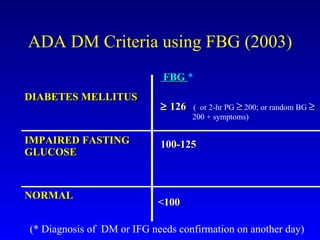
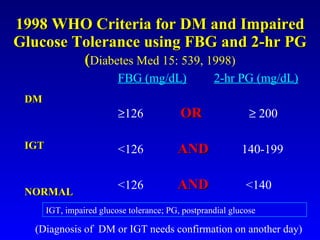
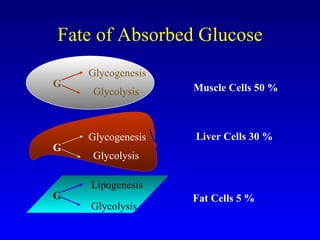
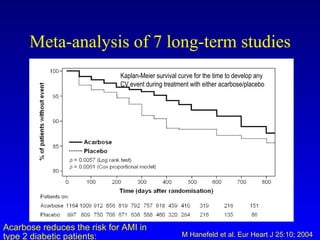
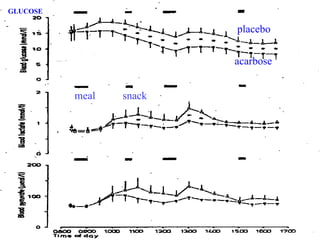
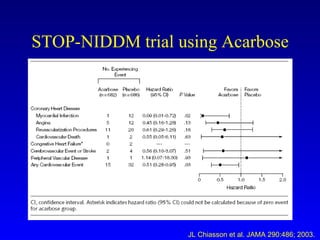
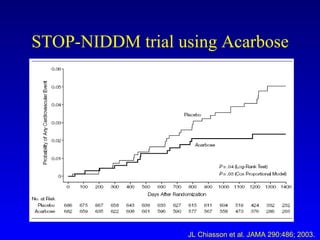
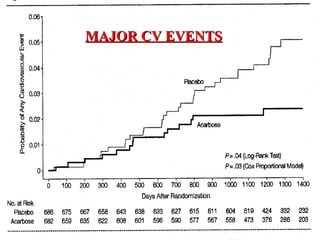
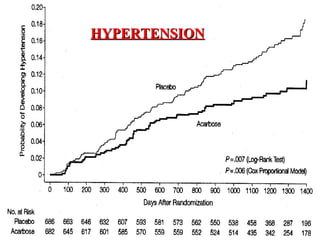
The document discusses acarbose, an oral medication for type 2 diabetes. It provides background on the development of diabetes treatments over time and criteria for diagnosing diabetes. It then focuses on acarbose, explaining that it works by inhibiting enzymes involved in breaking down carbohydrates, which reduces the rise in blood glucose after meals. Studies show acarbose can help lower HbA1c levels and may reduce cardiovascular risks when used long-term for people with type 2 diabetes or prediabetes. Common side effects include flatulence, diarrhea, and abdominal pain.