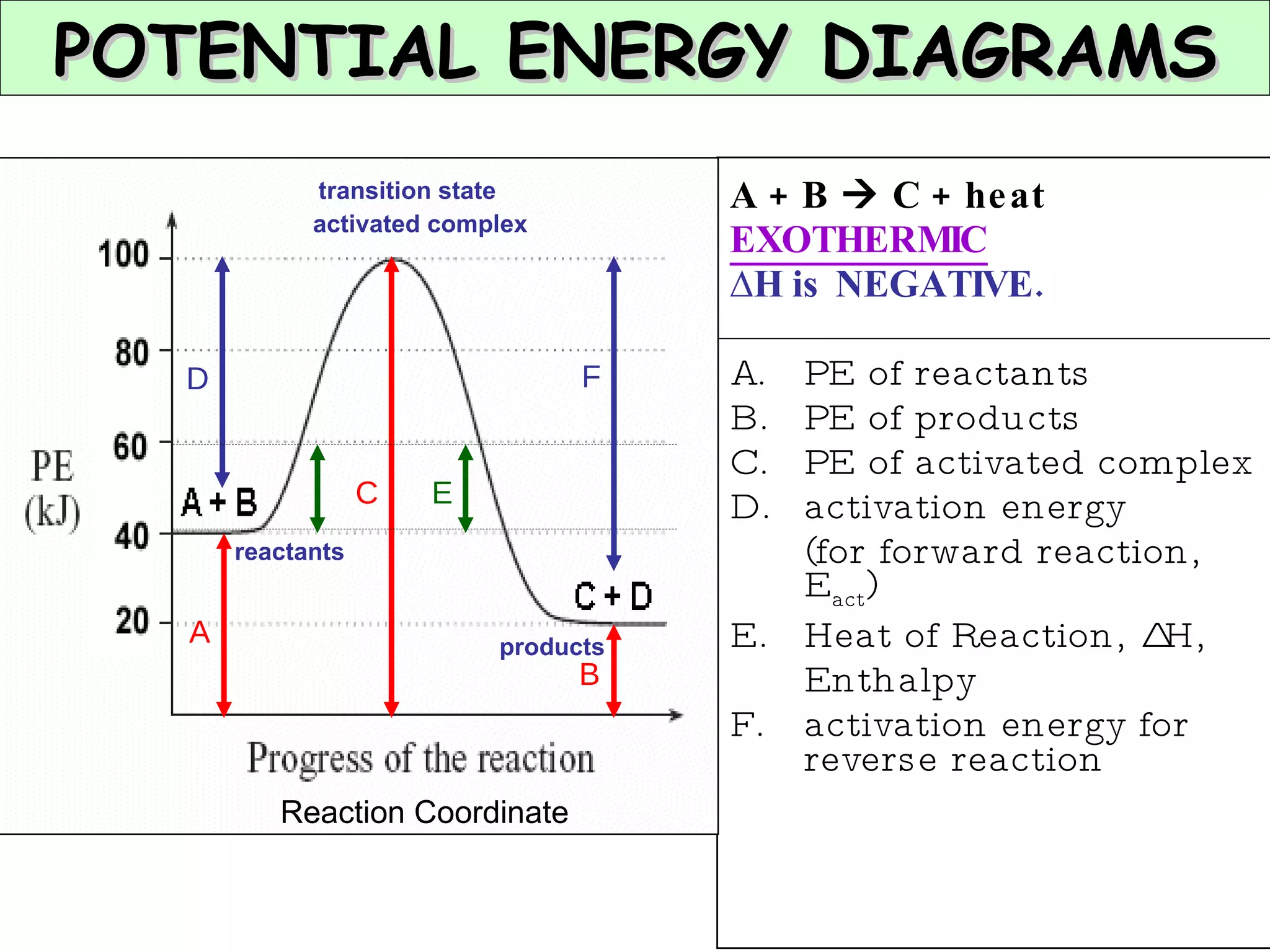
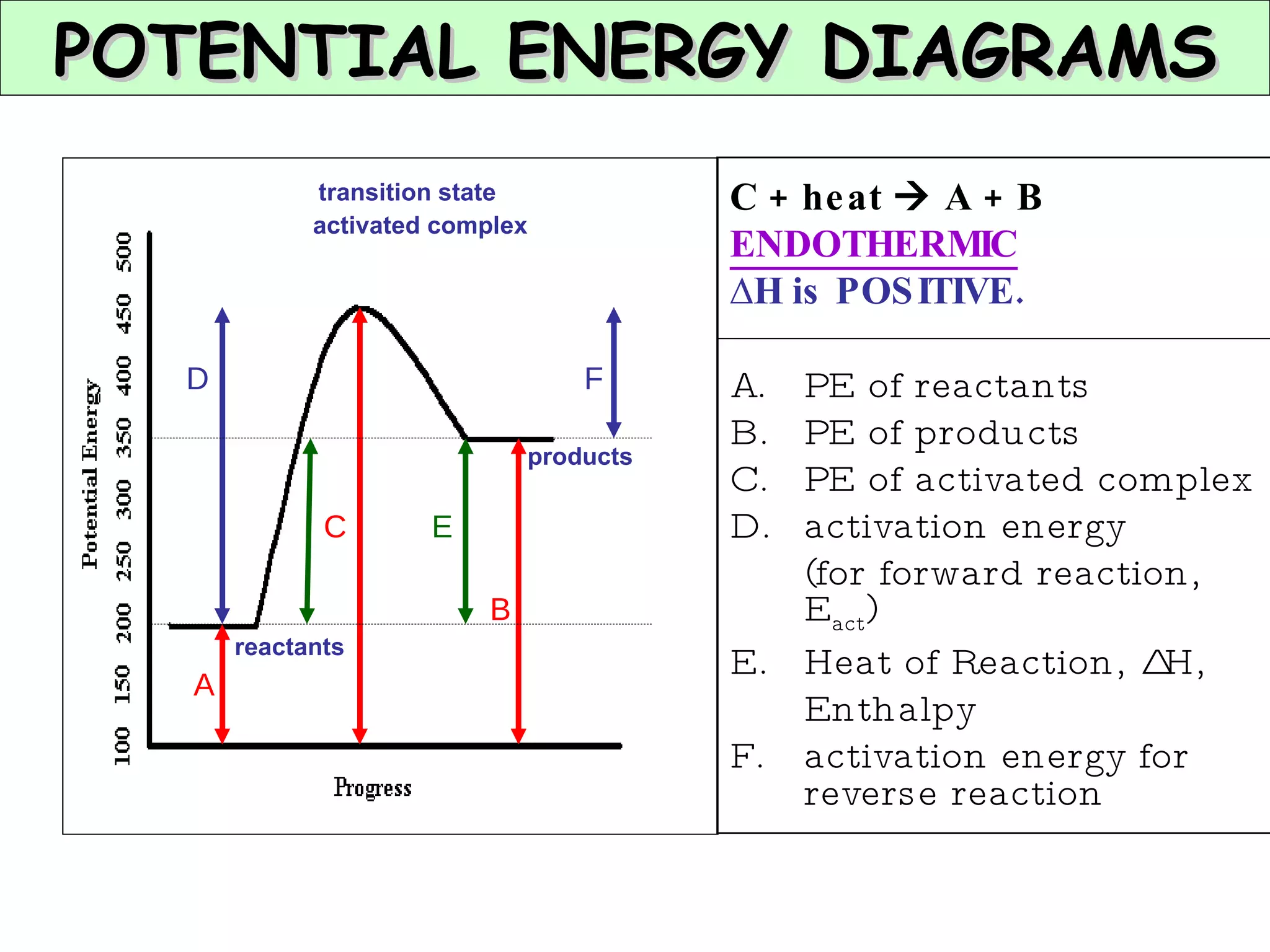
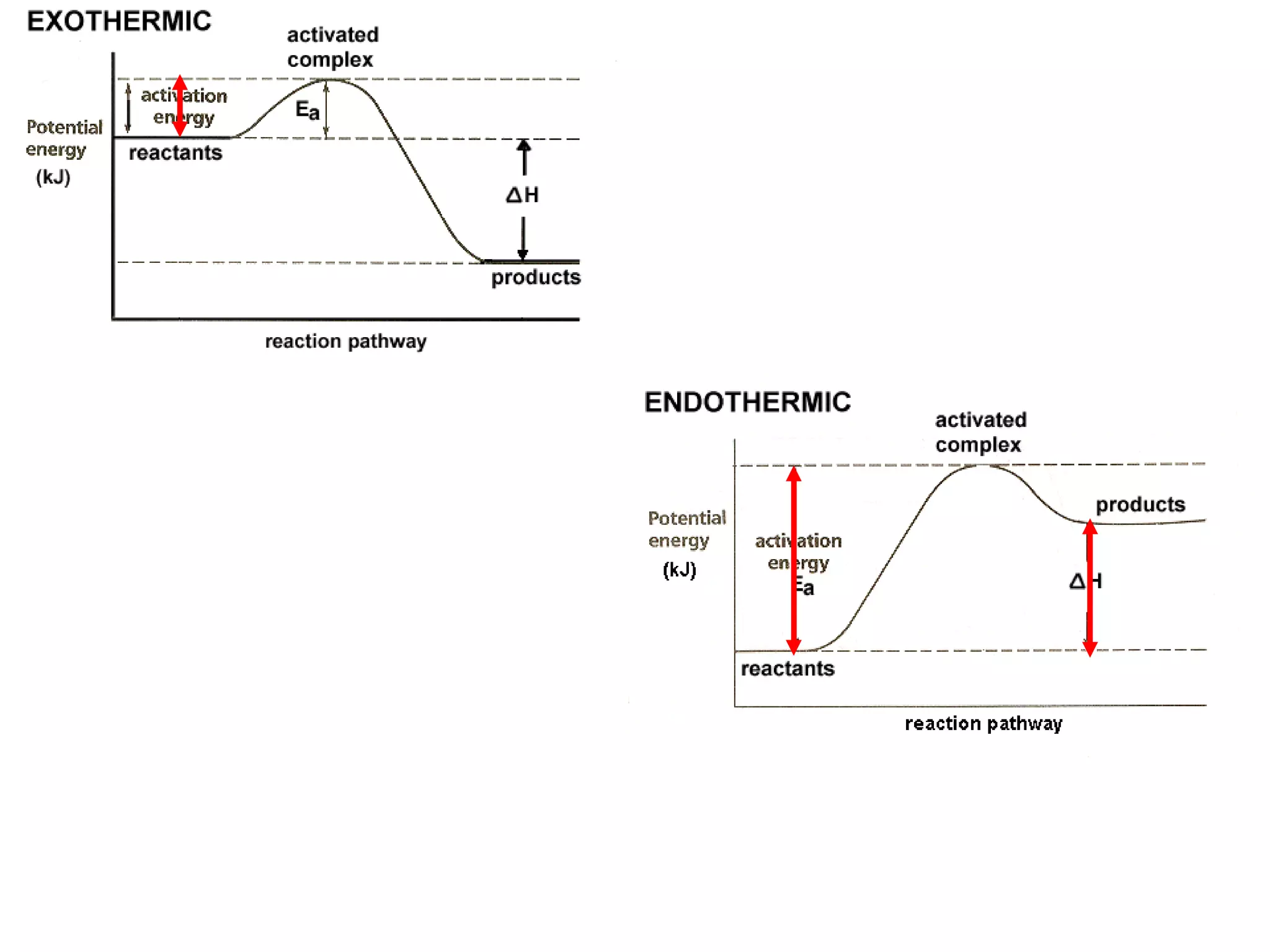
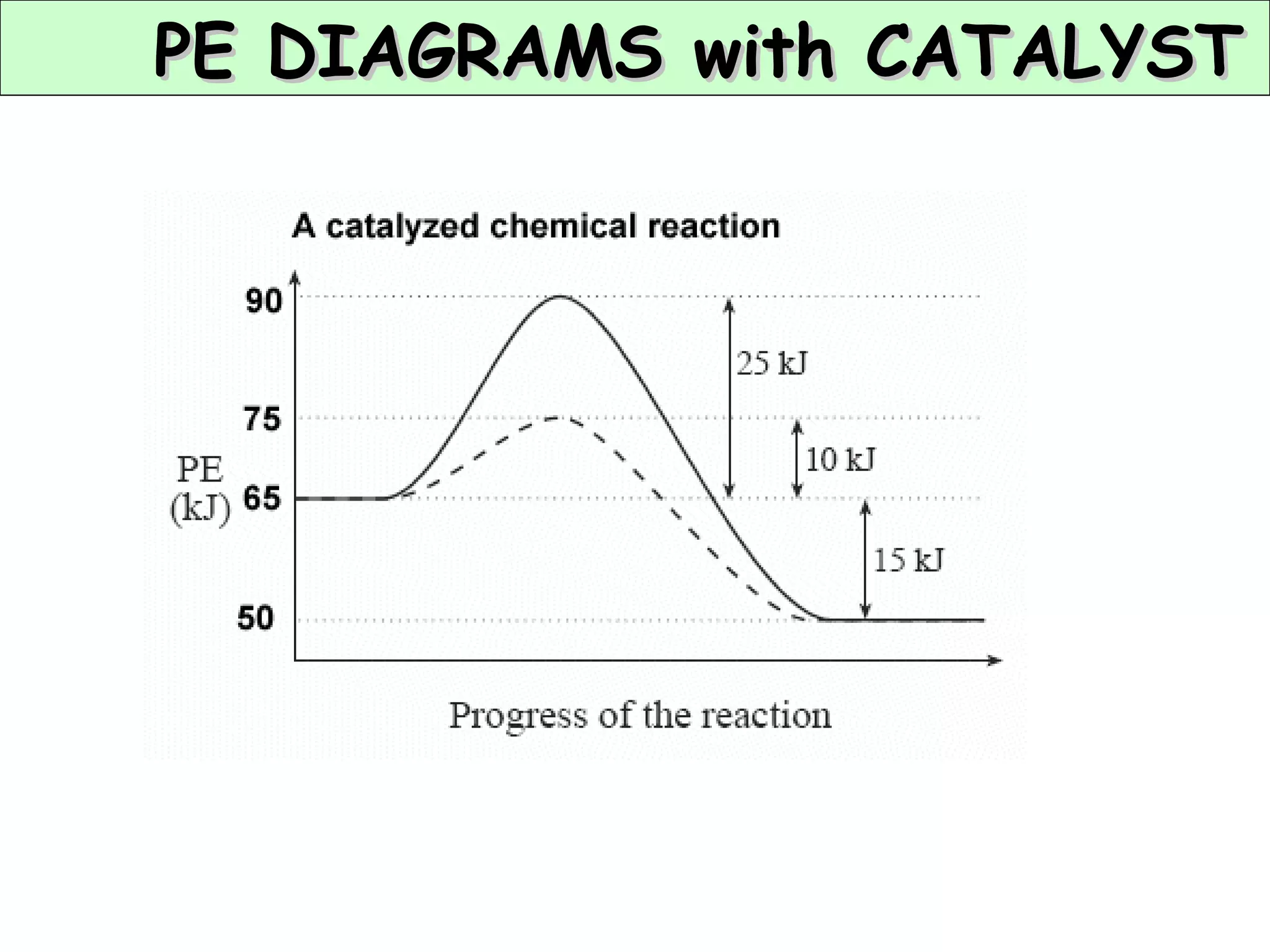
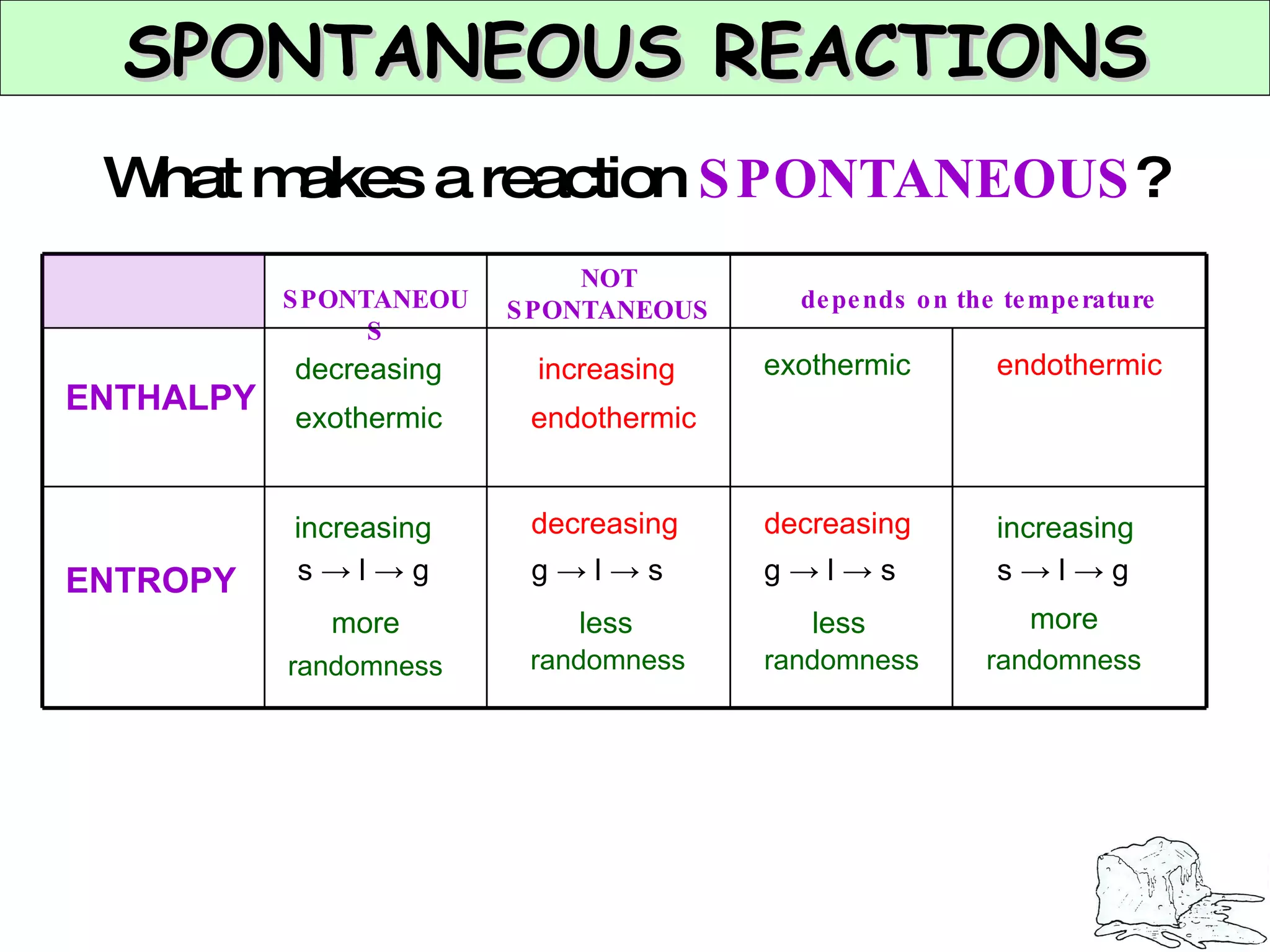
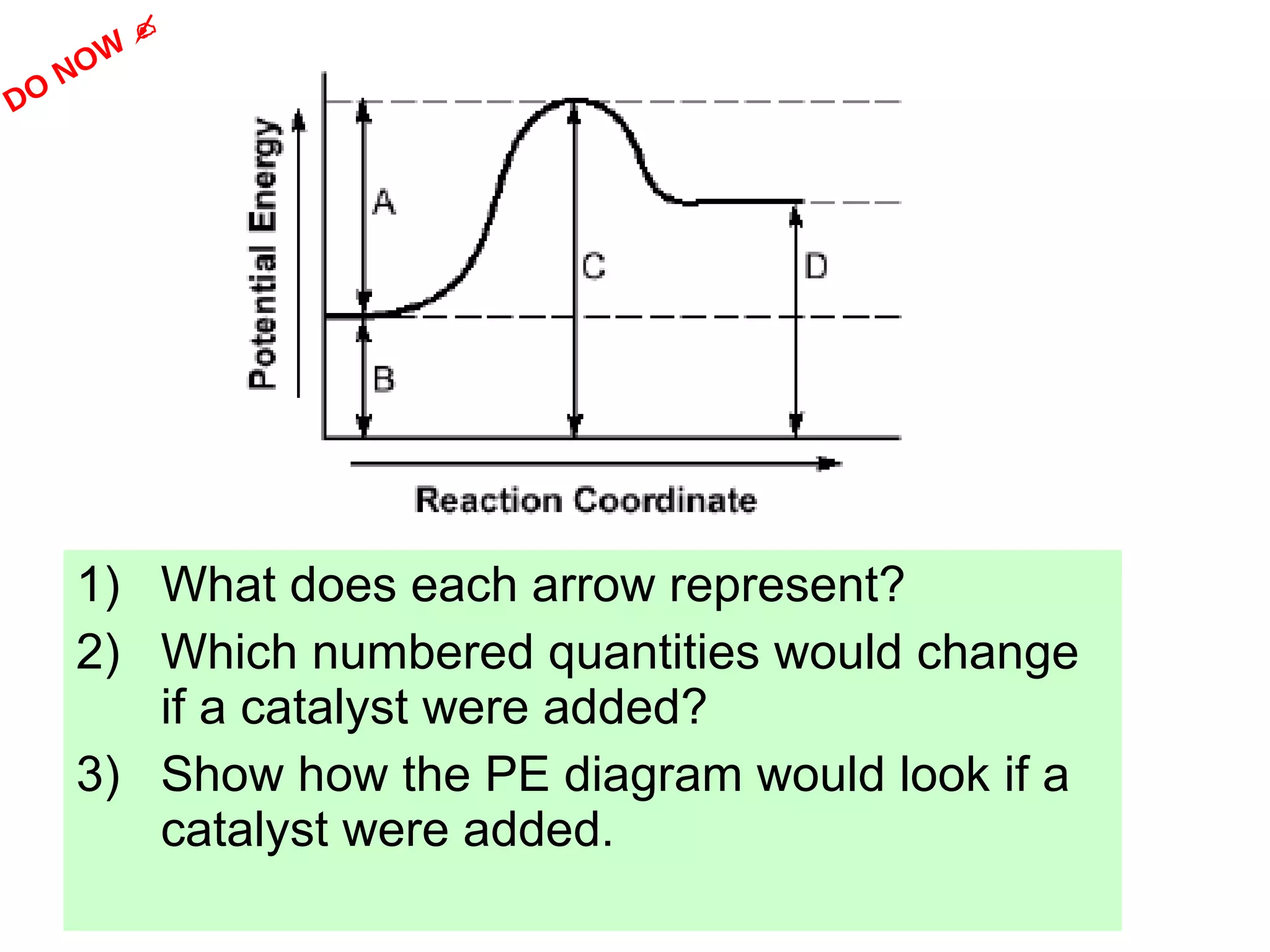
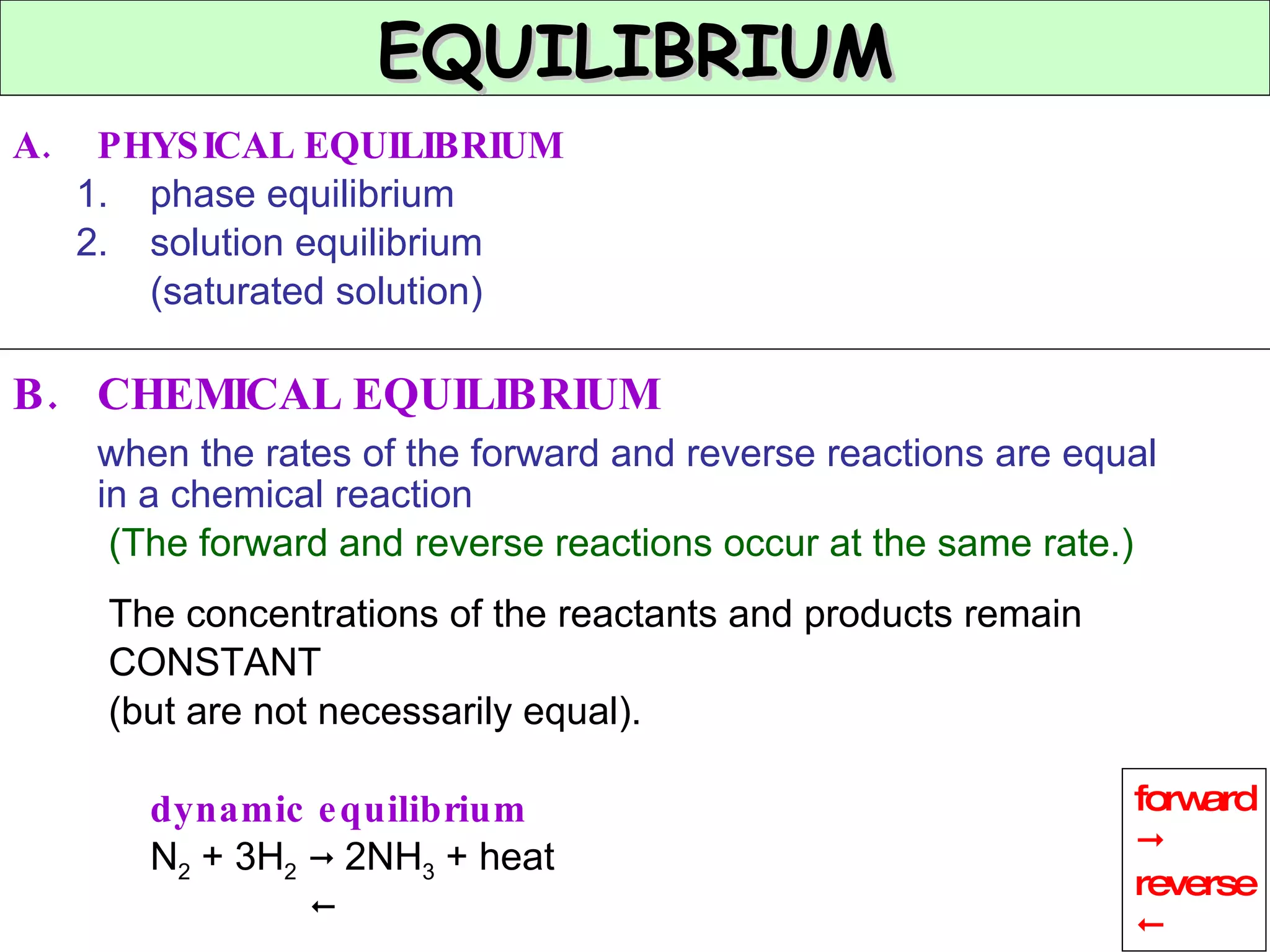
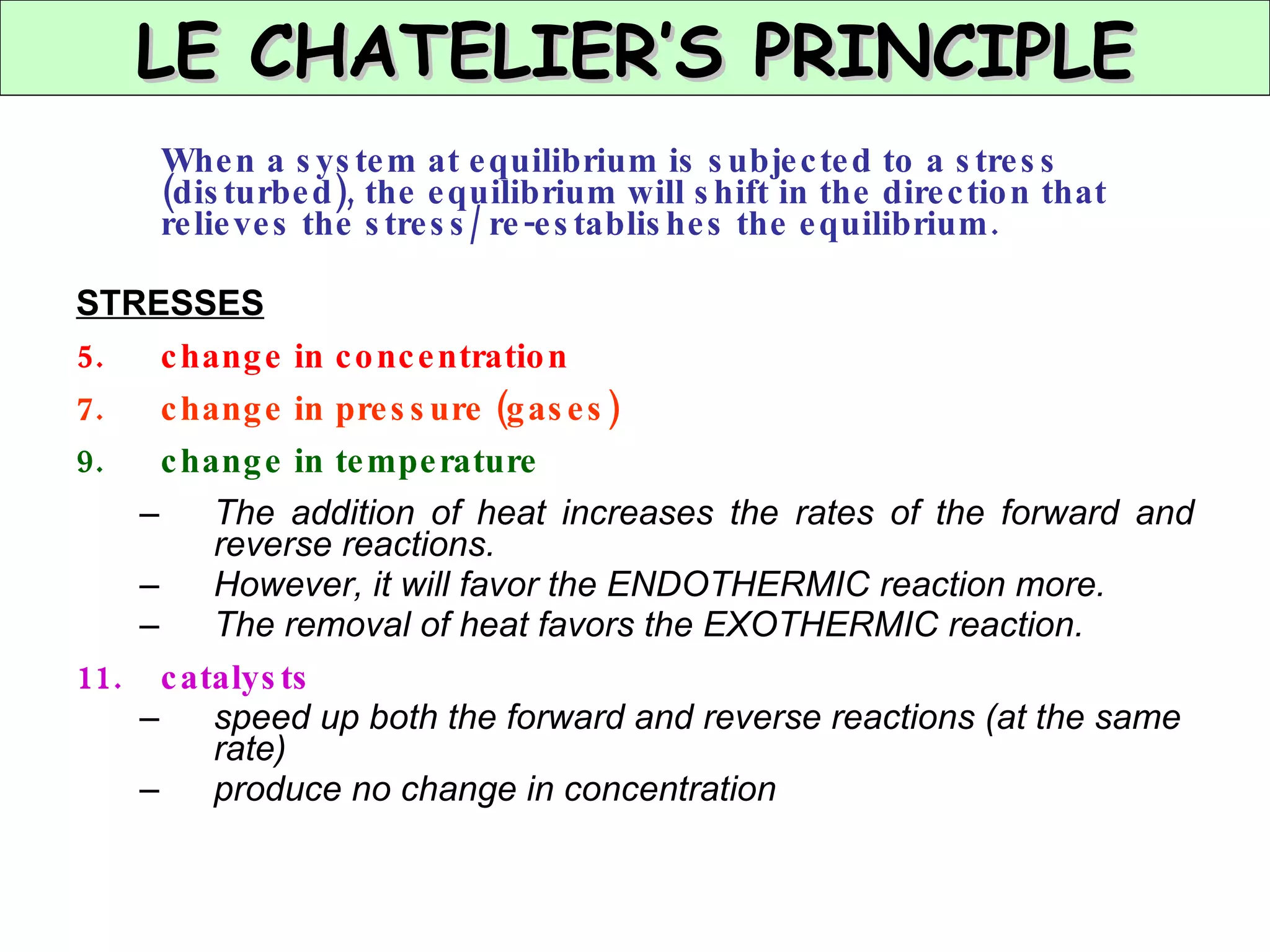
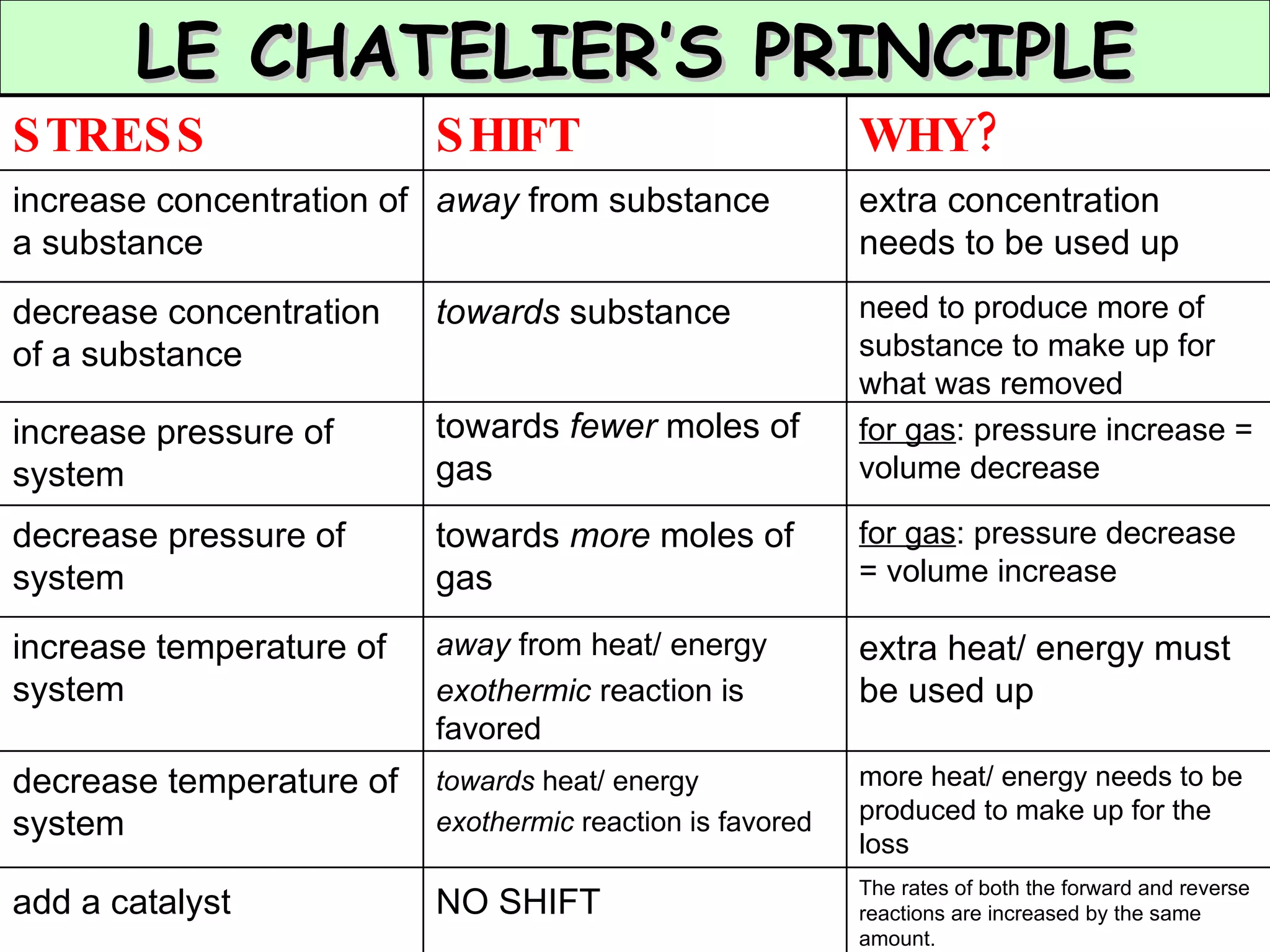
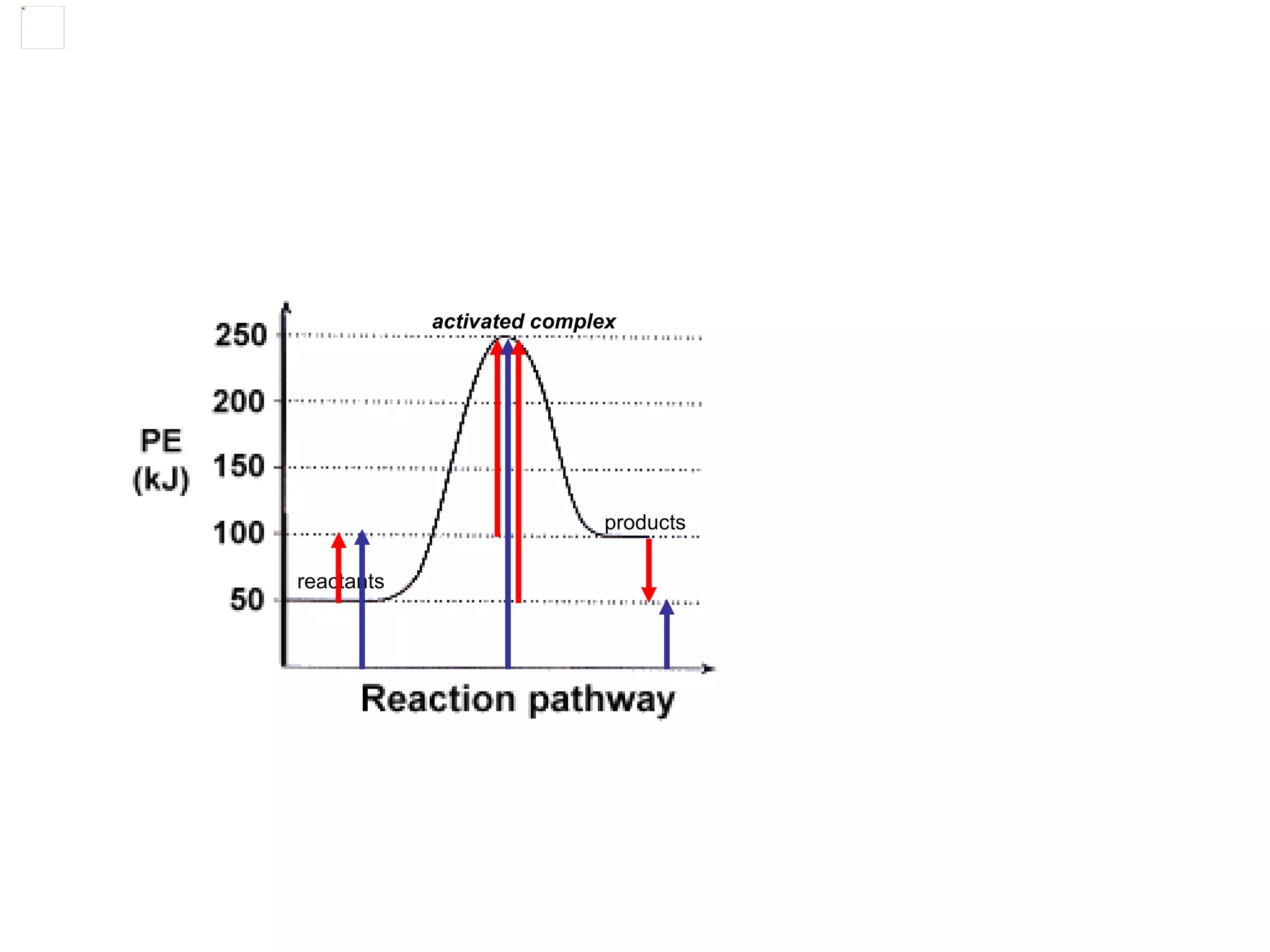
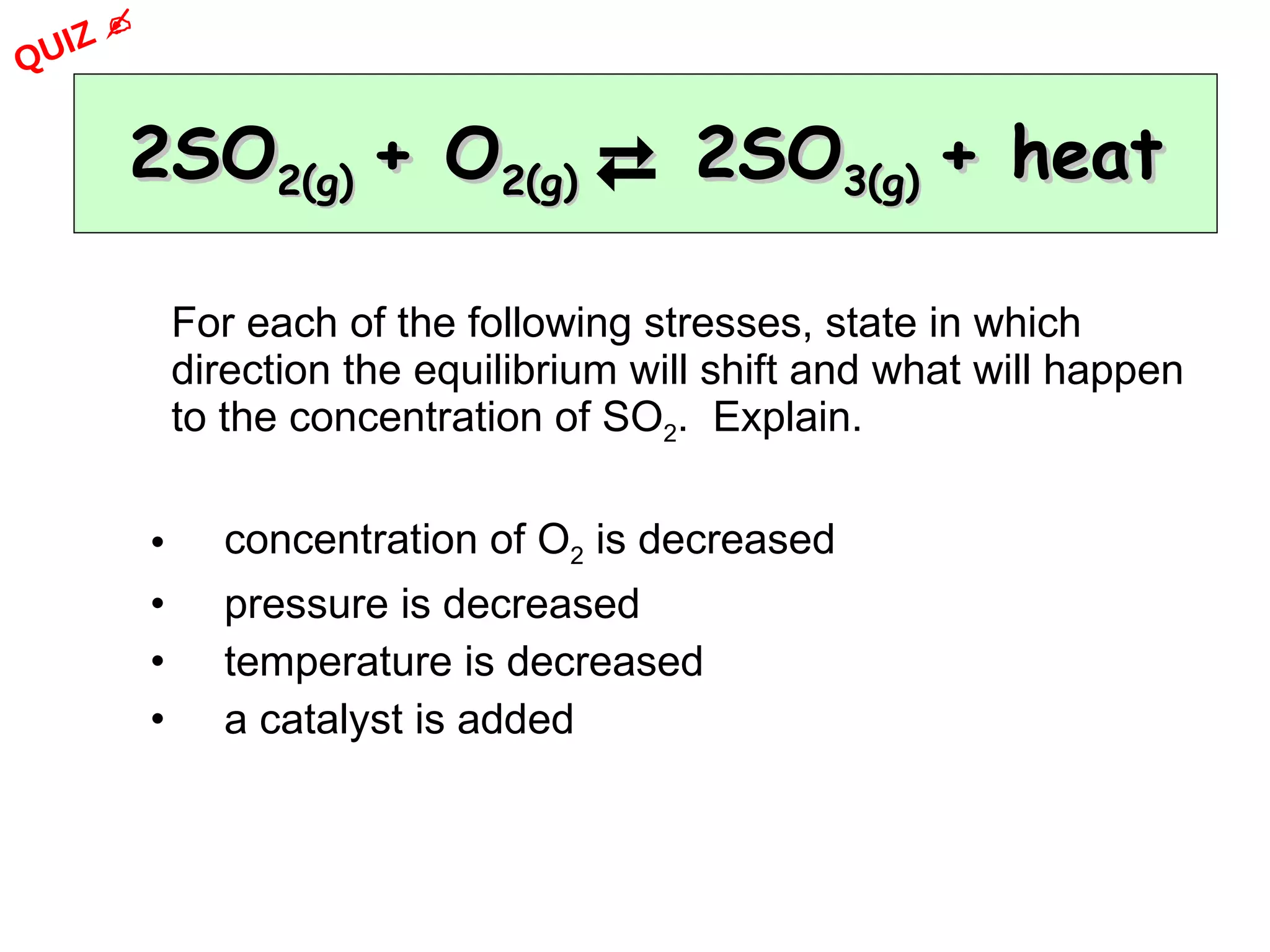
The document discusses factors that affect the rate of chemical reactions, including concentration, temperature, surface area, and catalysts. It explains collision theory and activation energy. Exothermic reactions release heat while endothermic reactions absorb heat. Le Chatelier's principle states that chemical equilibriums shift to counteract changes in concentration, temperature, pressure or addition of reactants/products.


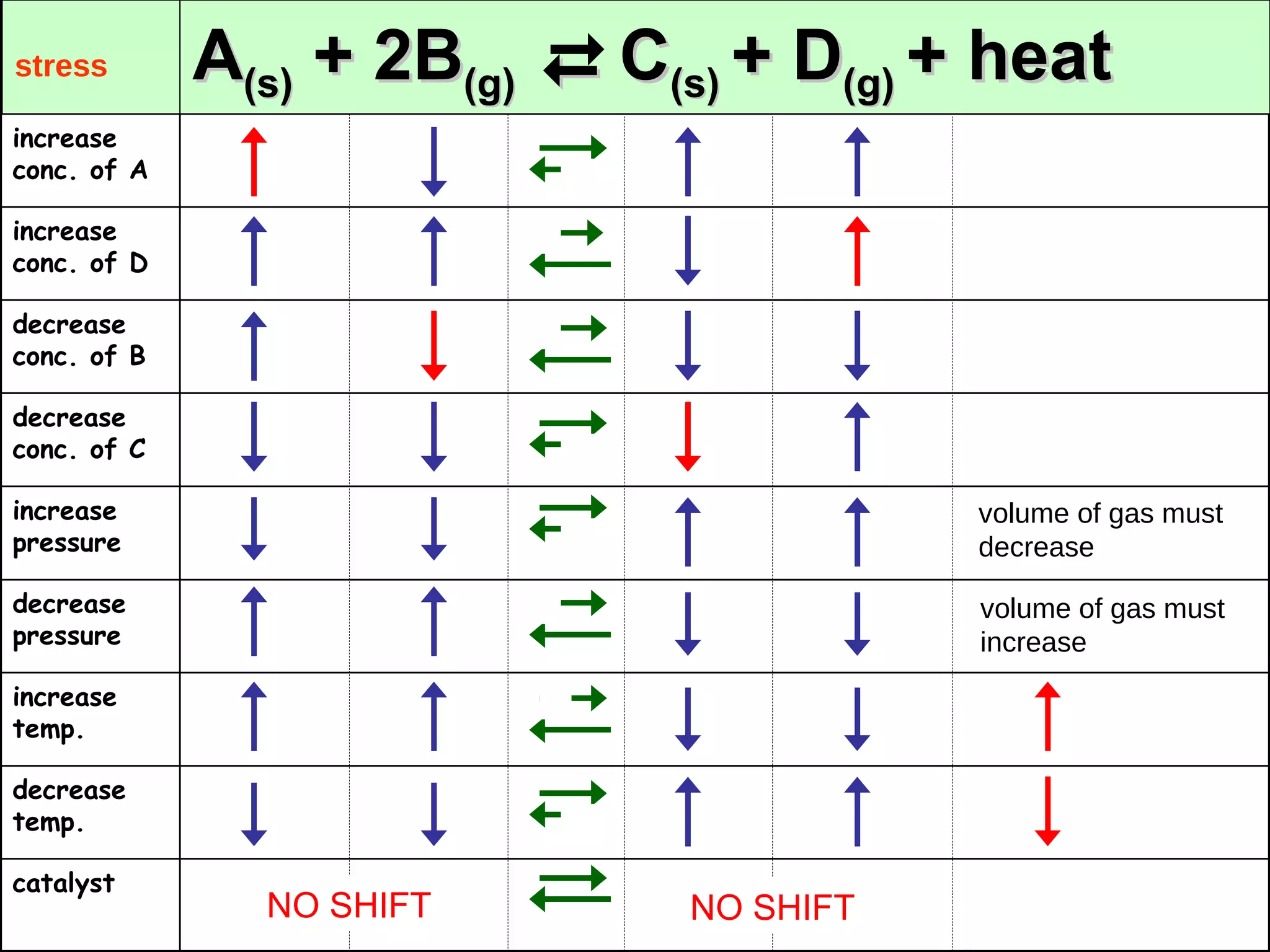
![N 2(g) + 3H 2(g) 2NH 3(g) + 22.0 kcal 10. Decrease press. 9. Increase press. 8. Decrease temp 7. Increase temp 6. Remove NH 3 5. Remove H 2 4. Remove N 2 3. Add NH 3 2. Add H 2 1. Add N 2 [NH 3 ] [H 2 ] [N 2 ] Shift Stress](https://image.slidesharecdn.com/chapter7kineticsandequilibrium-100415180947-phpapp02/75/Chapter-7-Kinetics-And-Equilibrium-24-2048.jpg)
![N 2(g) + 3H 2(g) 2NH 3(g) + 22.0 kcal _____ _____ _____ _____ _____ _____ 10. Decrease press. 9. Increase press. 8. Decrease temp 7. Increase temp 6. Remove NH 3 5. Remove H 2 4. Remove N 2 3. Add NH 3 2. Add H 2 1. Add N 2 [NH 3 ] [H 2 ] [N 2 ] Shift Stress](https://image.slidesharecdn.com/chapter7kineticsandequilibrium-100415180947-phpapp02/75/Chapter-7-Kinetics-And-Equilibrium-25-2048.jpg)


![2POCl 3(g) + energy 2PCl 3(g) + O 2(g) Which changes occur when O 2(g) is added to the system? 1) The equilibrium shifts right and [PCl 3 ] increases. 2) The equilibrium shifts right and [PCl 3 ] decreases. 3) The equilibrium shifts left and [PCl 3 ] increases. 4) The equilibrium shifts left and [PCl 3 ] decreases DO NOW ](https://image.slidesharecdn.com/chapter7kineticsandequilibrium-100415180947-phpapp02/75/Chapter-7-Kinetics-And-Equilibrium-29-2048.jpg)
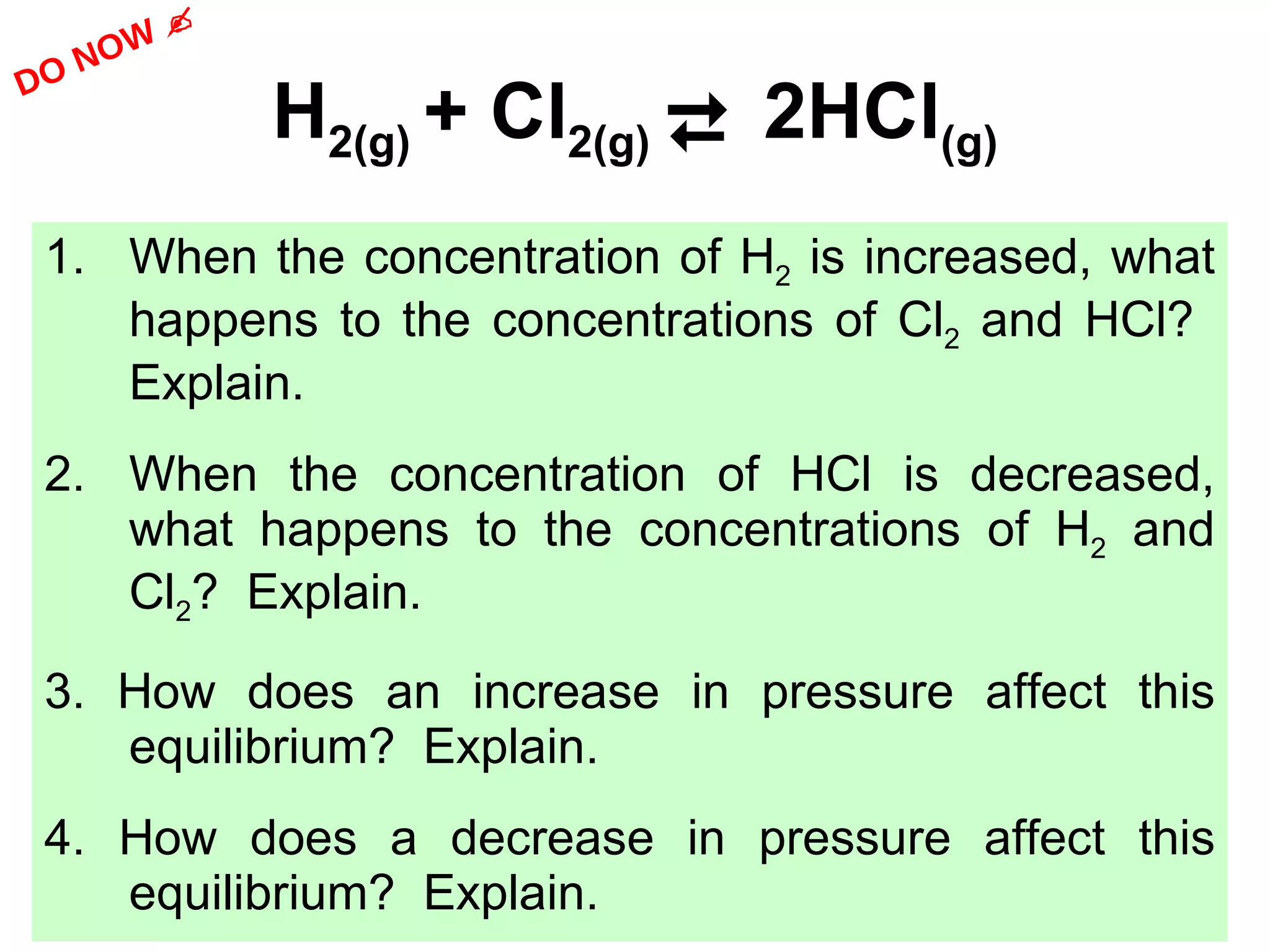
![12.6 kcal + H 2(g) + I 2(g) 2HI 10. Decrease press. 9. Increase press. 8. Decrease temp 7. Increase temp 6. Remove HI 5. Remove I 2 4. Remove H 2 3. Add HI 2. Add I 2 1. Add H 2 [HI] [I 2 ] [H 2 ] Shift Stress](https://image.slidesharecdn.com/chapter7kineticsandequilibrium-100415180947-phpapp02/75/Chapter-7-Kinetics-And-Equilibrium-30-2048.jpg)
![12.6 kcal + H 2(g) + I 2(g) 2HI _____ _____ _____ _____ _____ _____ = = NO CHANGE REMAINS THE SAME 10. Decrease press. 9. Increase press. 8. Decrease temp 7. Increase temp 6. Remove HI 5. Remove I 2 4. Remove H 2 3. Add HI 2. Add I 2 1. Add H 2 [HI] [I 2 ] [H 2 ] Shift Stress](https://image.slidesharecdn.com/chapter7kineticsandequilibrium-100415180947-phpapp02/75/Chapter-7-Kinetics-And-Equilibrium-31-2048.jpg)

![NaOH (s) Na + (aq) + OH - (aq) + 10.6 kcal 8. Decrease press 7. Increase press 6. Decrease temp 5. Increase temp 4. Add H + (removes OH + ) 3. Add KOH (adds OH - ) 2. Add NaCl (adds Na + ) 1. Add NaOH [OH - ] [Na + ] NaOH (s) Shift Stress](https://image.slidesharecdn.com/chapter7kineticsandequilibrium-100415180947-phpapp02/75/Chapter-7-Kinetics-And-Equilibrium-37-2048.jpg)
![NaOH (s) Na + (aq) + OH - (aq) + 10.6 kcal _____ _____ _____ _____ NO EFFECT …………………… REMAINS THE SAME 8. Decrease press 7. Increase press 6. Decrease temp 5. Increase temp 4. Add H + (removes OH + ) 3. Add KOH (adds OH - ) 2. Add NaCl (adds Na + ) 1. Add NaOH [OH - ] [Na + ] NaOH (s) Shift Stress](https://image.slidesharecdn.com/chapter7kineticsandequilibrium-100415180947-phpapp02/75/Chapter-7-Kinetics-And-Equilibrium-38-2048.jpg)