Embed presentation
Download to read offline



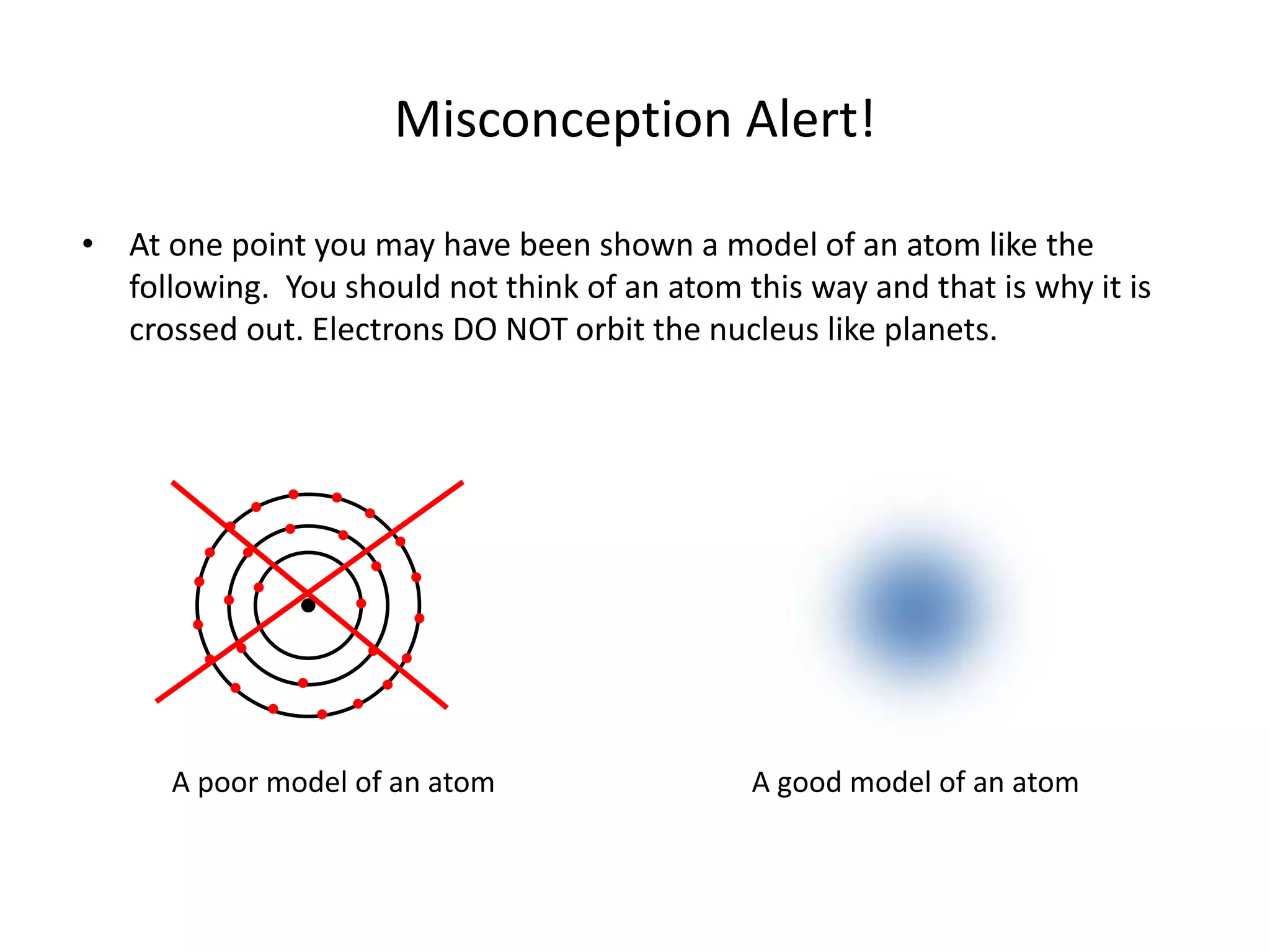

Electrons in atoms occupy specific energy states called orbitals. Orbitals represent the region where an electron is most likely to be found within its energy state. For example, the orbital occupied by the single electron in a hydrogen atom is shown as a cloud indicating a higher probability of finding the electron in the darker regions. While common diagrams depict electrons orbiting the nucleus like planets, this is not an accurate model - electrons occupy probabilistic regions described by orbitals rather than following precise orbits.




