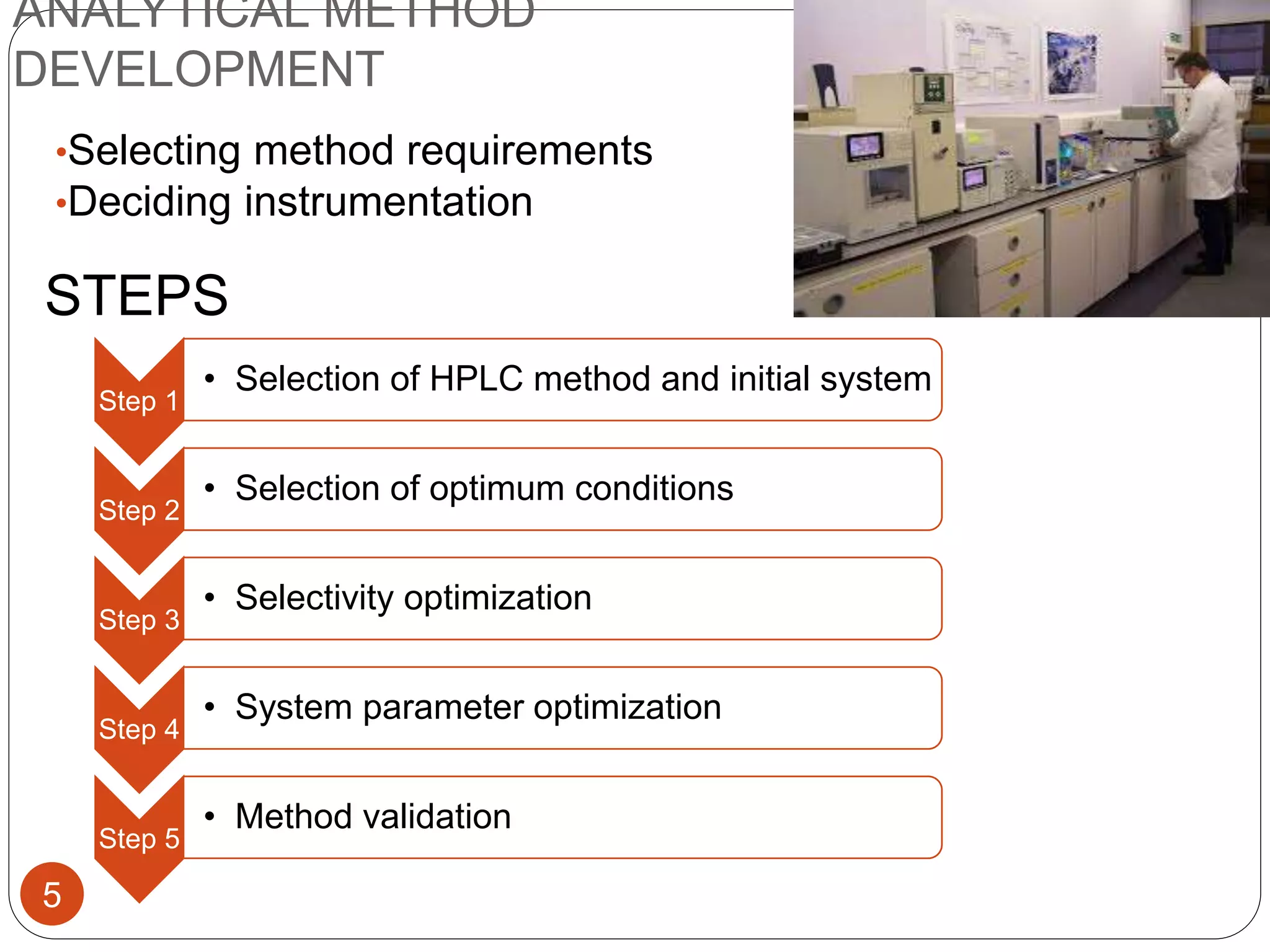
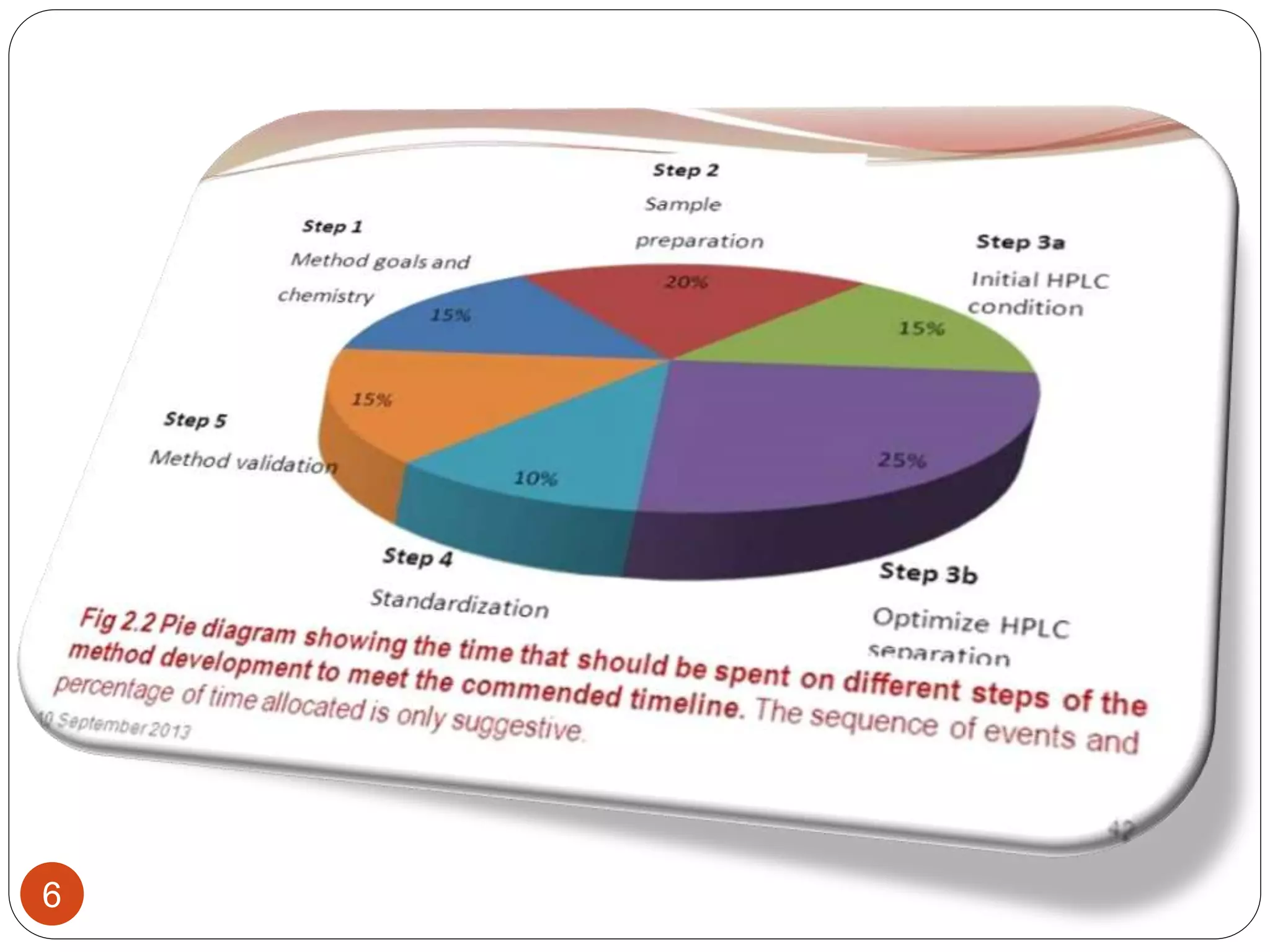
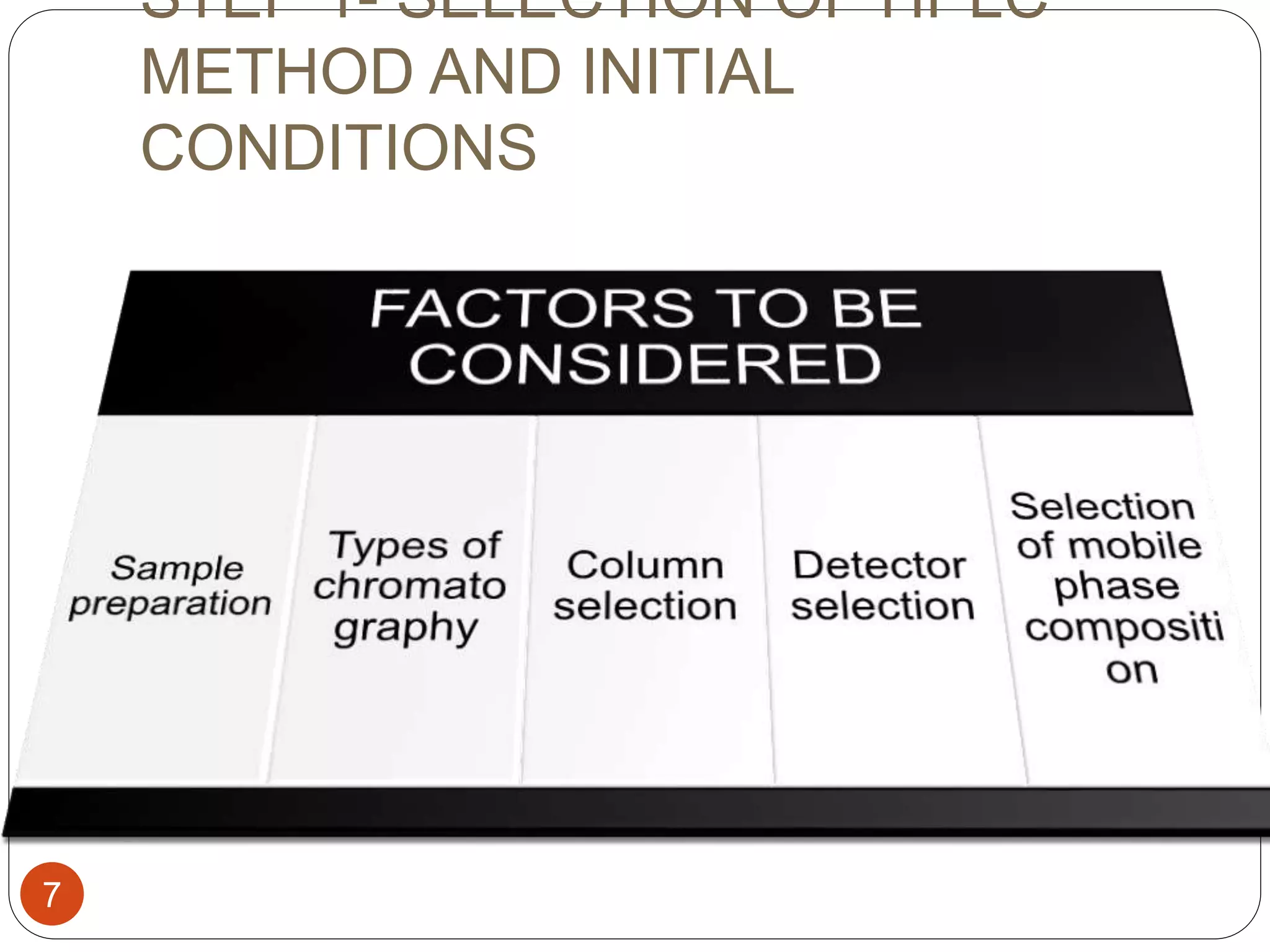
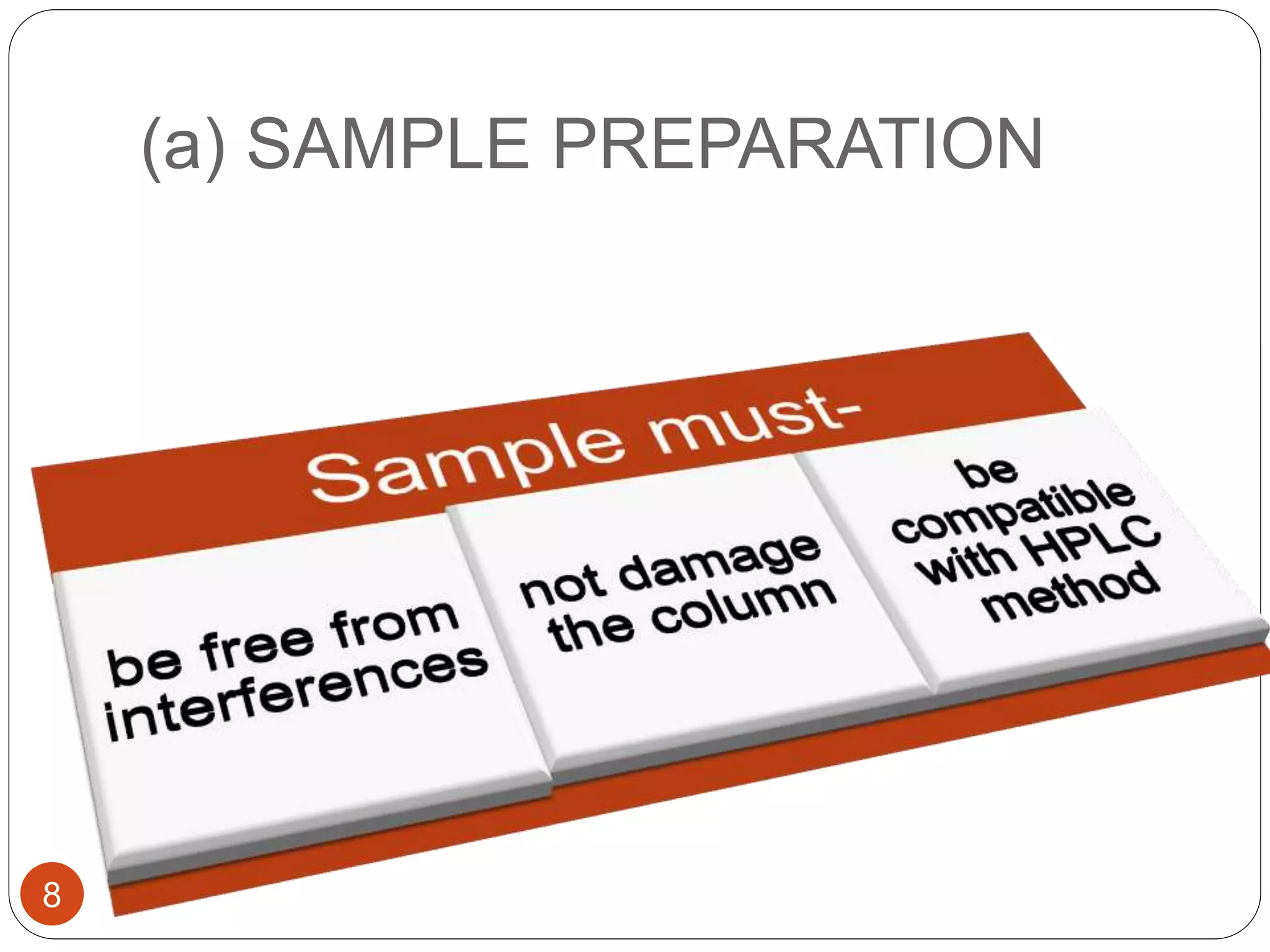
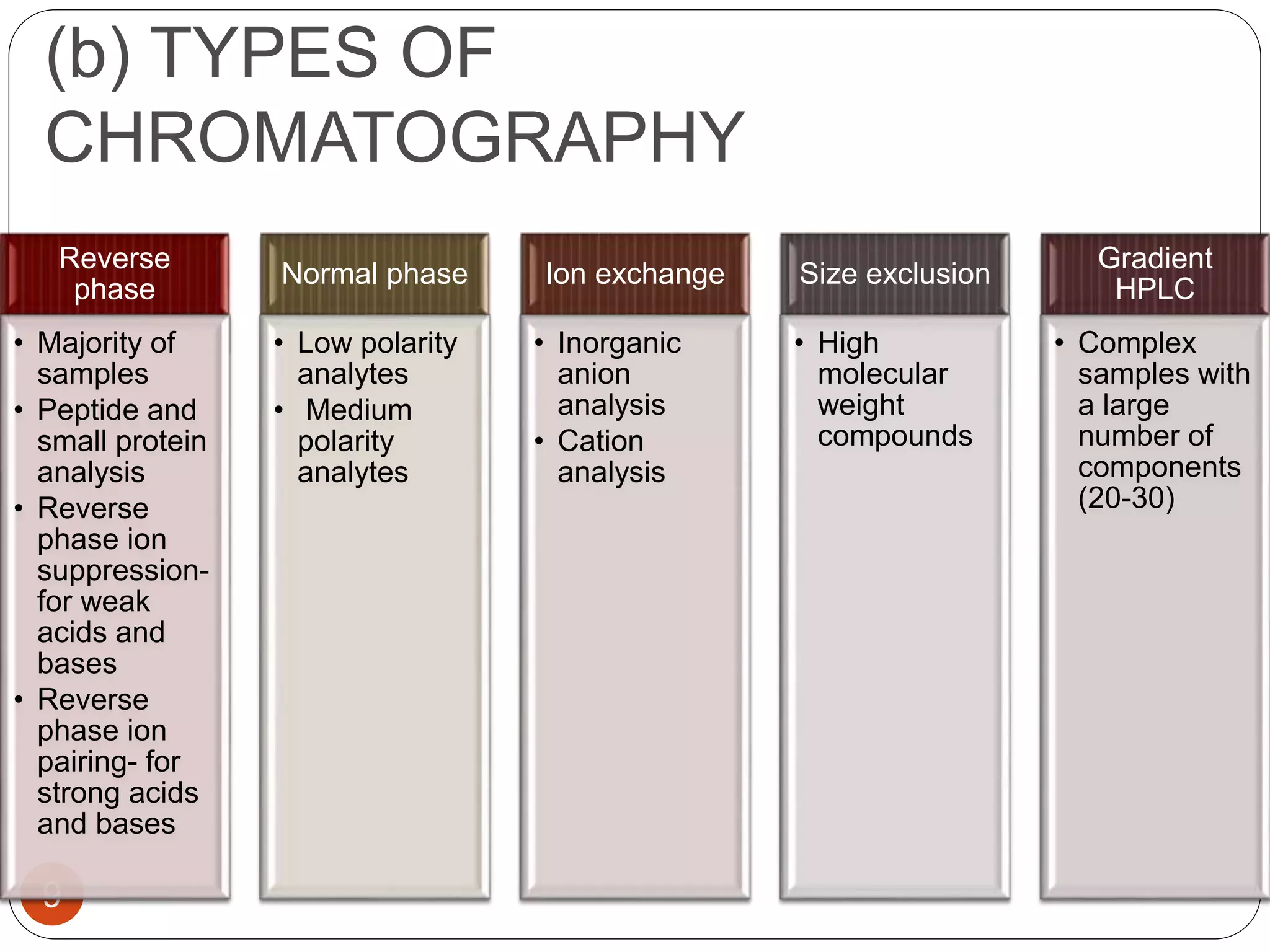
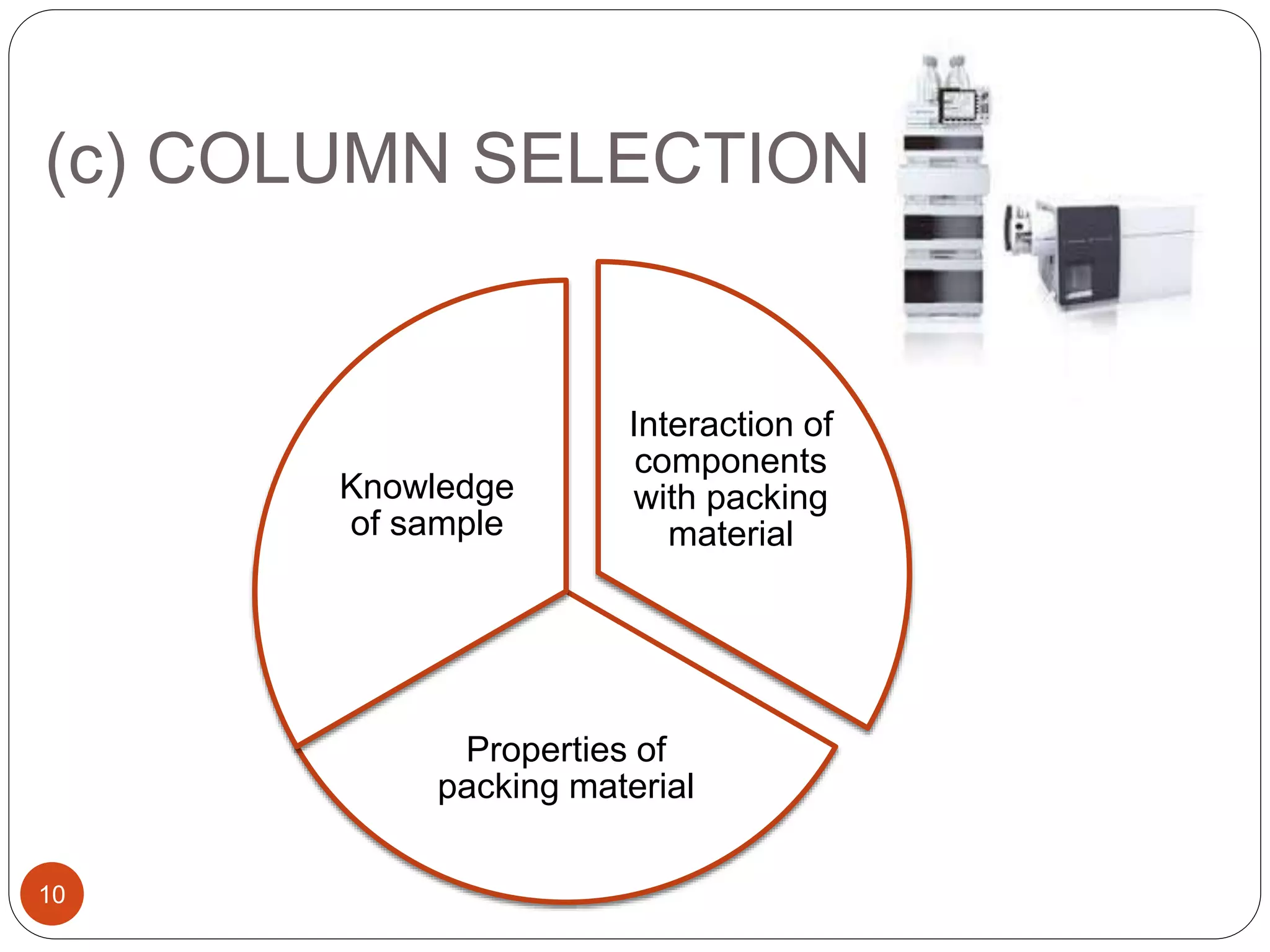
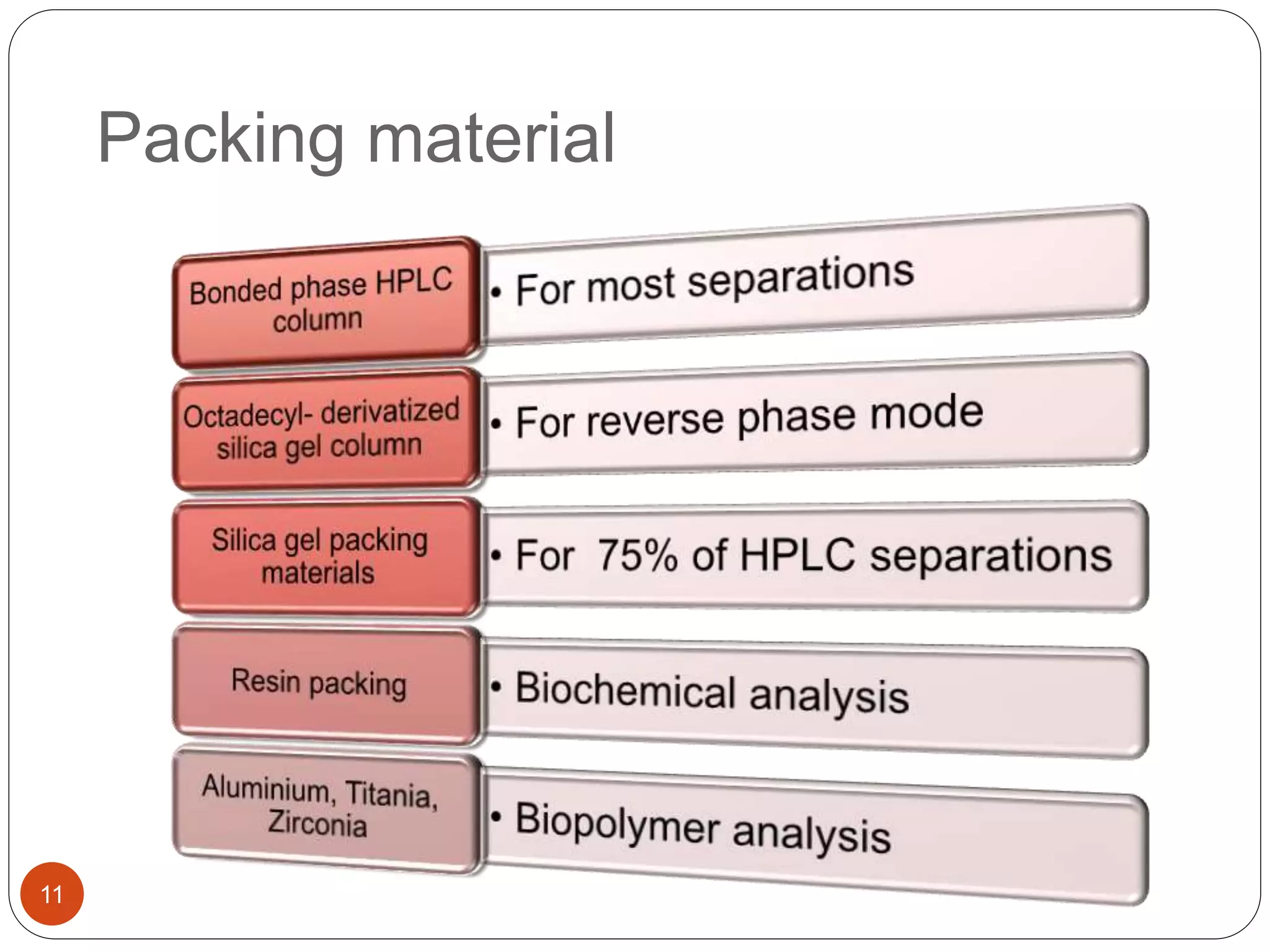
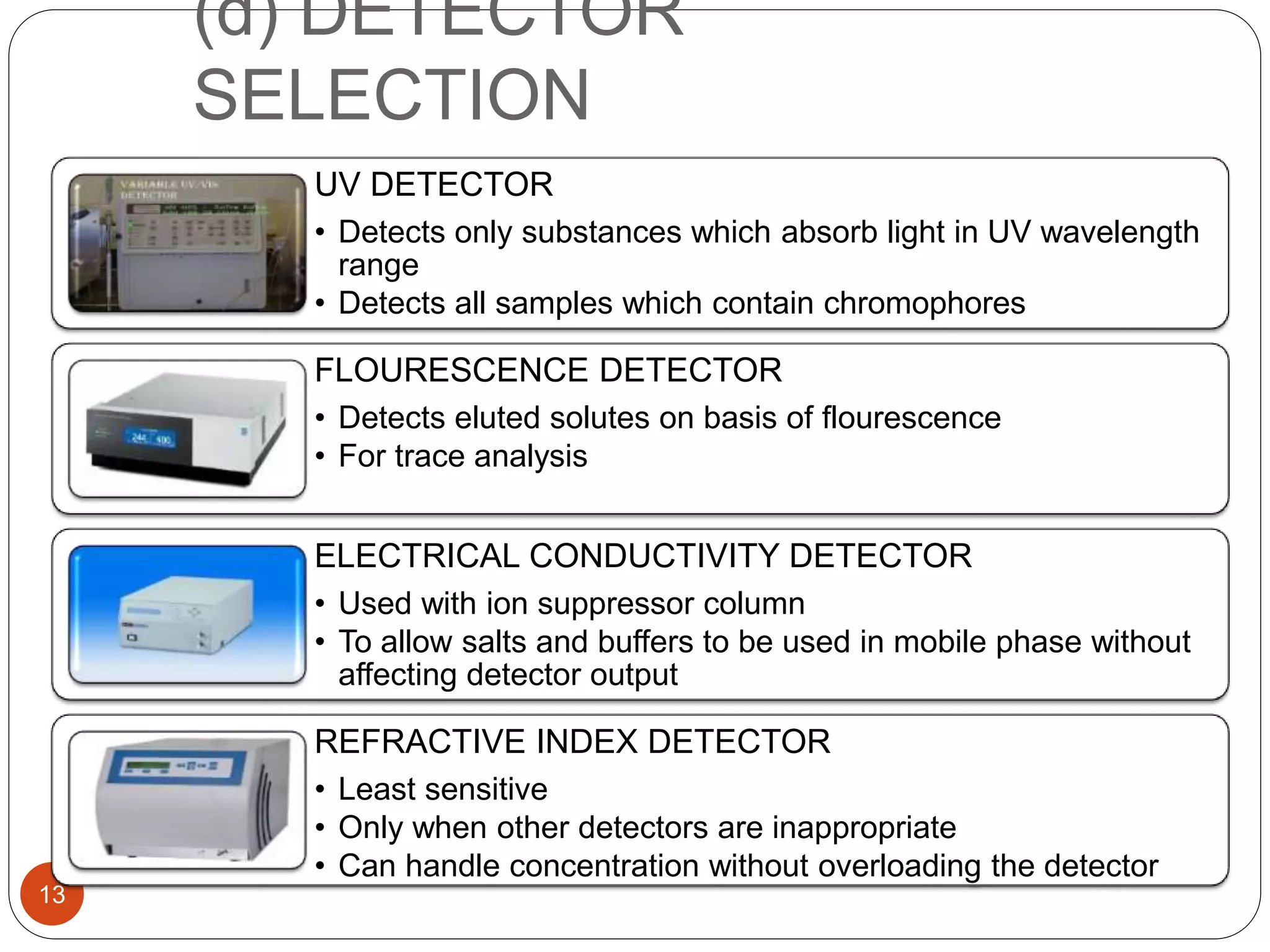
This document outlines the five main steps for developing an analytical HPLC method: 1) selecting the initial HPLC method and conditions, 2) selecting the initial chromatographic conditions, 3) optimizing selectivity, 4) optimizing system parameters, and 5) validating the method. Key aspects of each step are discussed, including selecting the type of chromatography, column, detector, and mobile phase based on the analytes. The goal is to develop a validated method that provides adequate resolution and selectivity within a desired analysis time.