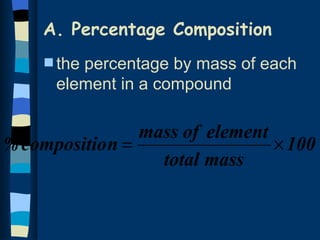
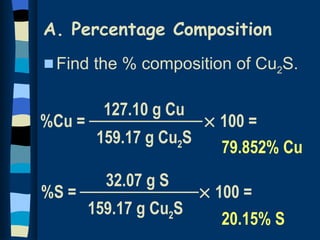
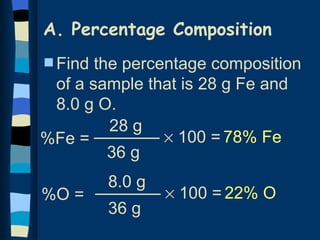
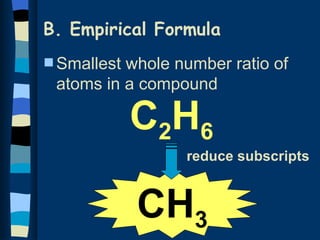
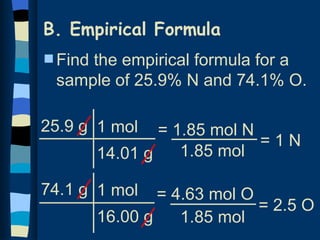
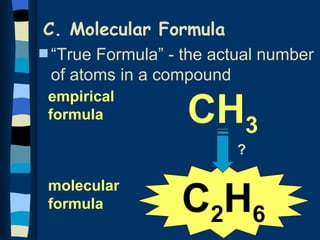
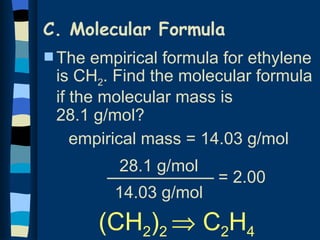
The document discusses methods for determining the percentage composition, empirical formula, and molecular formula of chemical compounds from experimental data. It provides examples of calculating percentage composition from mass data and determining the empirical and molecular formulas of samples. The key steps outlined are calculating percentage composition from element masses, determining moles of each element and reducing ratios to simplest whole numbers for empirical formulas, and relating molecular mass to empirical formula mass to derive molecular formulas.