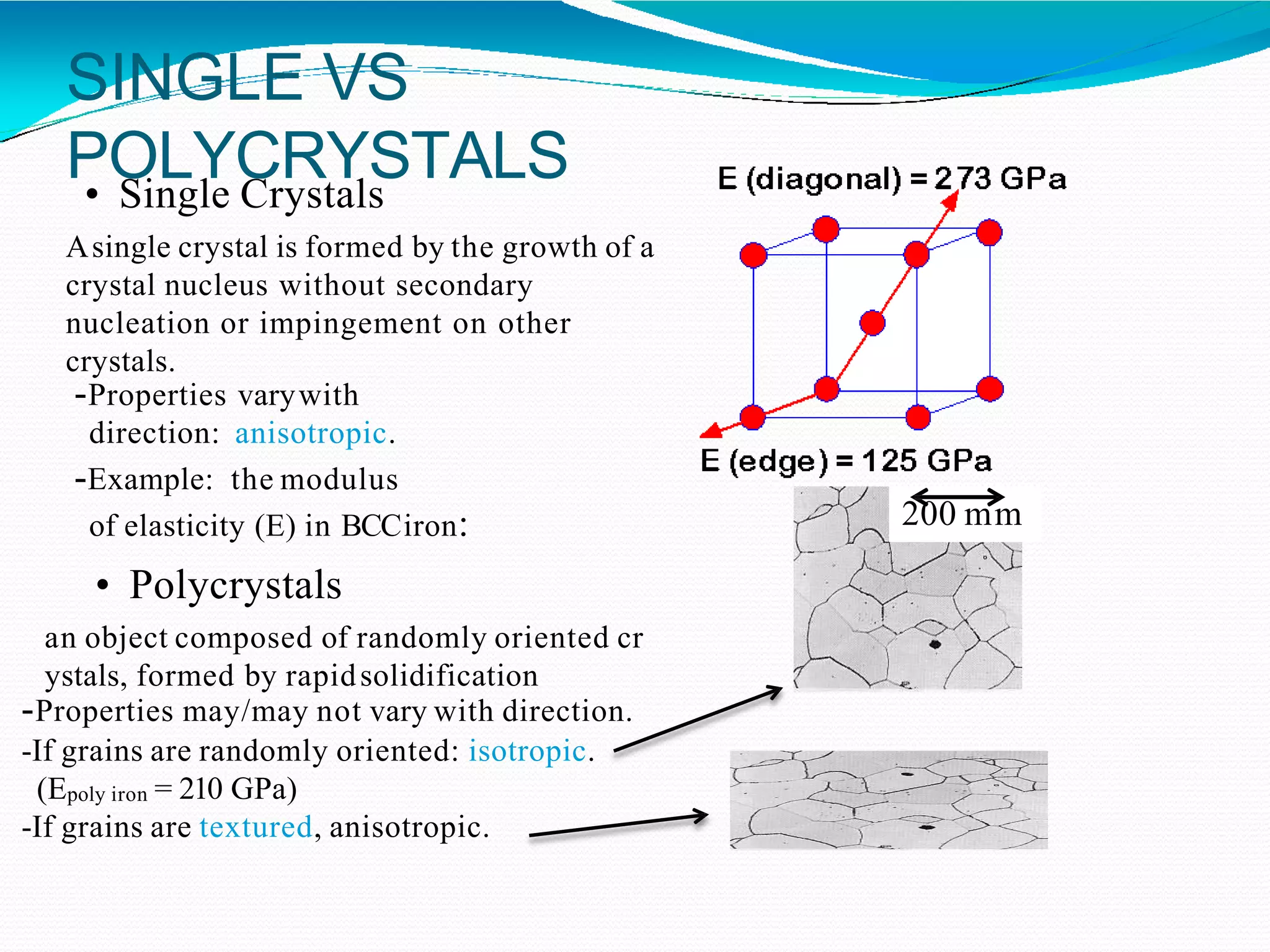
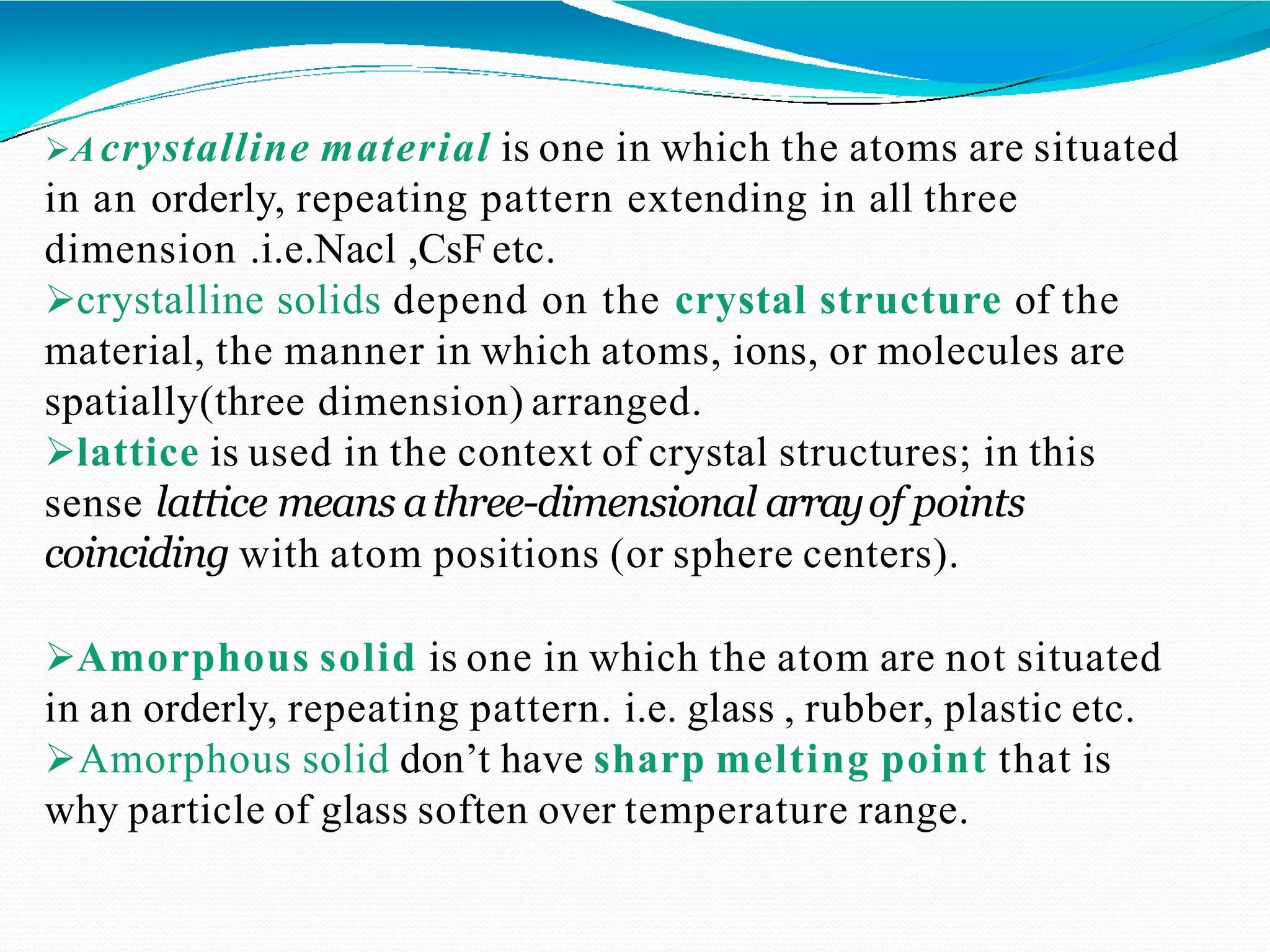
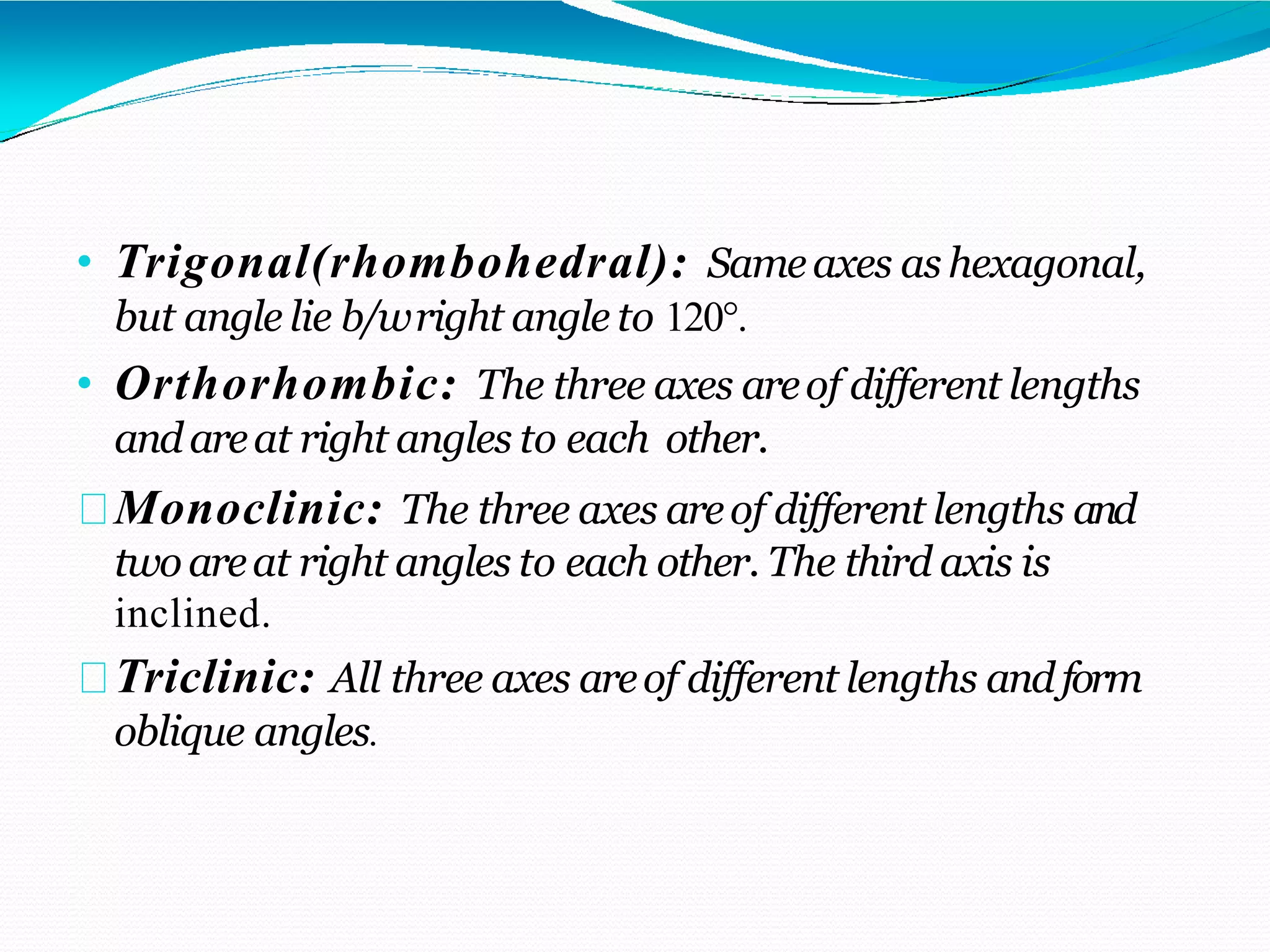
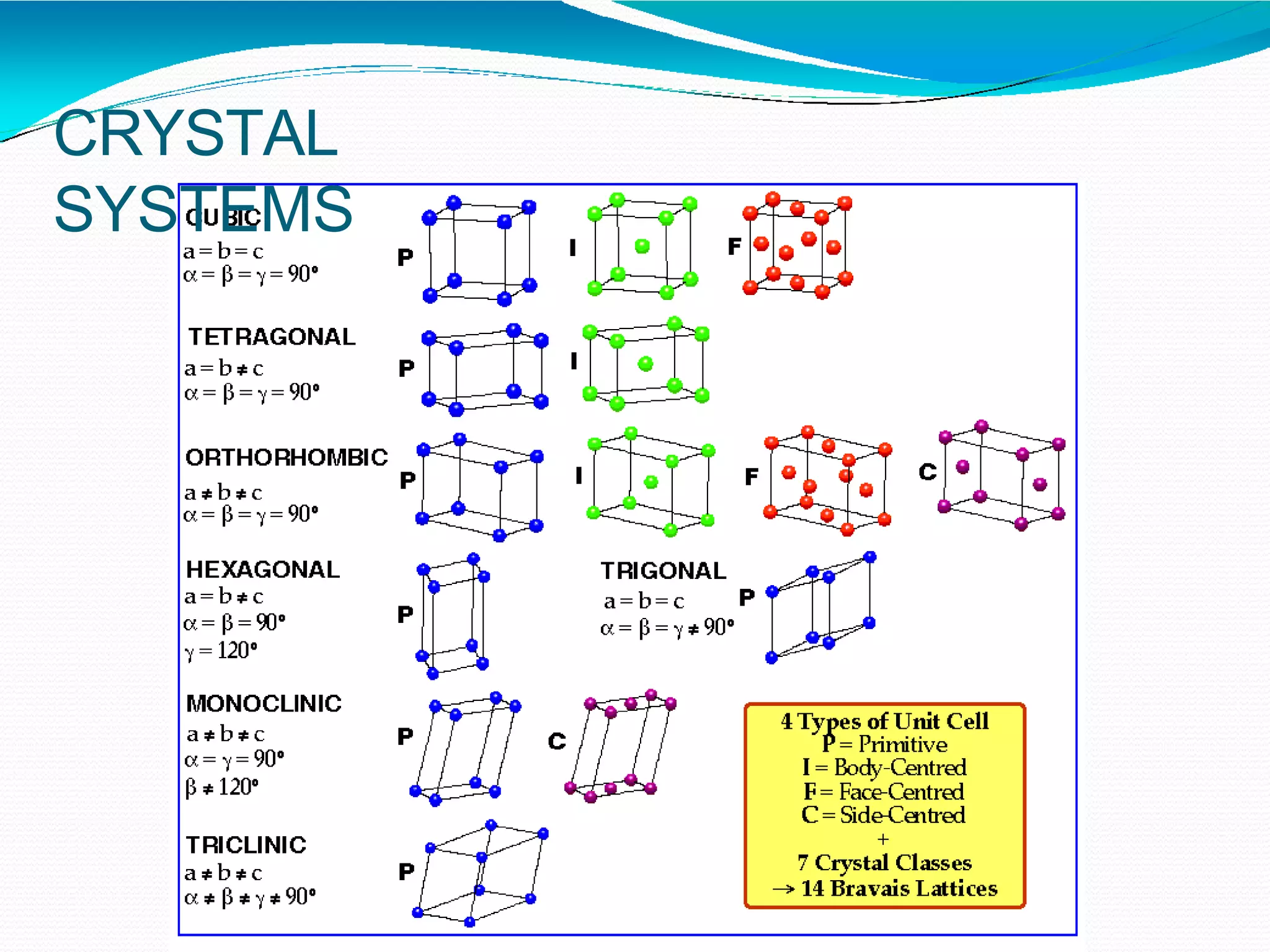
The document discusses the structure of crystalline solids. It explains that crystalline materials have orderly repeating atomic patterns extending in three dimensions, forming crystal structures like FCC, BCC, and HCP. Properties depend on crystal structure. Polycrystalline materials contain randomly oriented crystals and typically have isotropic properties, while single crystals are anisotropic. Understanding crystal structure helps explain material properties and transformations during processes like heat treatment of steel.












![Close packed crystals
…ABCABCABC…packing
[Face Centered Cubic(FCC)]
…ABABAB…packing
[Hexagonal Close Packing (HCP)]
Aplane
Bplane
C plane
Aplane](https://image.slidesharecdn.com/crystalstructureofmetal-230103050446-2c219c3f/75/crystal-structure-of-metal-pptx-17-2048.jpg)