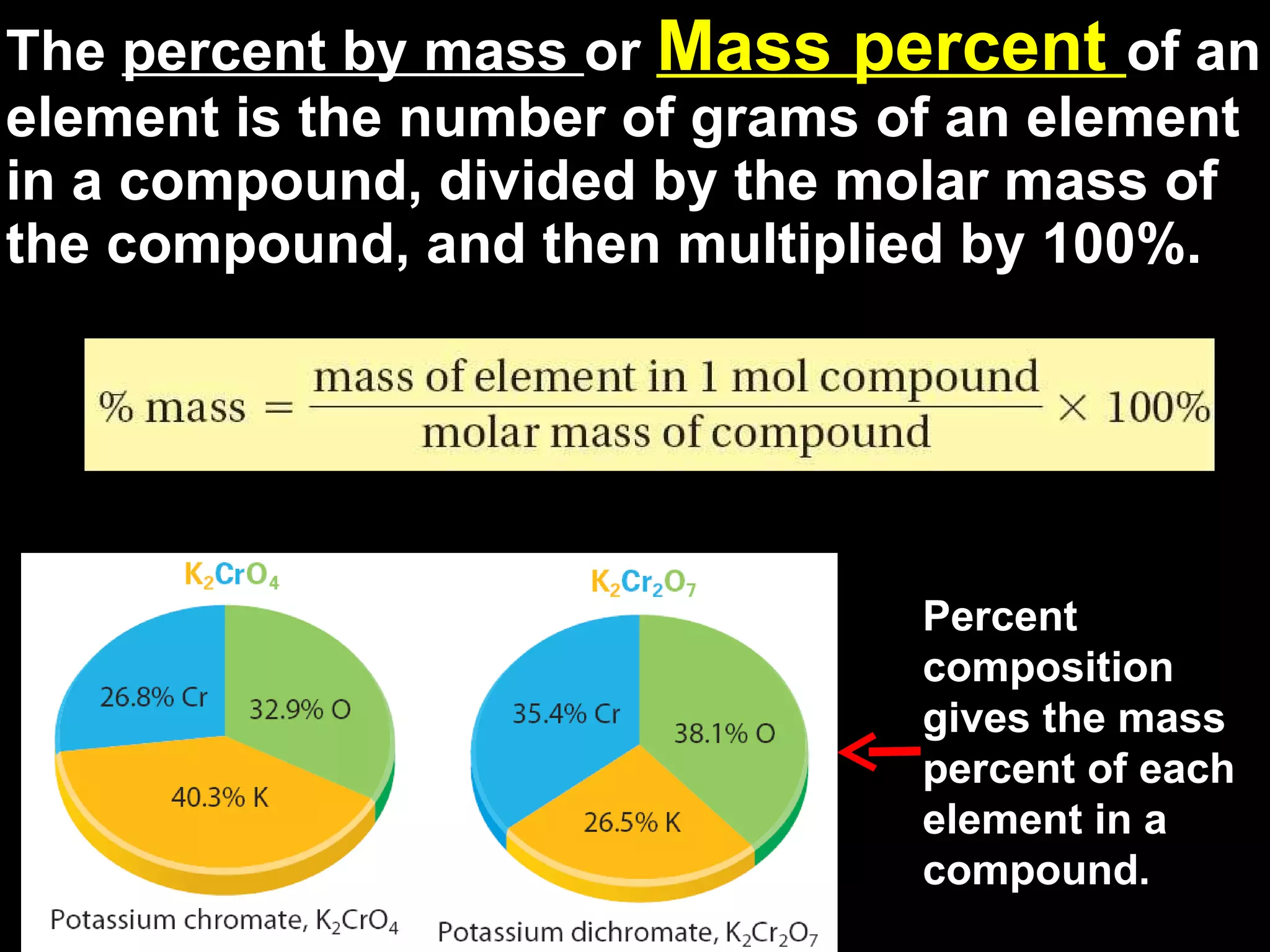
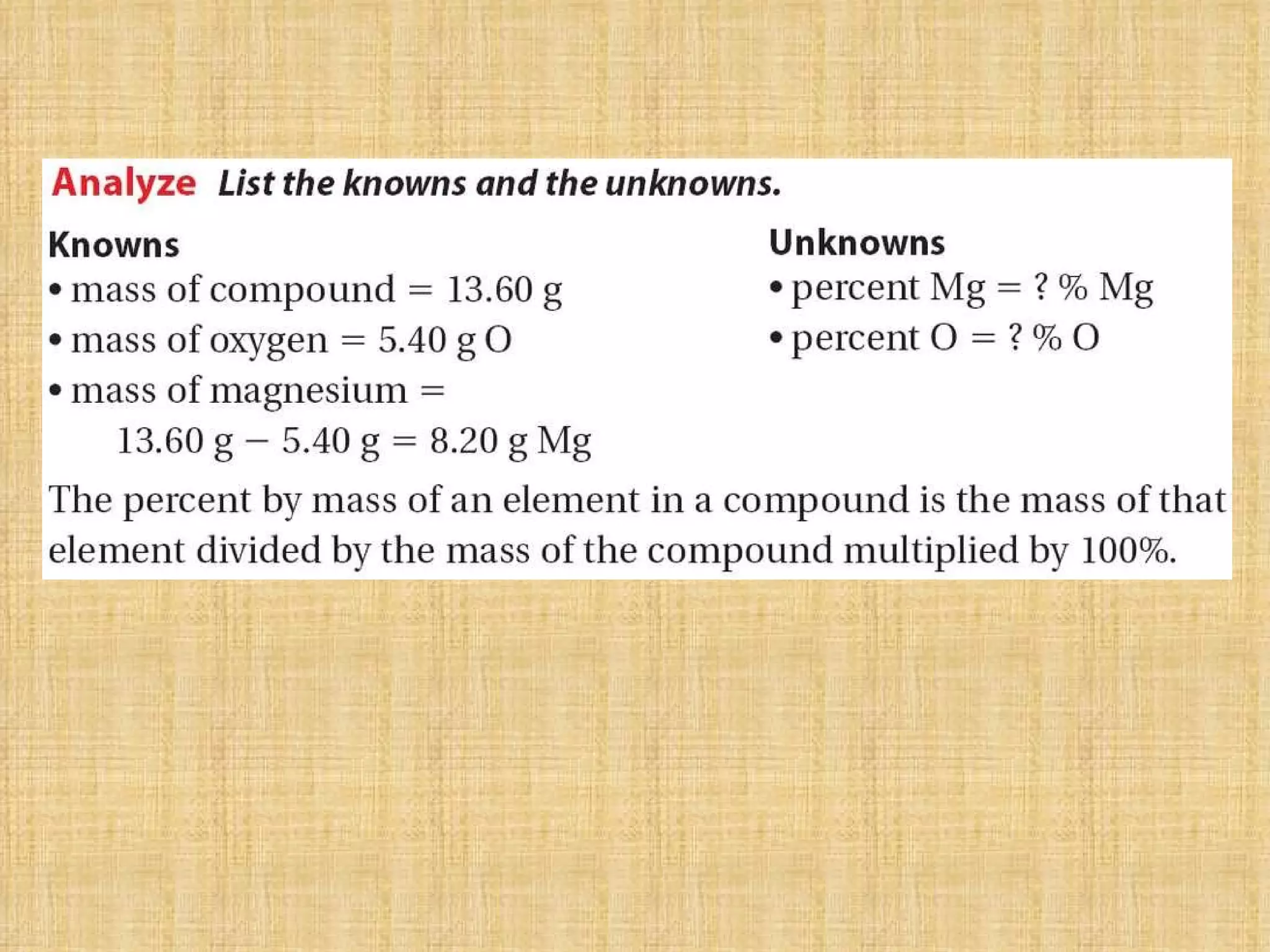
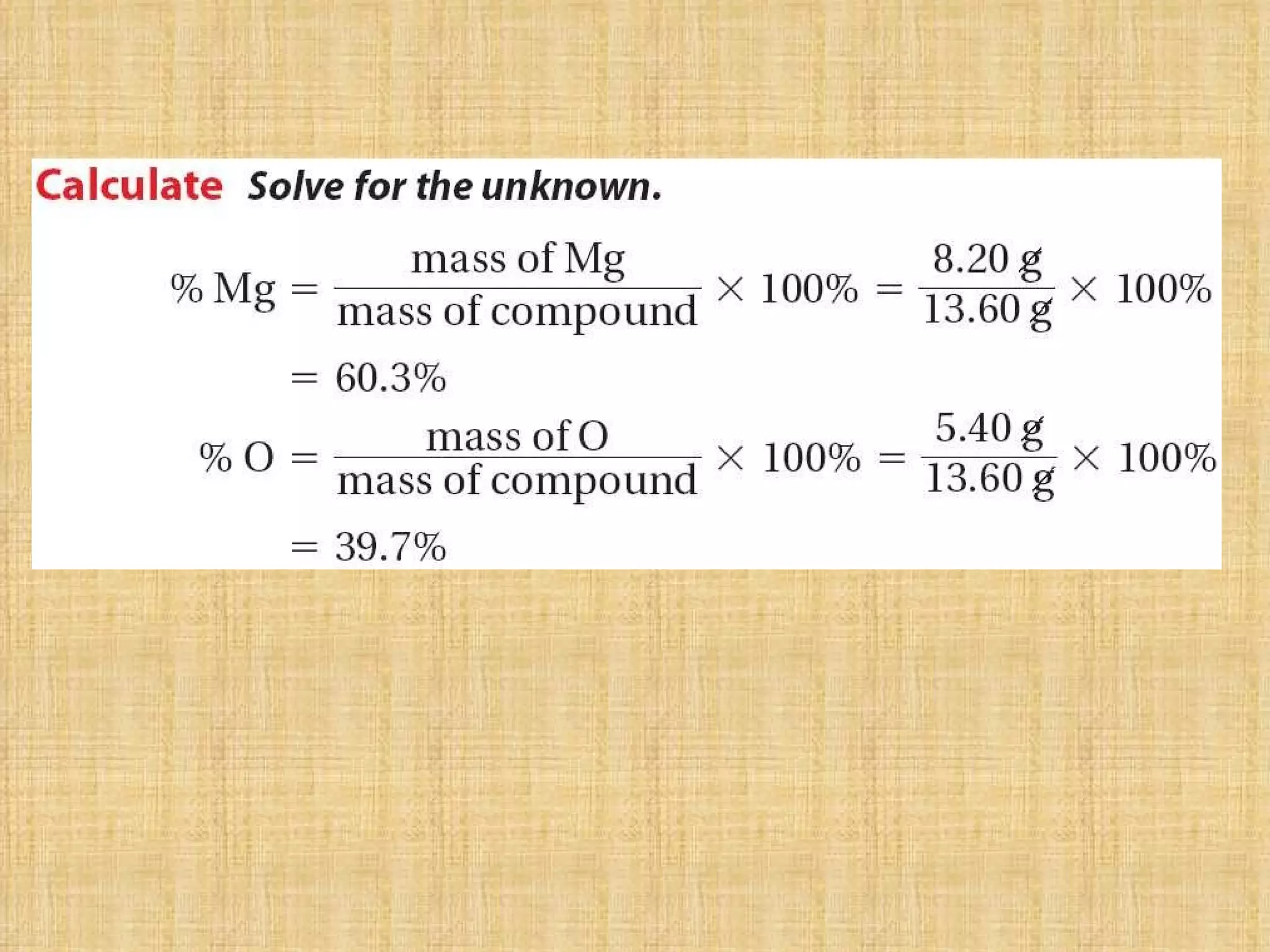
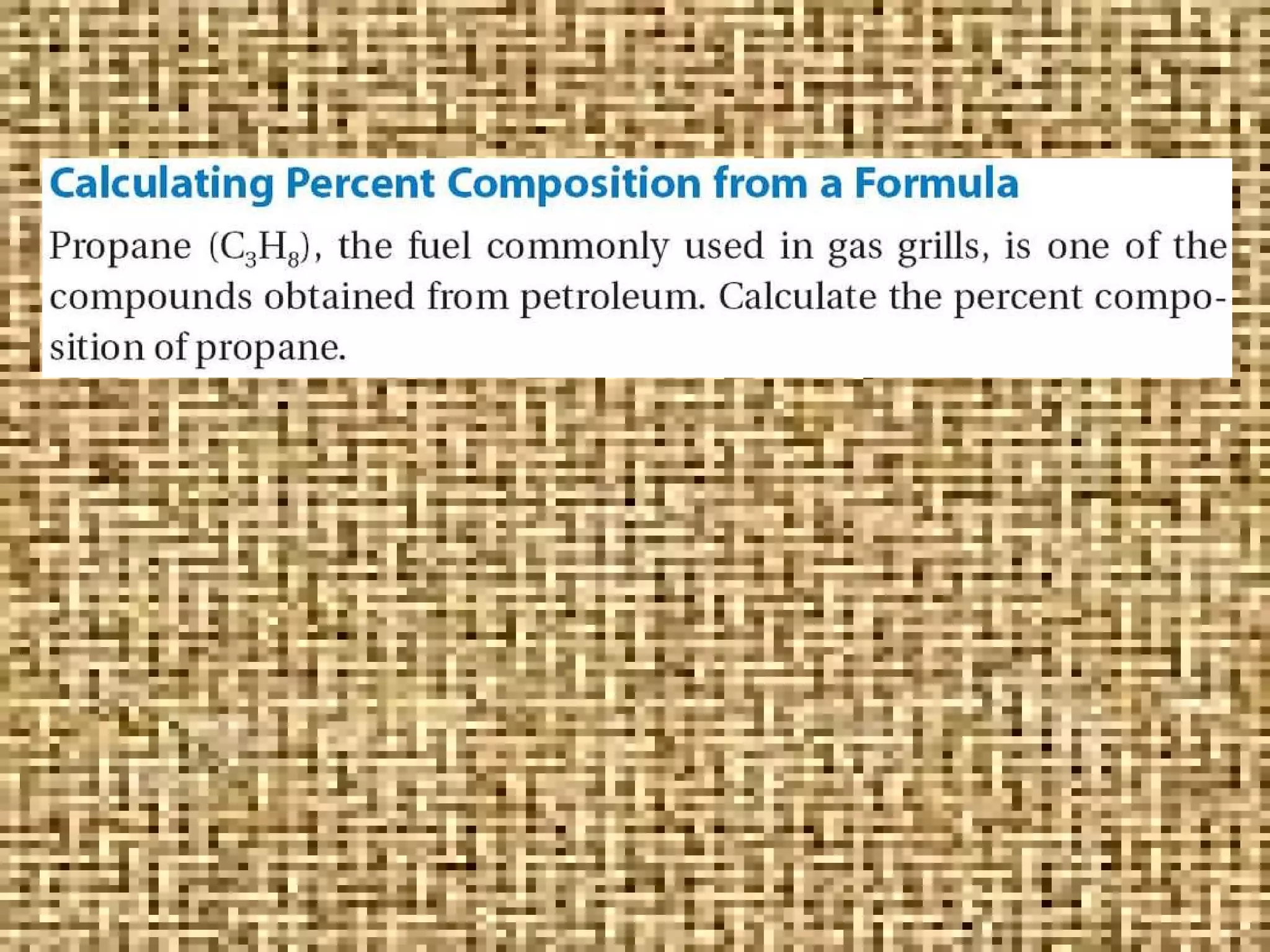
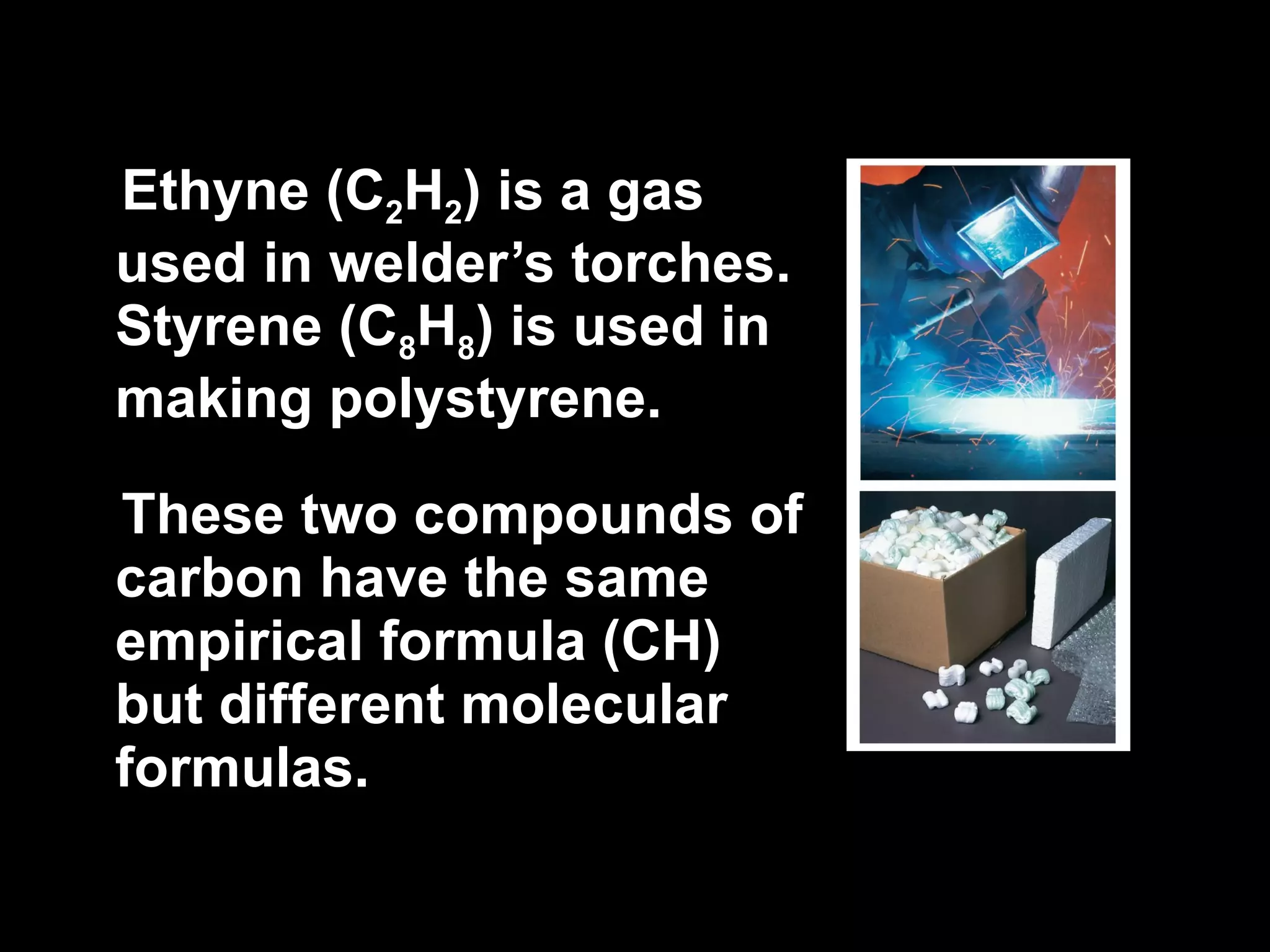
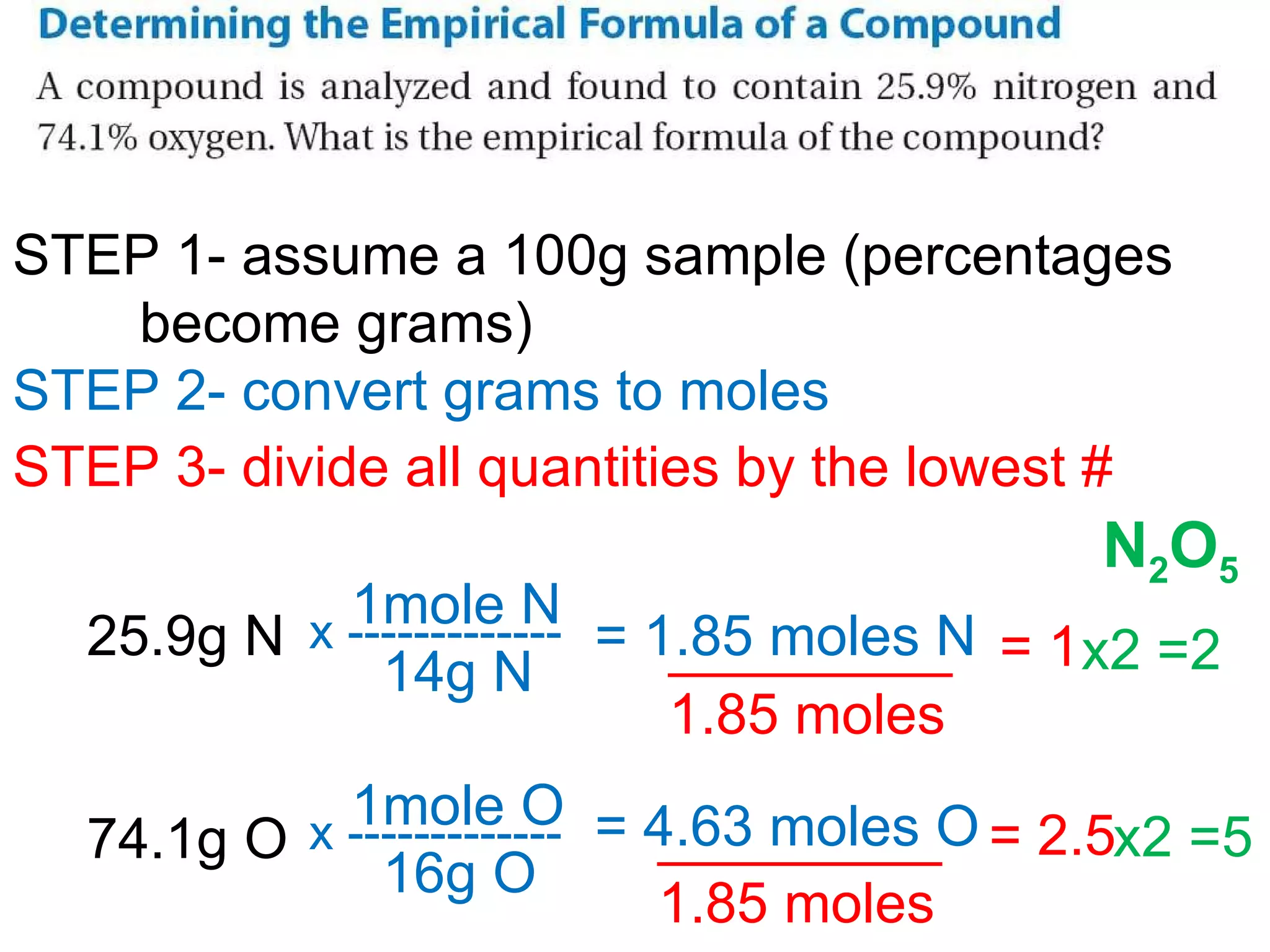
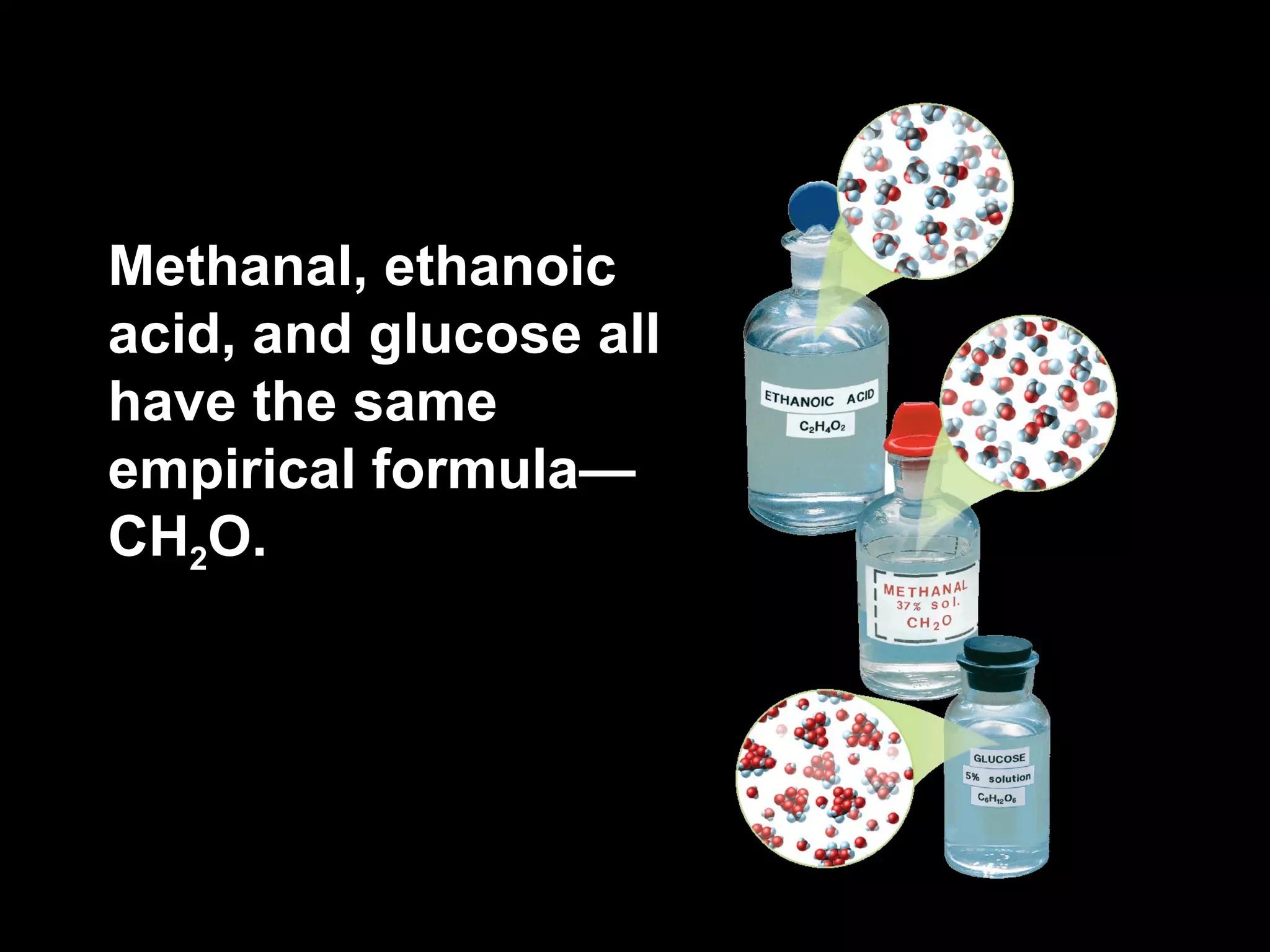
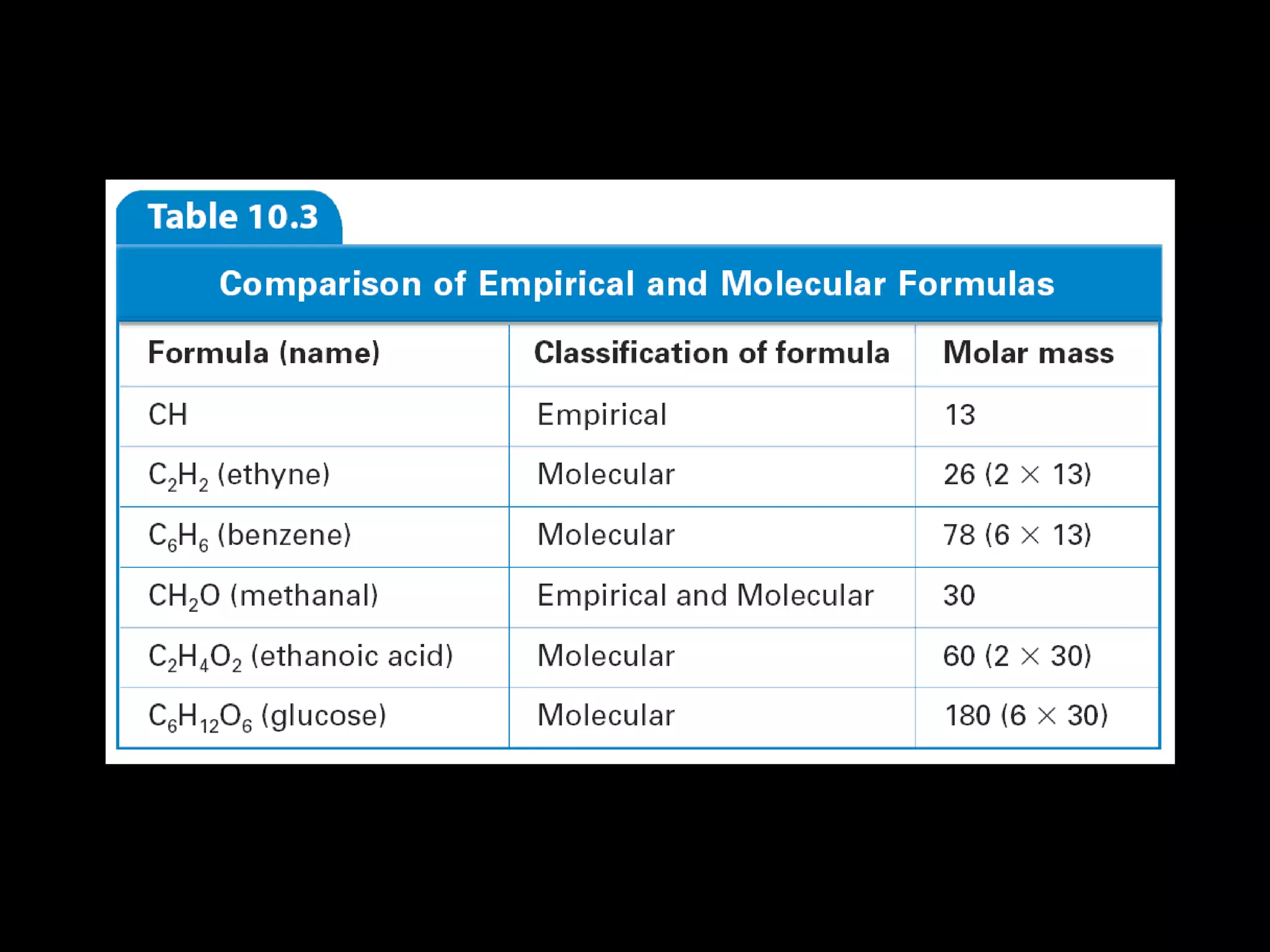
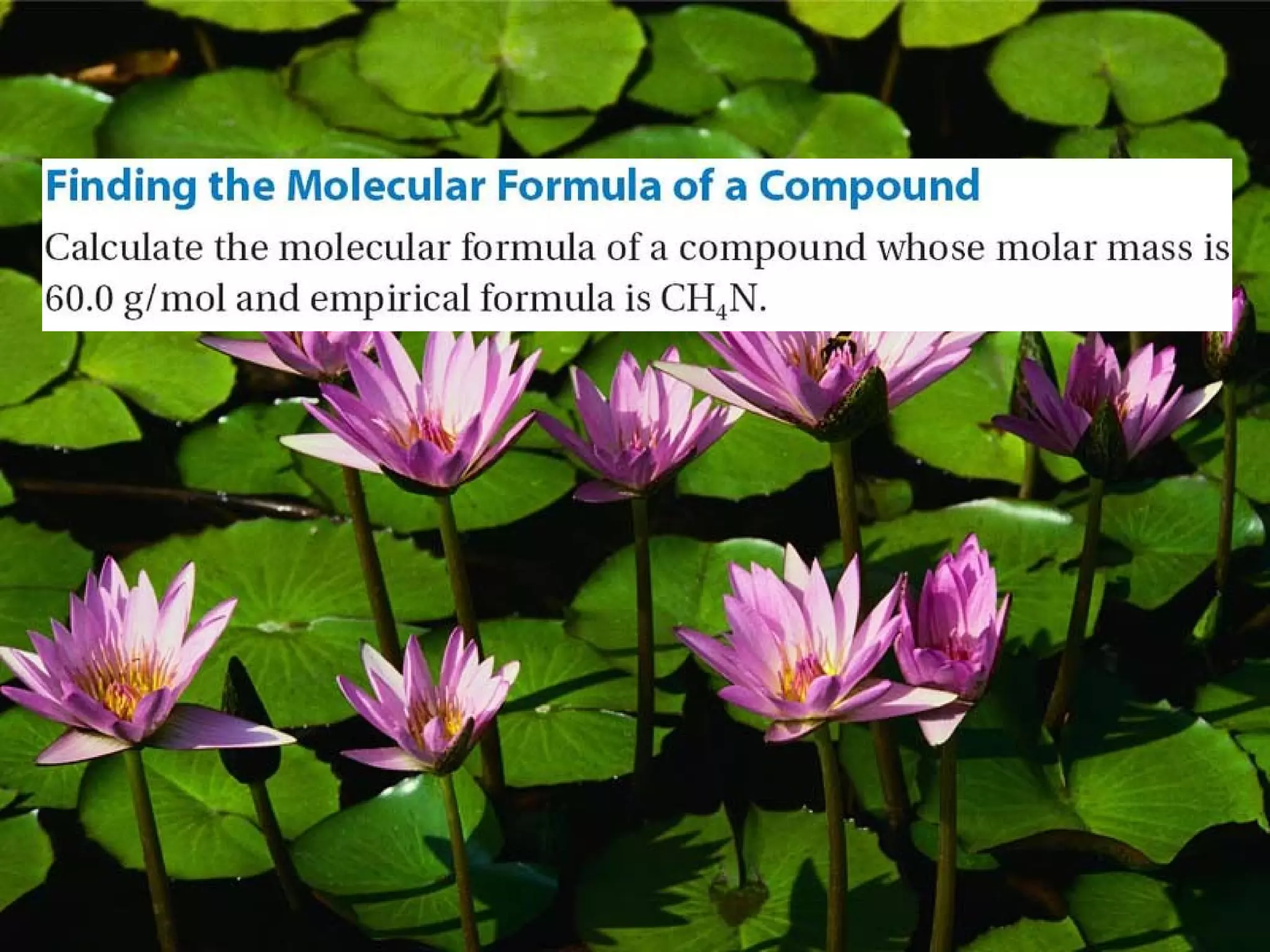
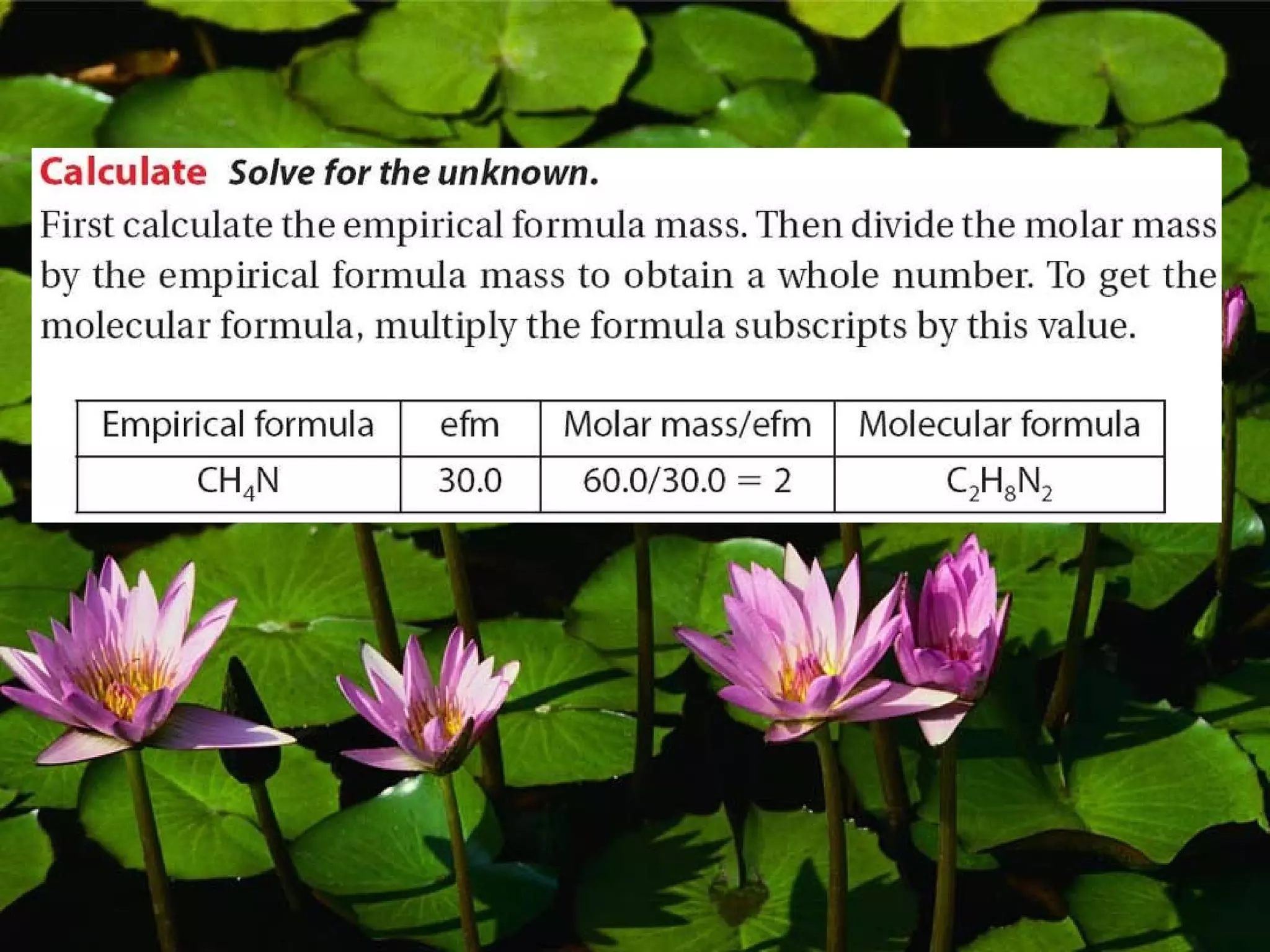
The document discusses empirical formulas, molecular formulas, and percent composition. It provides examples of compounds with the same empirical formula but different molecular formulas, like ethyne and styrene which both have an empirical formula of CH but different molecular formulas. It also gives examples of compounds like methanal, ethanoic acid, and glucose that have the same empirical formula of CH2O.