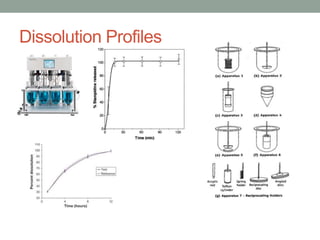
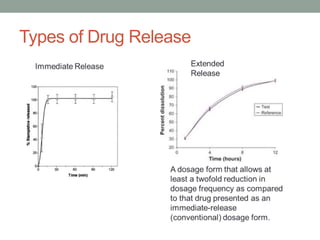
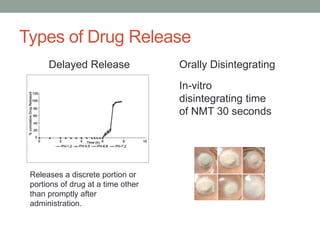
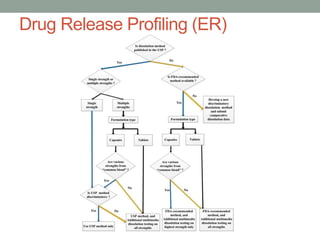
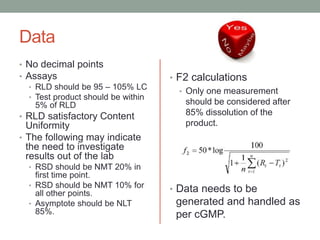
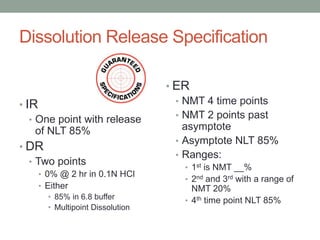
This document discusses dissolution profiles and specifications. It describes different types of drug release and provides guidance on generating dissolution profiles for immediate release, delayed release, and extended release products. Key steps include following the FDA dissolution method database, using the same apparatus and conditions as the reference listed drug, and demonstrating stability in various pH conditions. It also addresses alcohol-induced dose dumping tests in various ethanol concentrations. Specifications are typically based on achieving at least 85% dissolution by certain time points.