Embed presentation
Downloaded 29 times


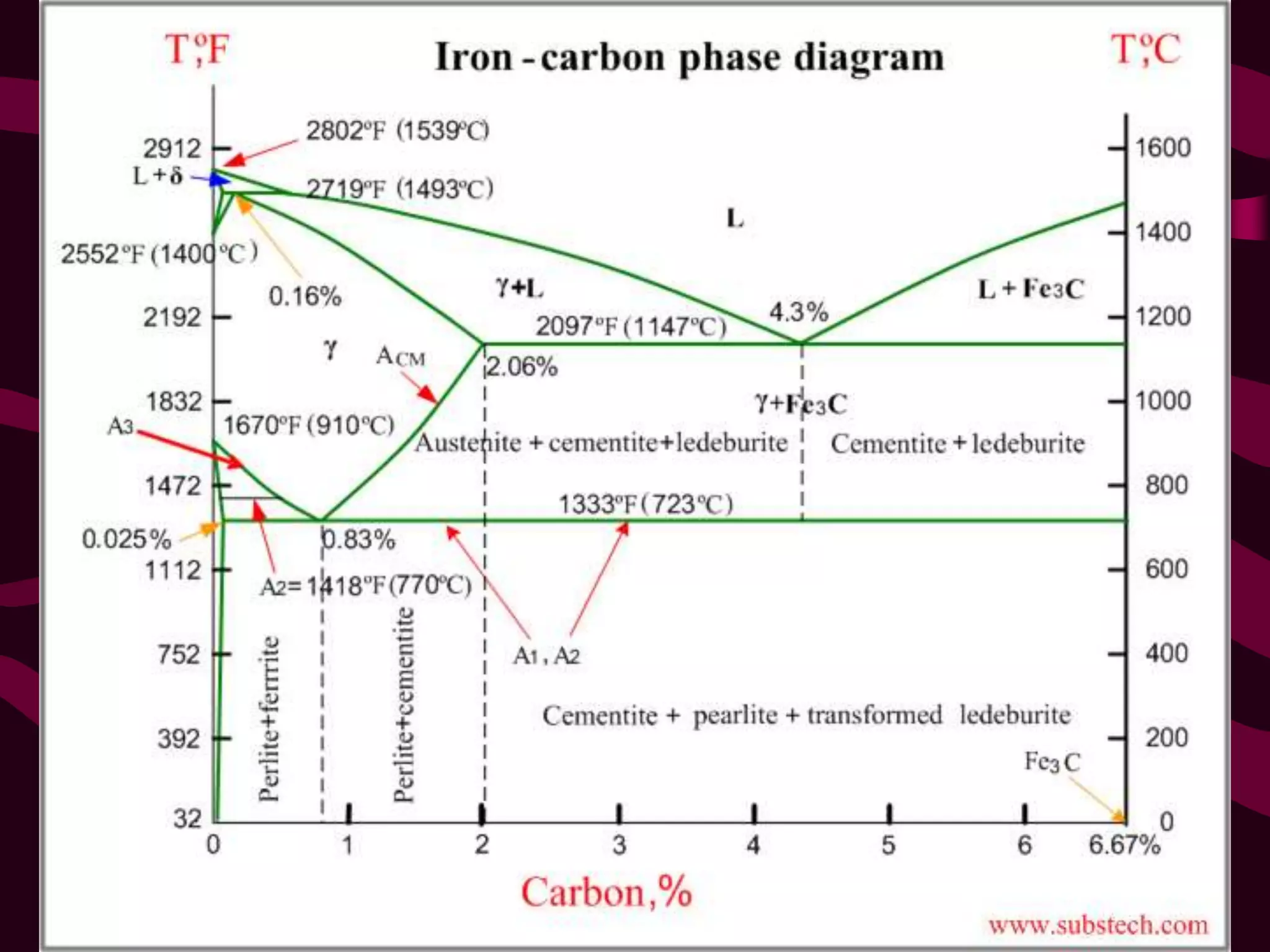
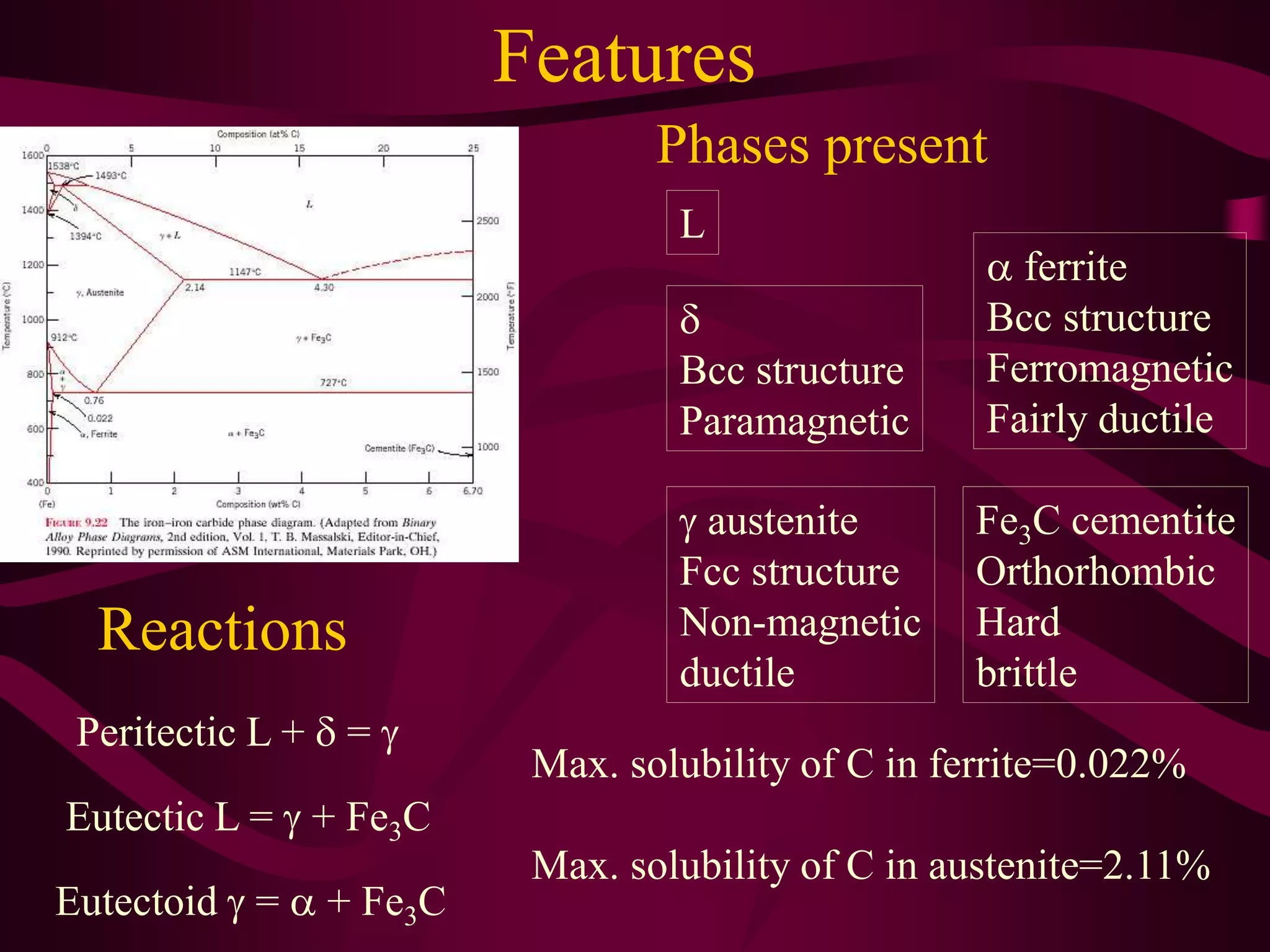
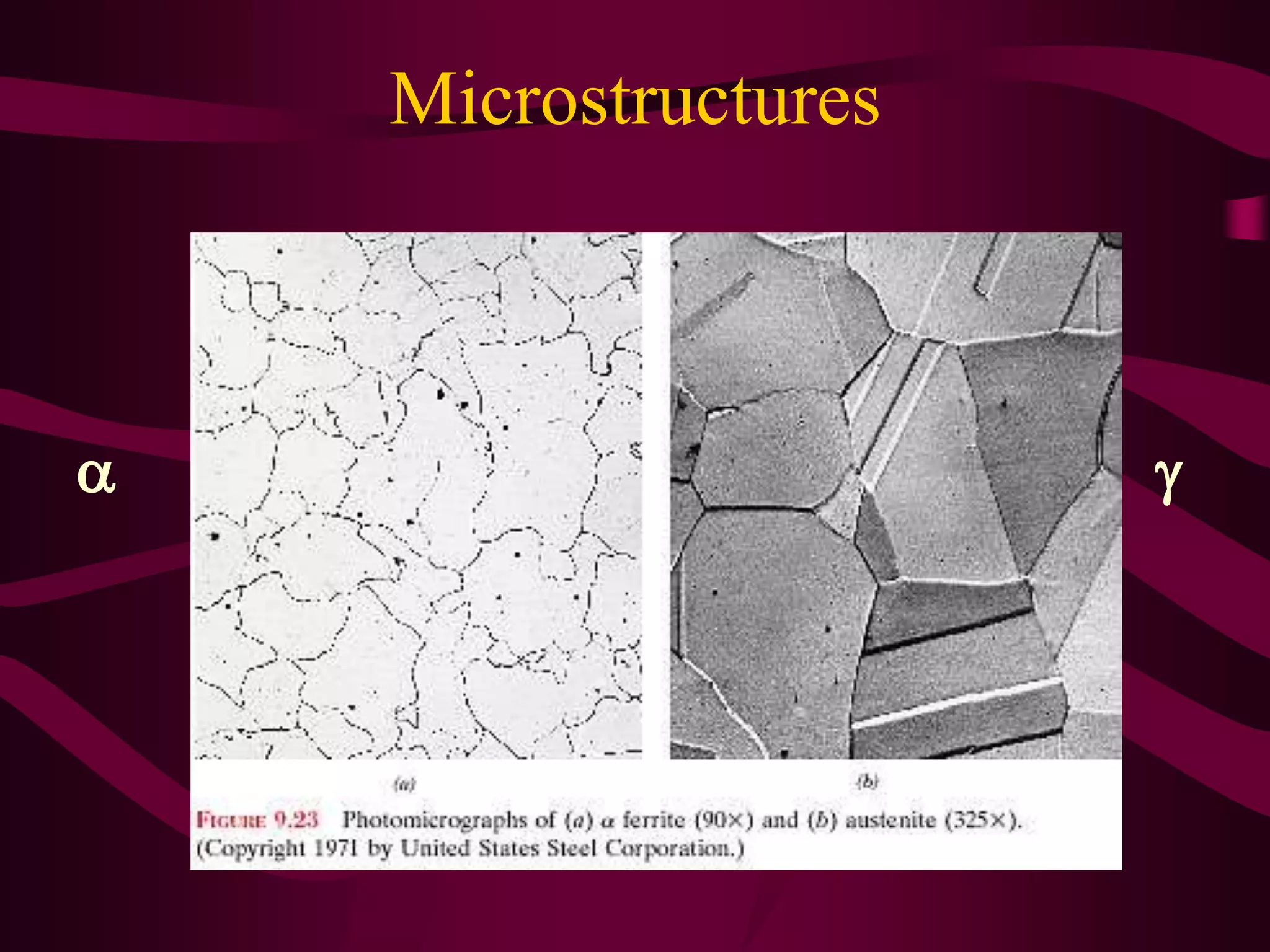


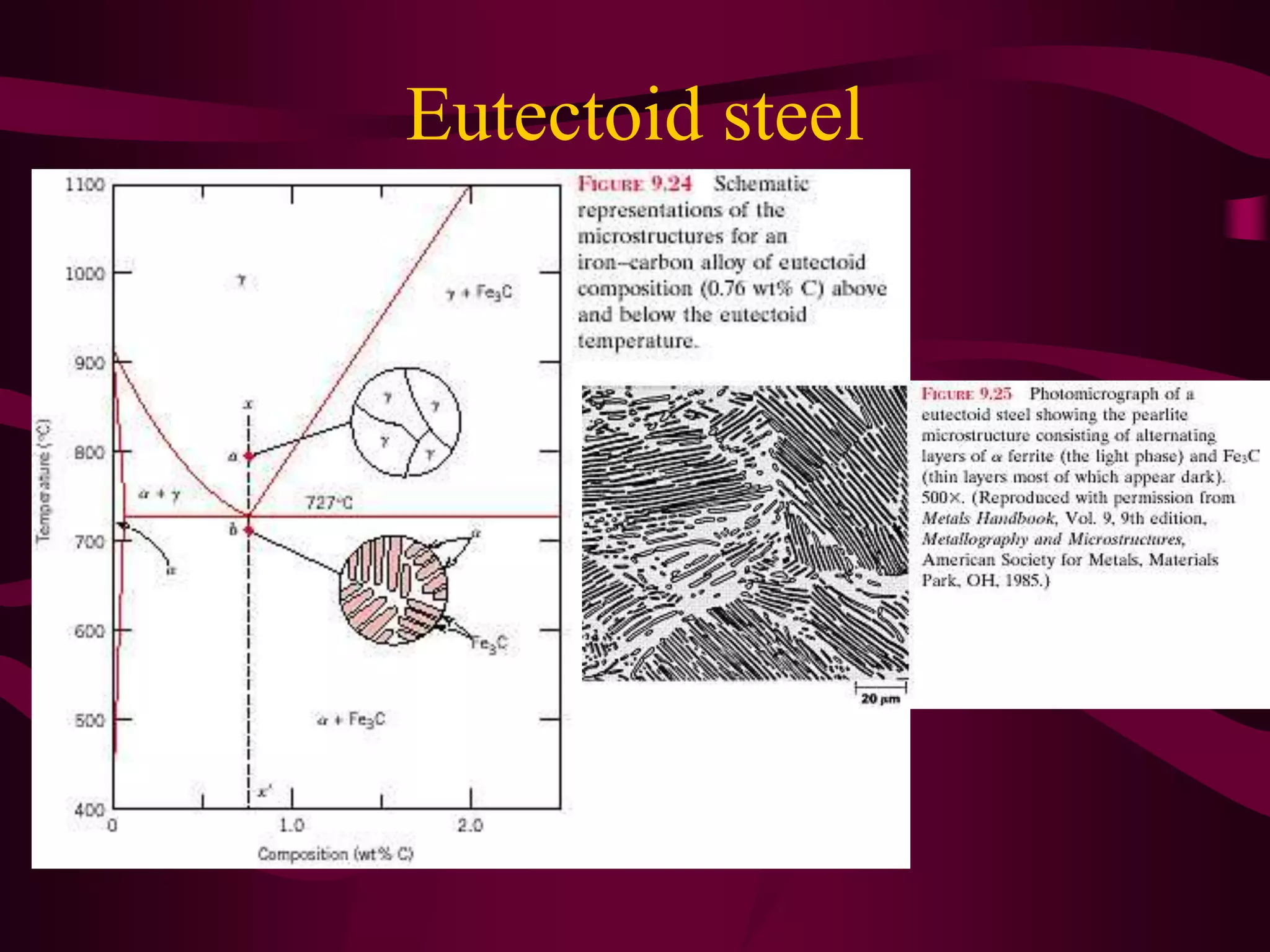
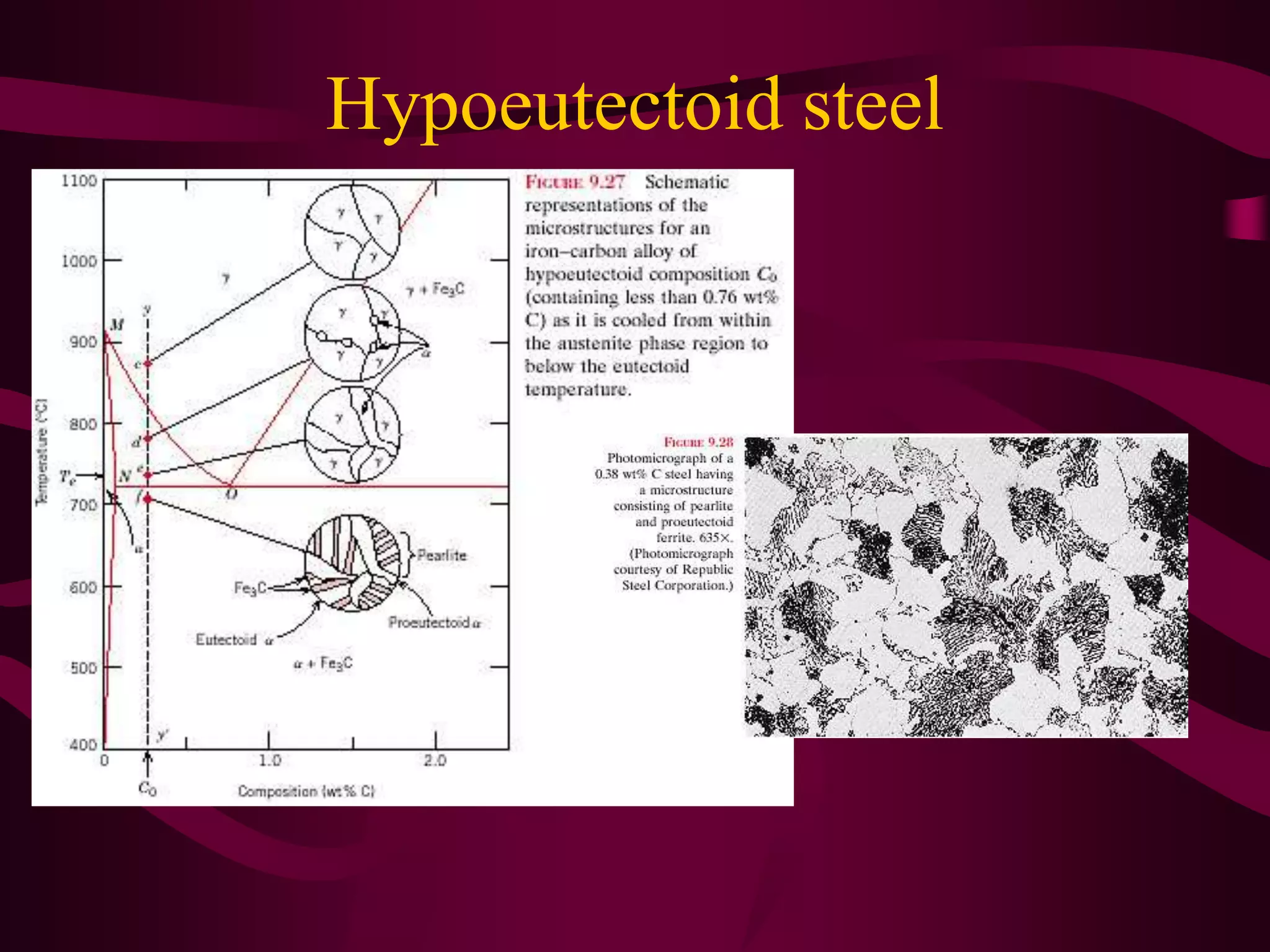


The document discusses the iron-iron carbide phase diagram and the microstructural changes that occur in steels of different carbon compositions during heating and cooling. It outlines the phases present - ferrite, austenite, cementite - and their structures. Steels are defined as solid solutions of carbon in iron, with classifications based on carbon content: low carbon <0.2%, medium 0.2-0.4%, and high carbon >0.4%. The diagram shows eutectic, peritectic, and eutectoid reactions that occur, and how the microstructures of eutectoid, hypoeutectoid, and hypereutectoid steels change during cooling.









