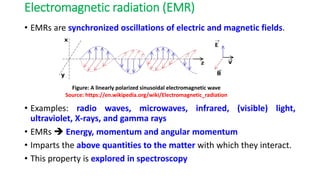
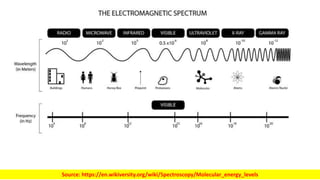
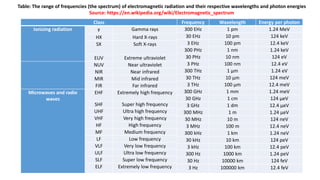
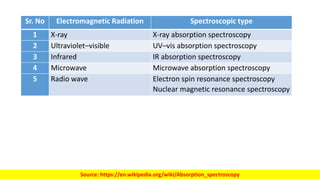
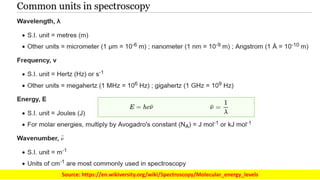
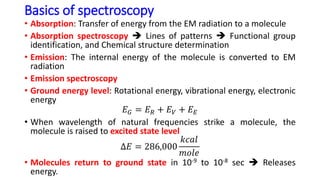
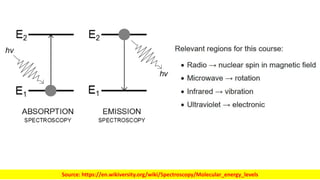
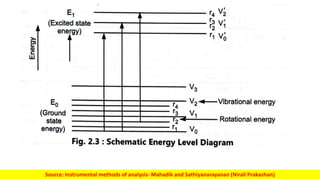
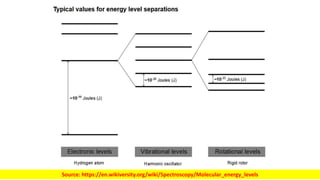
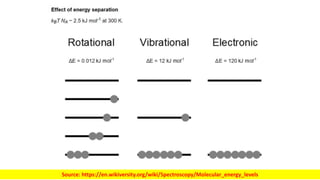
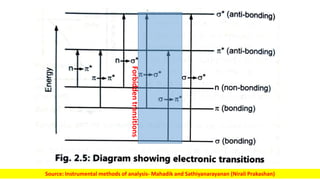
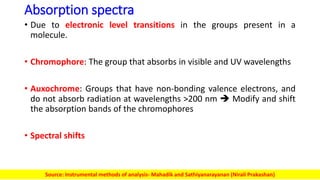
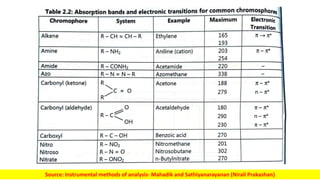
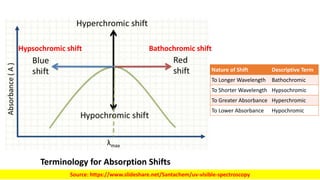
The document discusses electromagnetic radiation and absorption spectroscopy. It provides information on different types of electromagnetic radiation, including their frequencies, wavelengths and photon energies. It also discusses the basic principles of spectroscopy, including absorption and emission. It describes how absorption spectroscopy can be used to obtain structural information about analytes and determine unknown concentrations. It outlines different spectroscopic techniques used for various regions of the electromagnetic spectrum, including UV-visible absorption spectroscopy, infrared absorption spectroscopy and nuclear magnetic resonance spectroscopy.