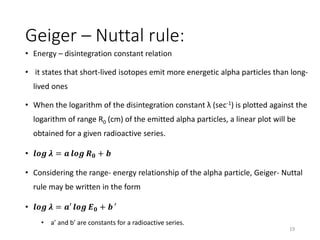
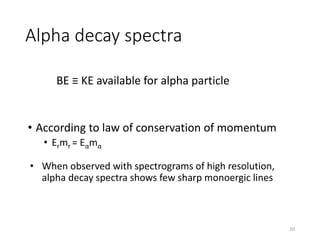
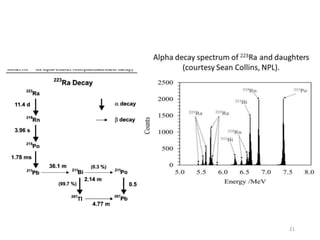
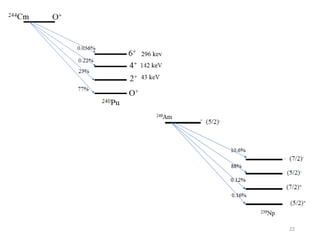
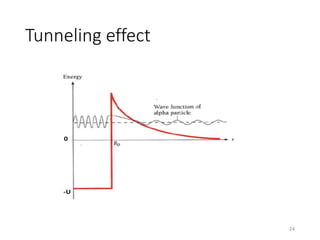
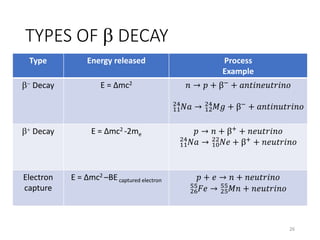
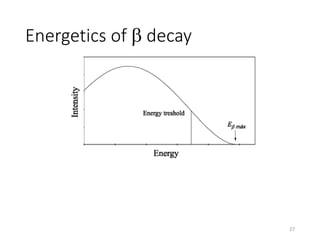
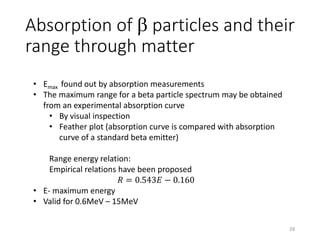
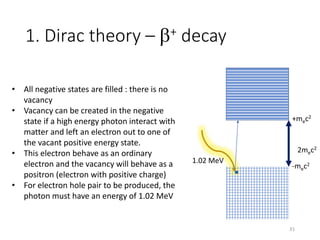
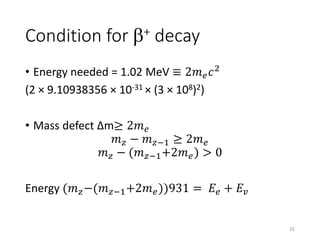
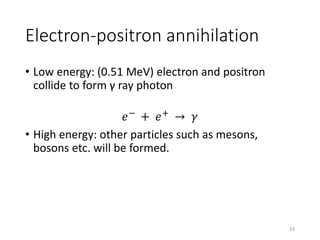
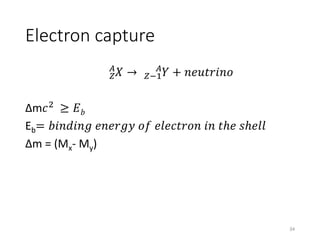
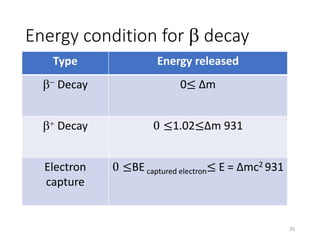
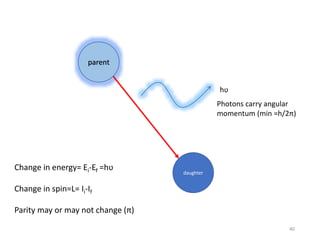
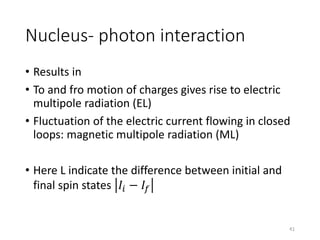
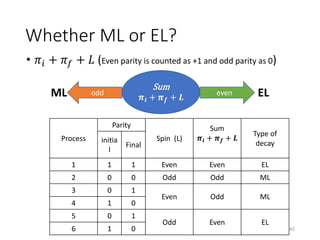
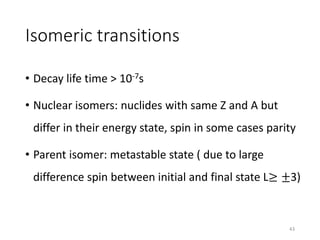
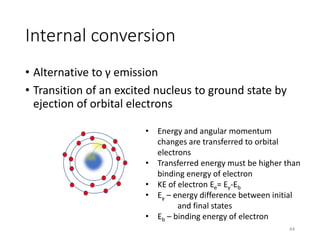
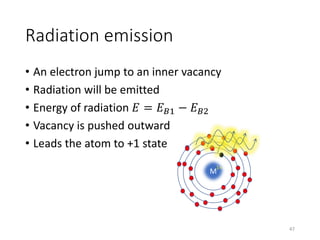
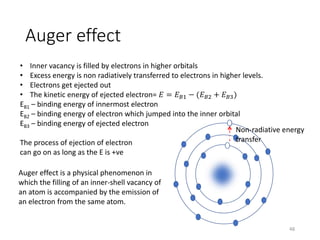
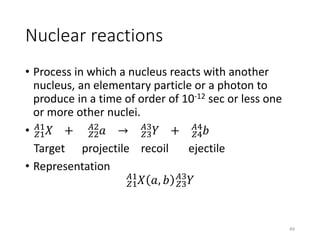
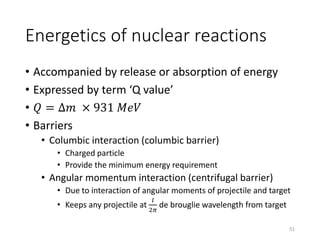
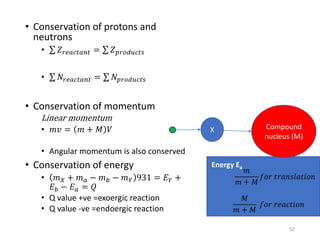
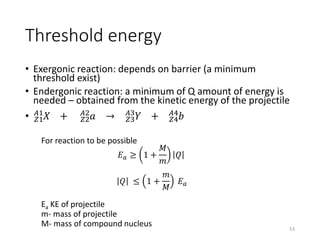
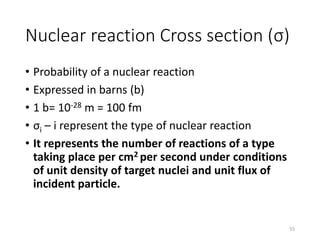
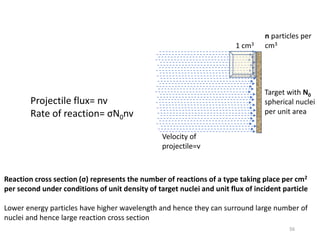
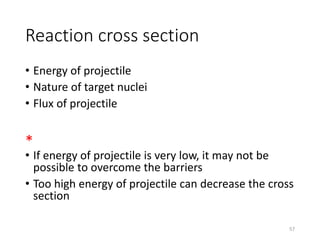
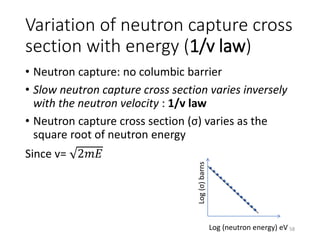
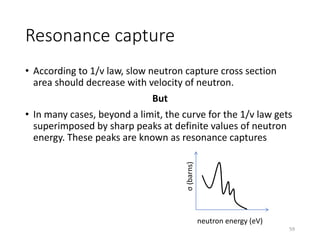
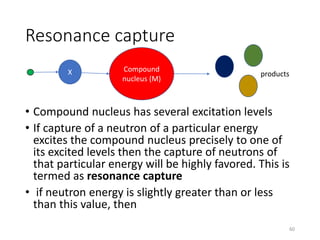
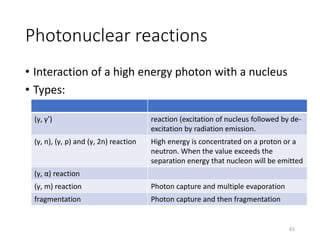
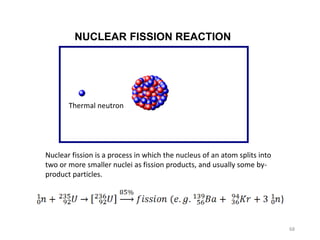
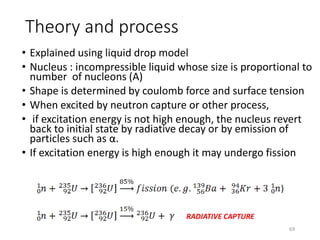
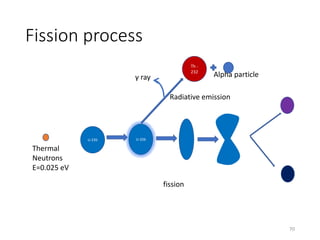
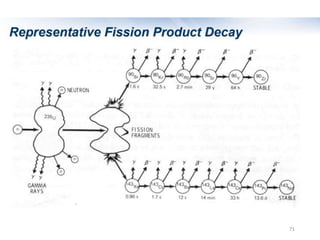
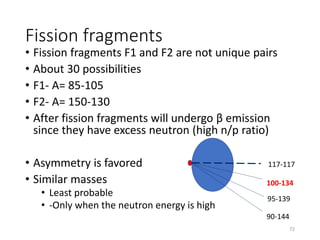
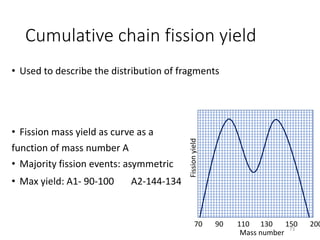
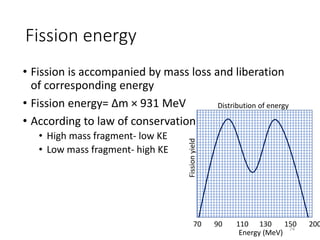
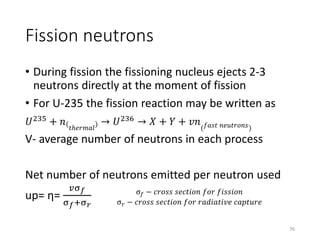
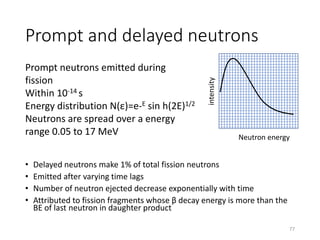
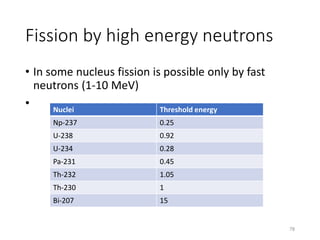
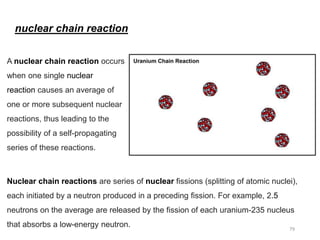
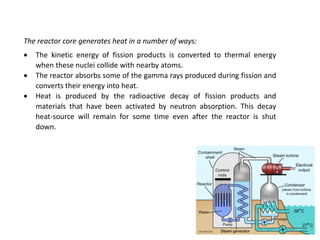
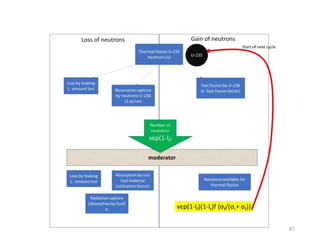
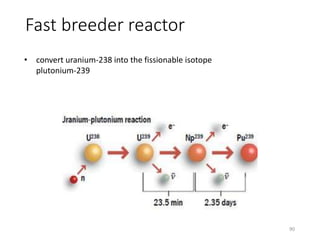
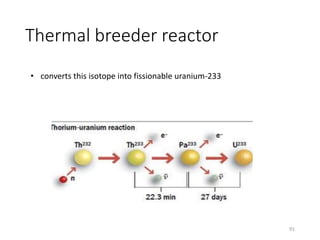
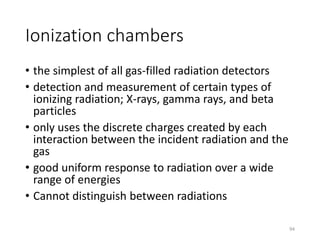
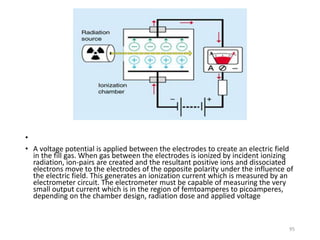
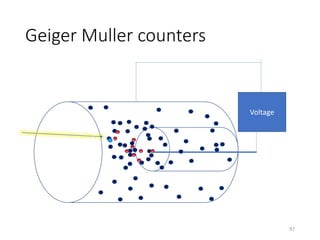
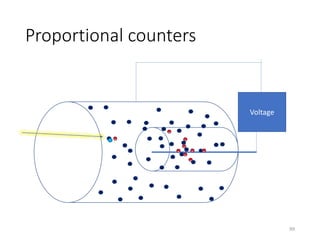
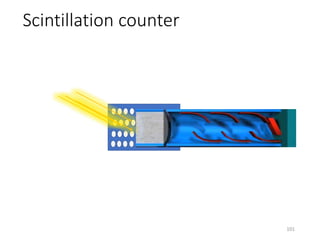
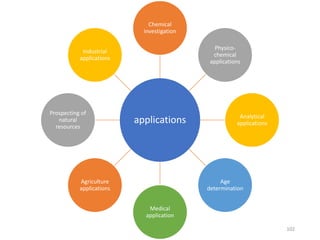
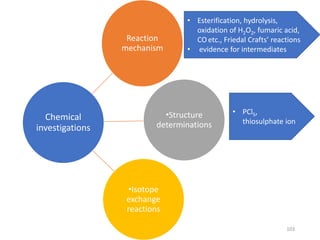
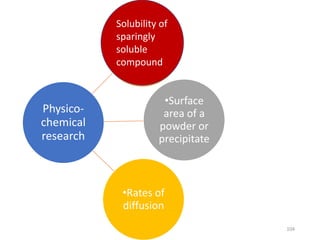
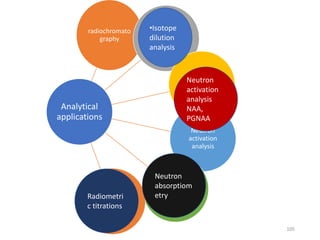
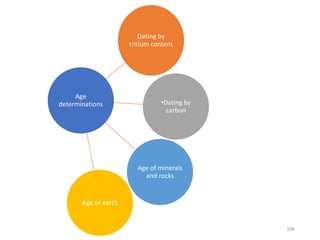
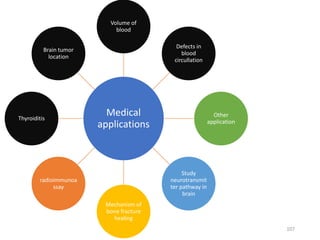
Nuclear chemistry deals with radioactive processes and transformations that occur within atom nuclei. It involves the study of radioactive decay, nuclear reactions, nuclear reactors, radioactive waste management, and applications of radioisotopes. Some key areas covered include alpha and beta decay, nuclear stability factors like binding energy and neutron-to-proton ratio, and nuclear reaction terms such as cross-section and fission.







![Energy of alpha particle
• The maximum energy available for alpha particle is
equivalent to the mass defect
𝐸 = [𝑚𝑝 − (𝑚𝑟 + 𝑚α)] 931 MeV
• 𝑚 𝑝- mass of parent nucleus
• 𝑚 𝑟- mass of daughter (recoil) nucleus
• 𝑚α− mass of alpha particle
• E= Er +Eα
15](https://image.slidesharecdn.com/nuclearchemistry1-200209184446/85/Nuclear-chemistry-15-320.jpg)