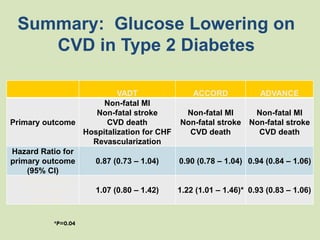
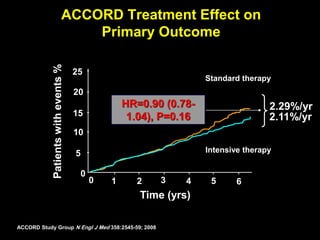
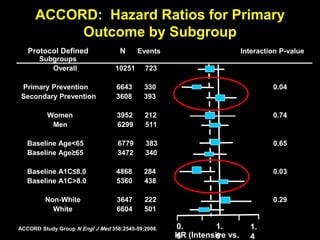
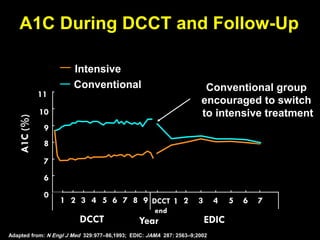
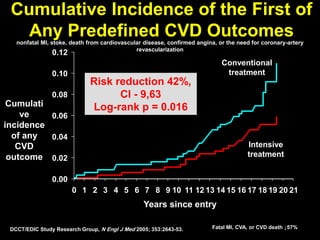
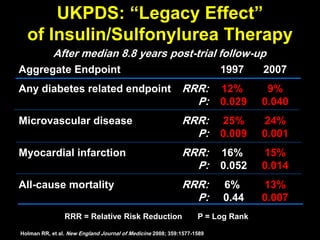
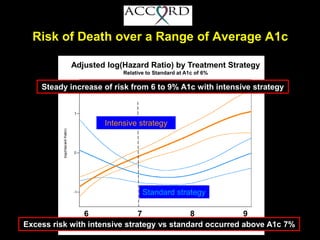
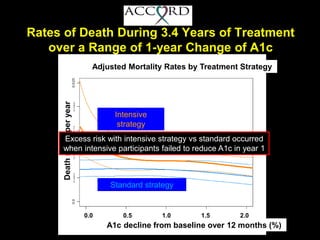
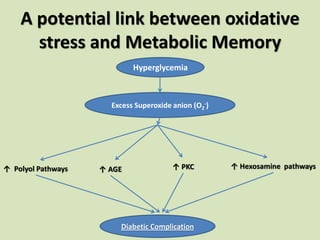
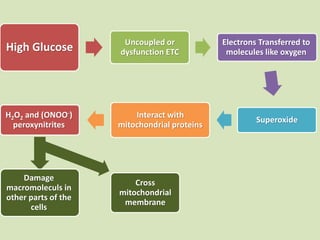
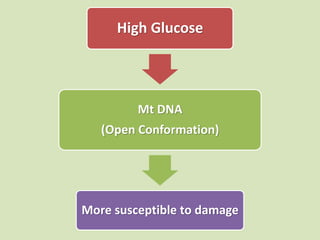
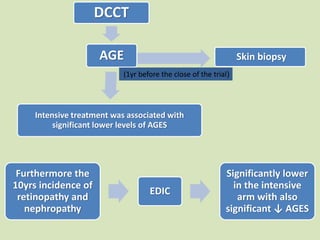
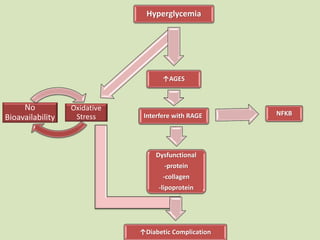
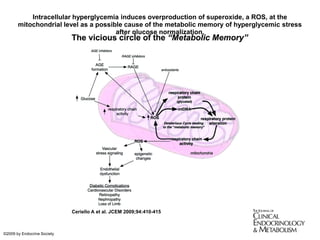
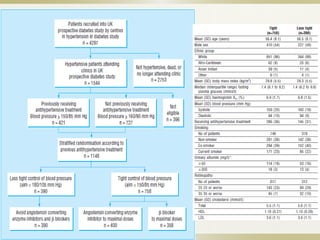
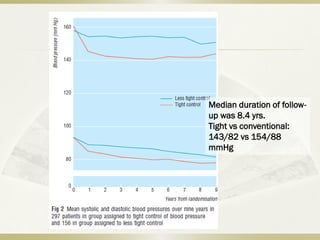
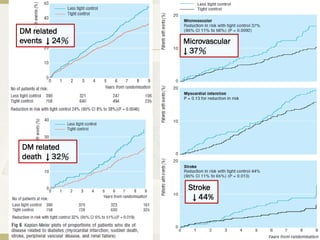
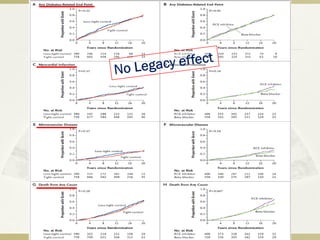
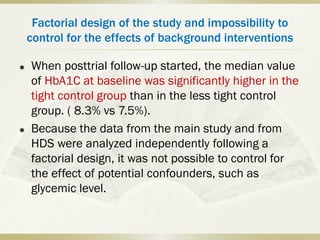
The document discusses the concept of "metabolic memory" where the risks of diabetic complications can persist even after glucose levels have returned to normal. It provides evidence from animal and human studies in the 1980s and 2000s supporting this concept. It then summarizes findings from several major clinical trials that compared intensive glucose control to standard control and found reductions in microvascular and macrovascular outcomes with intensive control, though the benefits took years to emerge after trial completion.