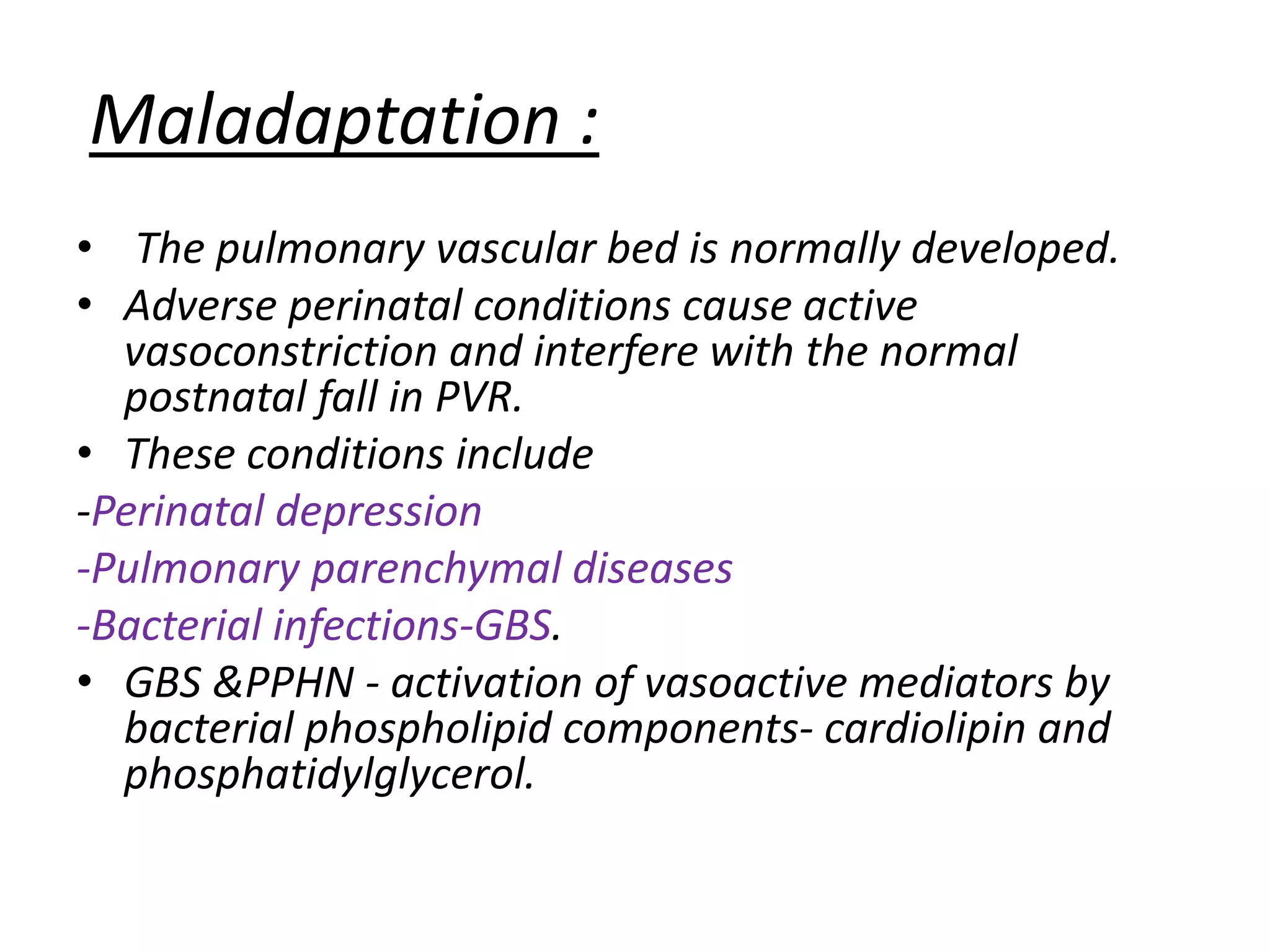
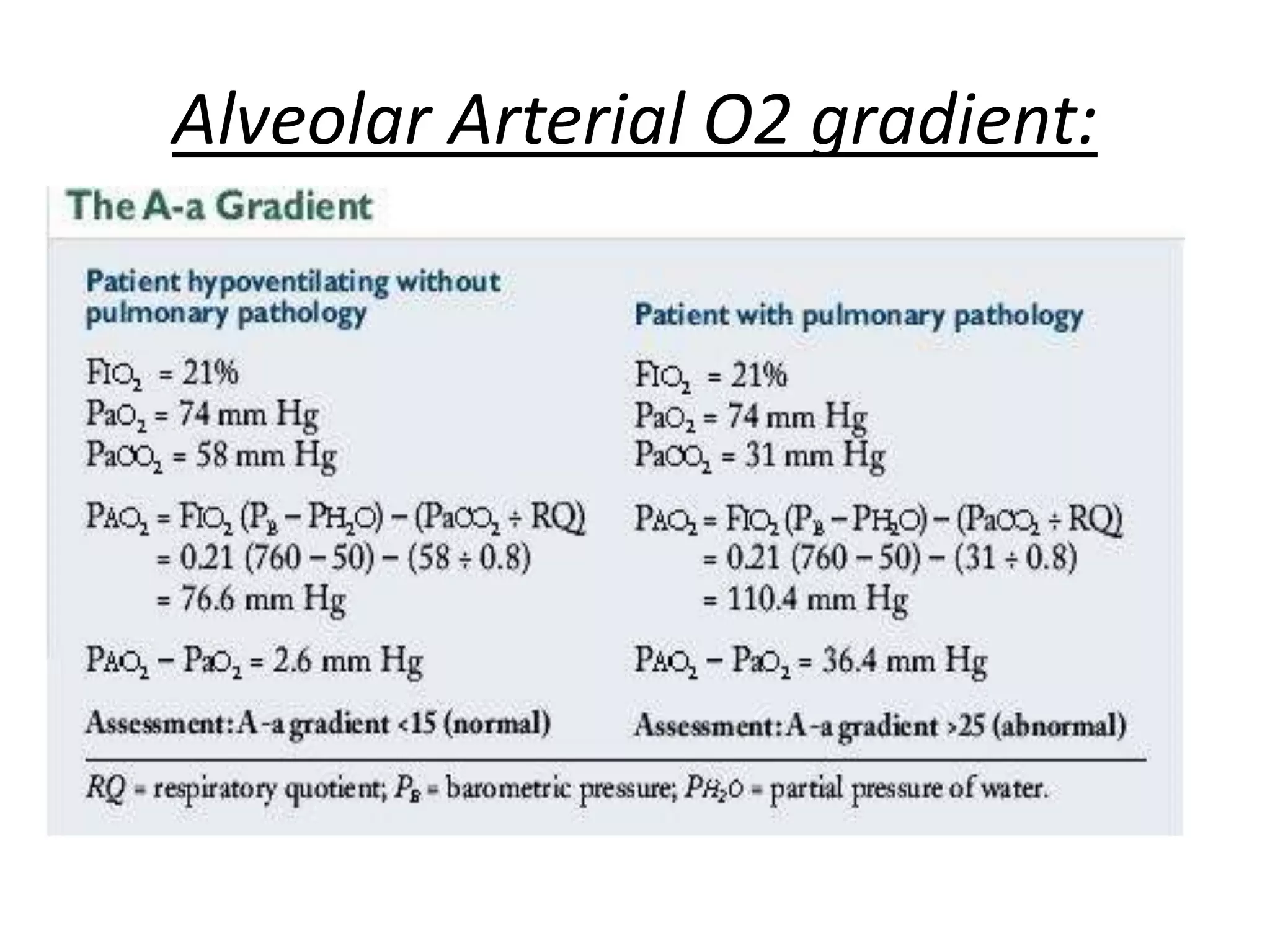
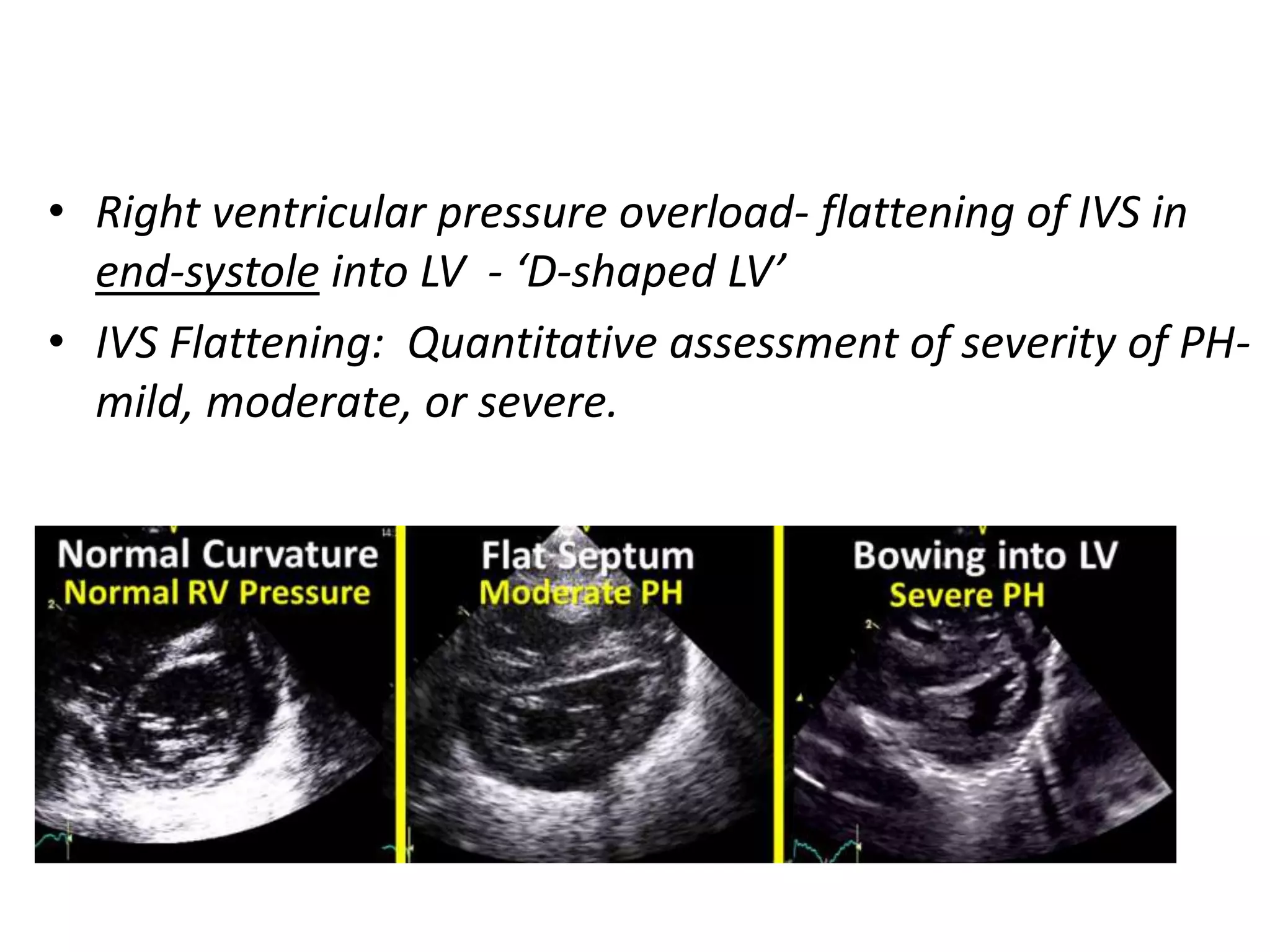
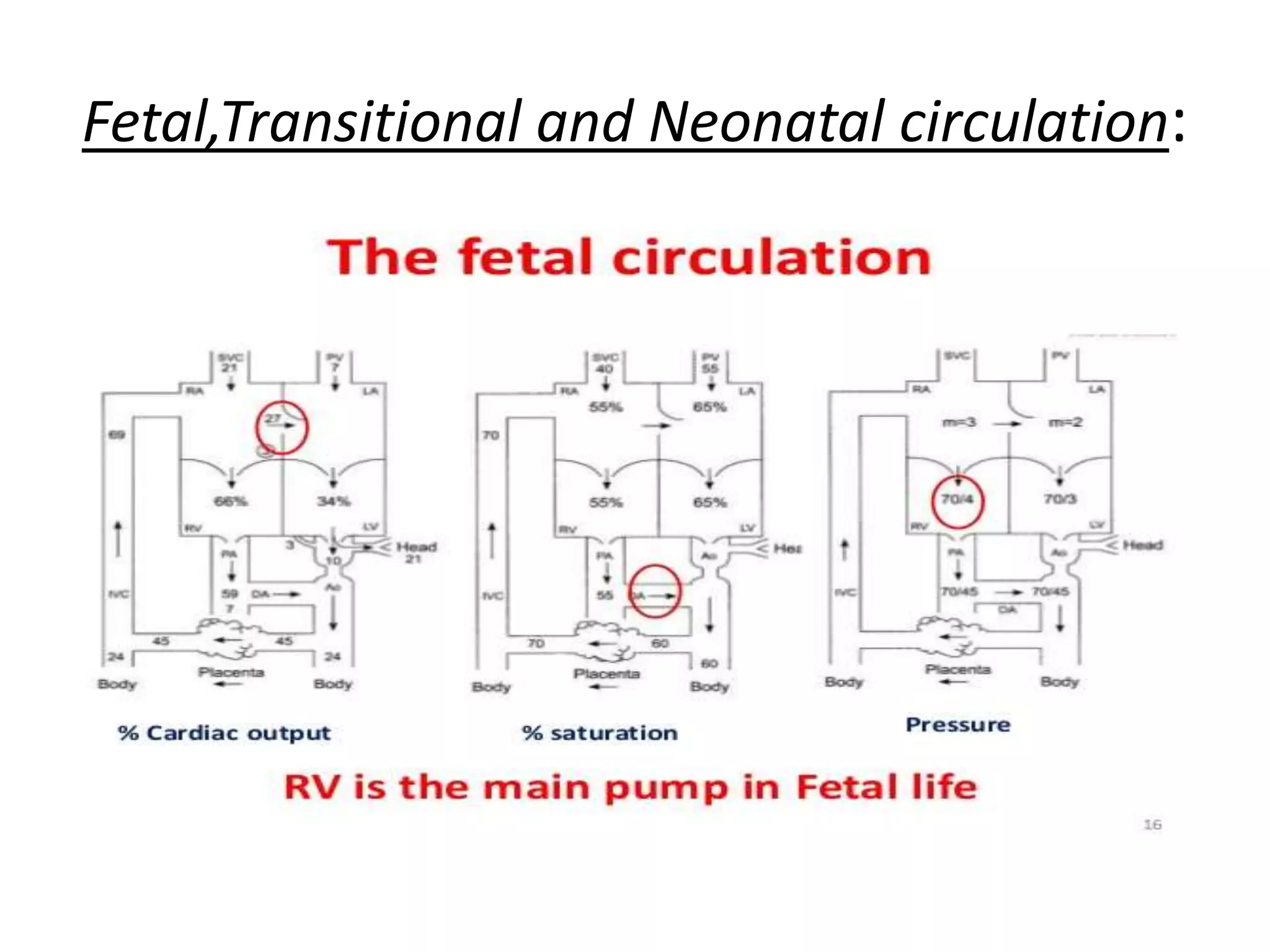
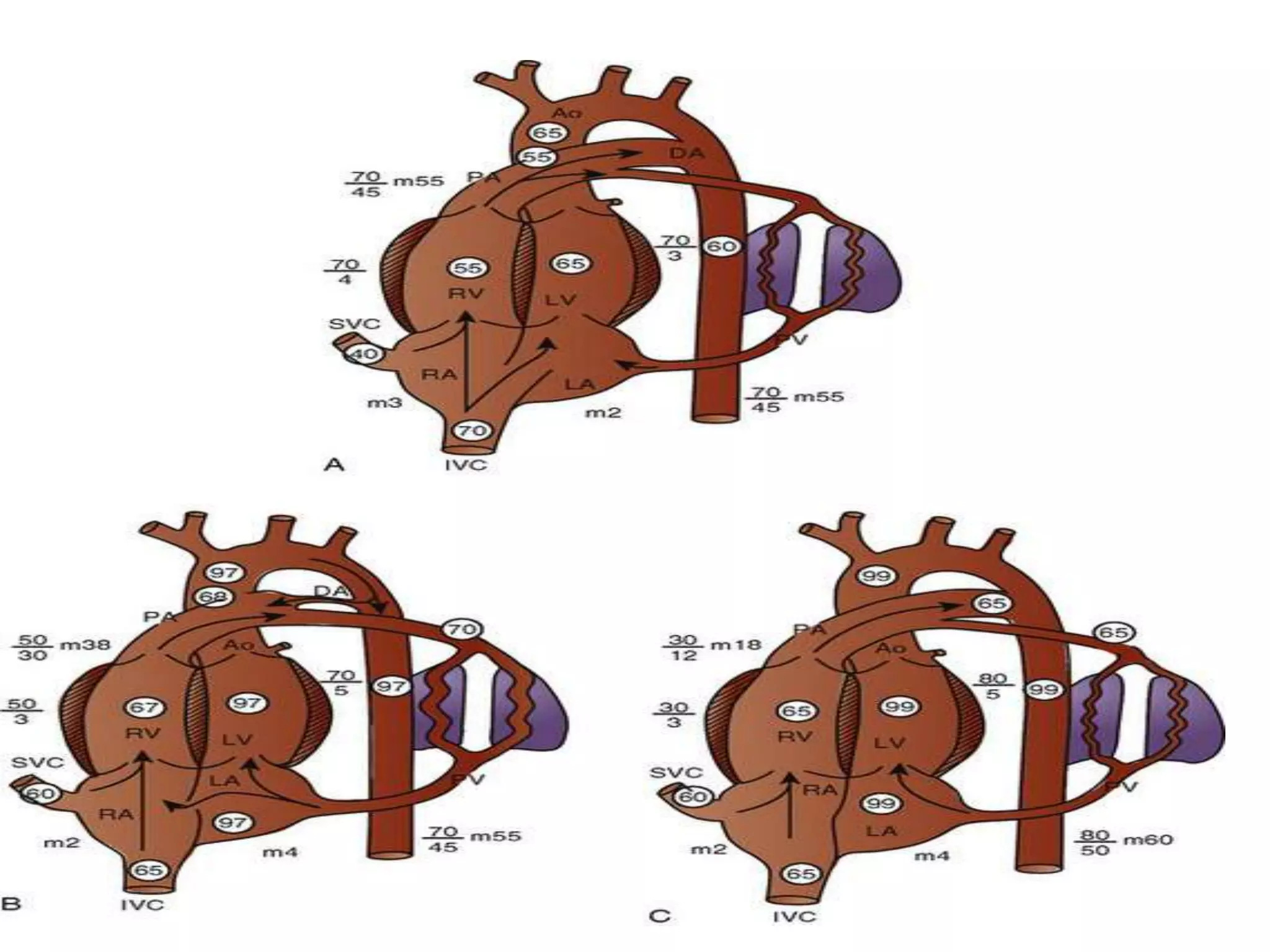
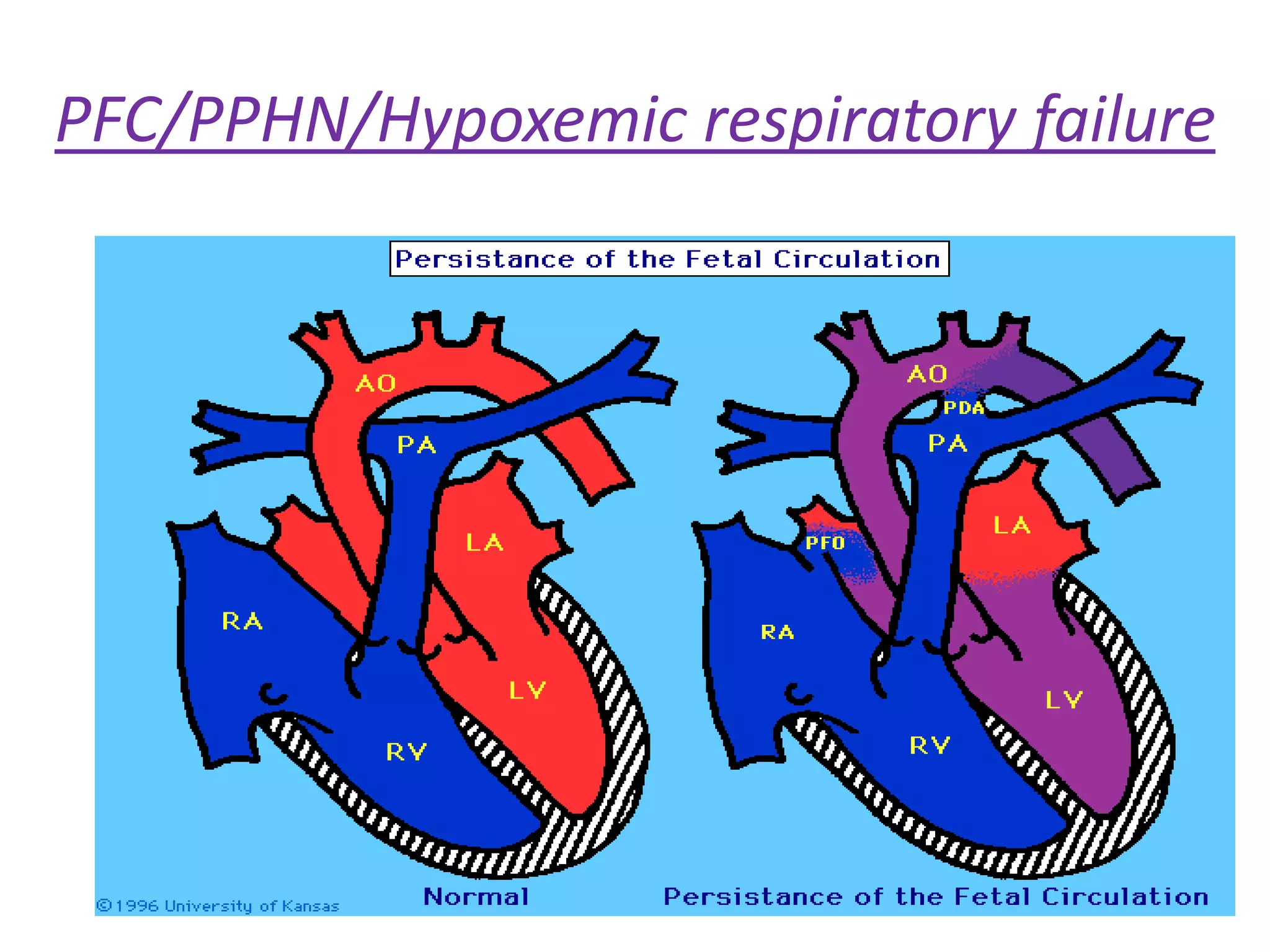
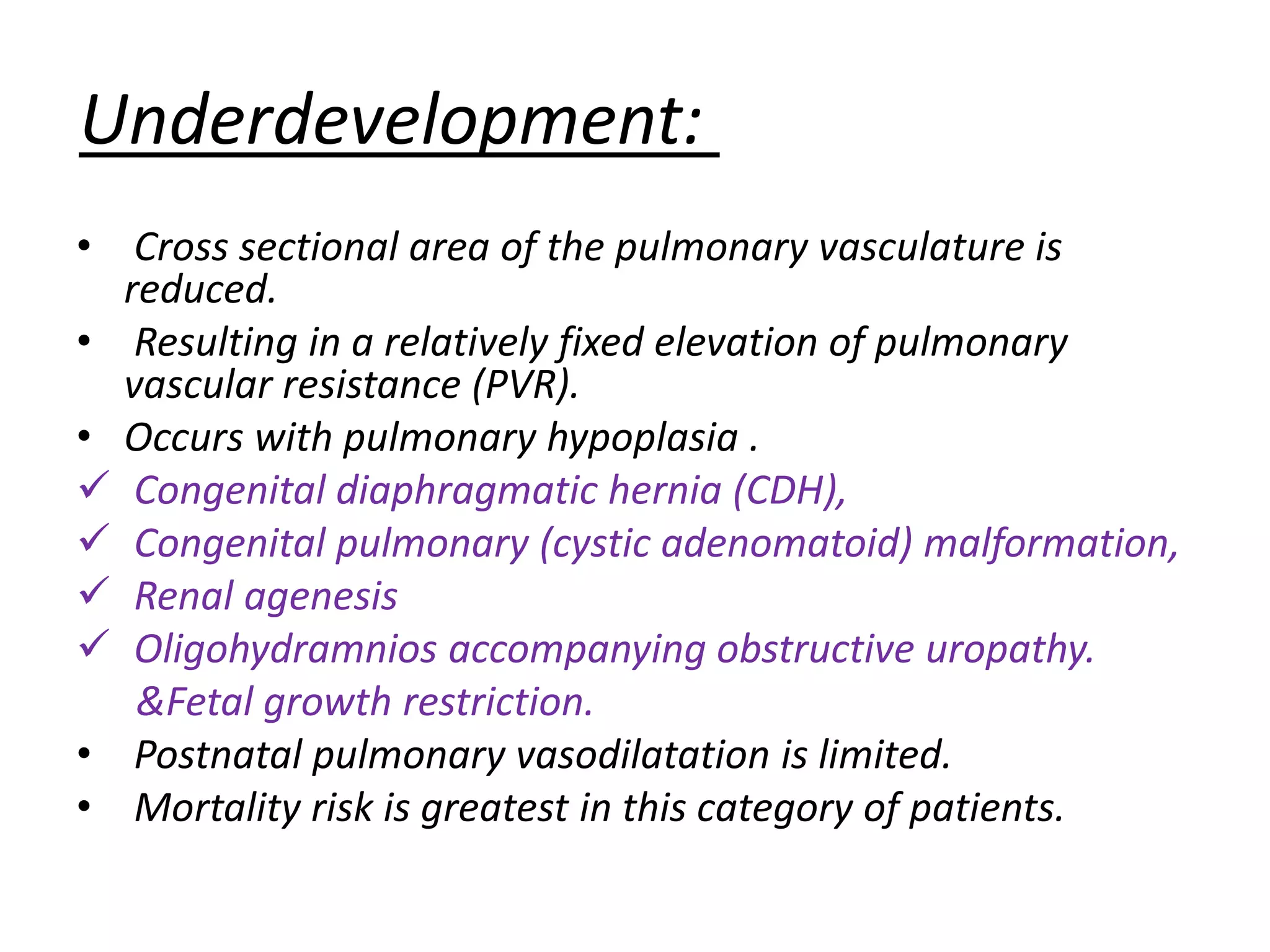
Persistent pulmonary hypertension of the newborn (PPHN) results from failure of the normal decrease in pulmonary vascular resistance after birth, causing right-to-left shunting of blood and hypoxemia. It can be caused by underdevelopment, maldevelopment, or maladaptation of the pulmonary vasculature. Clinical features include cyanosis and respiratory distress within the first 24 hours of life. Diagnosis involves echocardiography demonstrating elevated pulmonary pressures and responding poorly to oxygen challenges. Treatment aims to reduce PVR through ventilation strategies, medications, and potentially extracorporeal membrane oxygenation.







![Maldevelopment:
• Conditions associated with PPHN caused by
vascular maldevelopment include
Post term delivery.
Meconium staining & meconium aspiration
syndrome (MAS)
IU exposure to SSRI.
• Excessive perfusion of the fetal lung also may
predispose to vascular maldevelopment.
Premature closure of the ductus arteriosus
[NSAIDs]
High placental vascular resistance
TAPVC](https://image.slidesharecdn.com/persistentpulmonaryhypertensionofnewborn-pphn-170516192942/75/Persistent-pulmonary-hypertension-of-newborn-PPHN-12-2048.jpg)