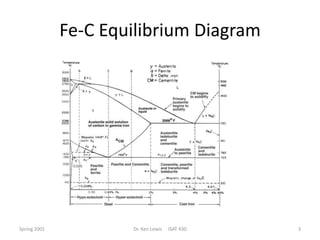
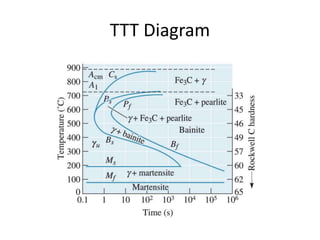
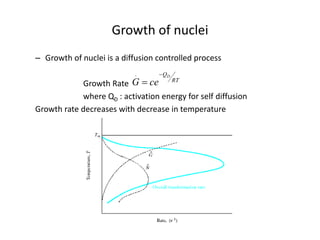
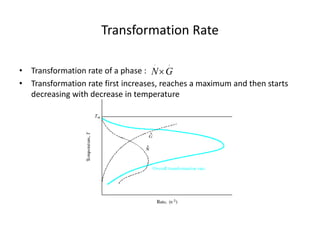
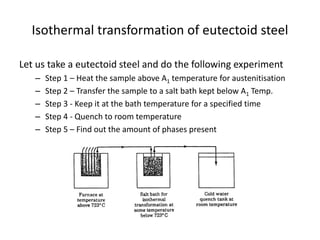
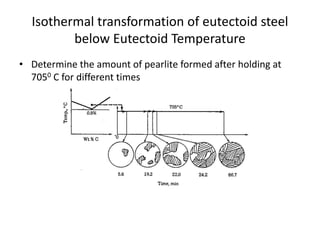
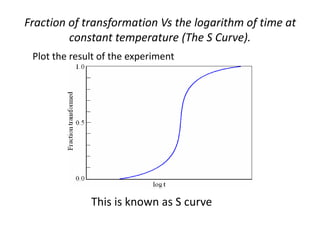
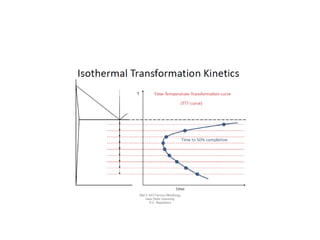
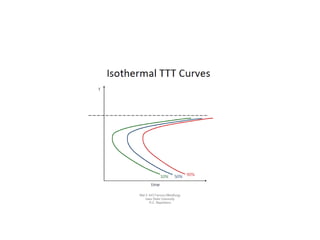
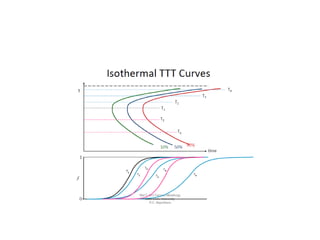
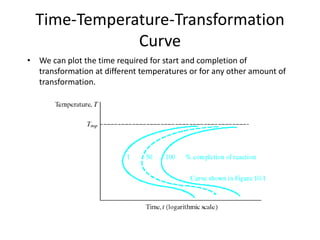
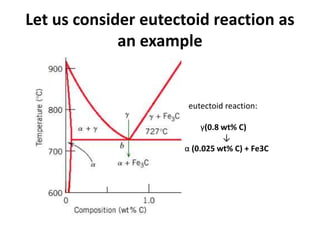
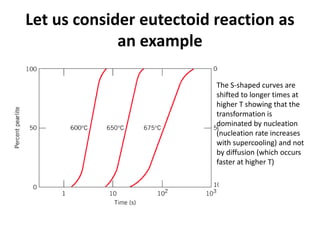
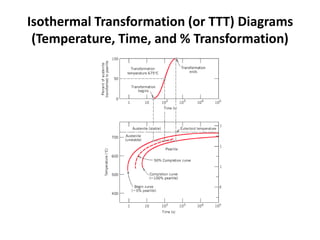
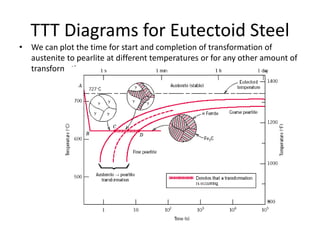
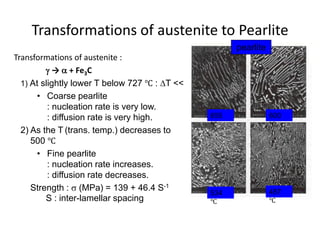
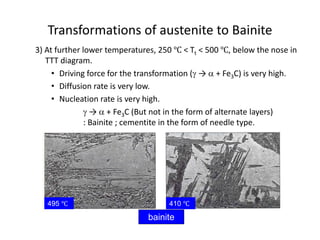
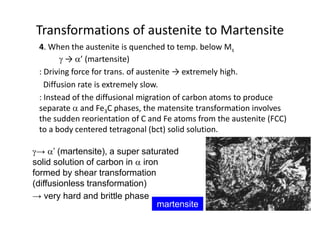
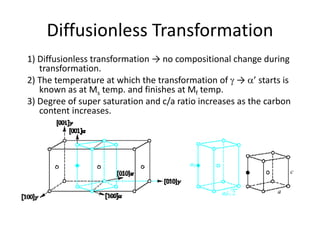
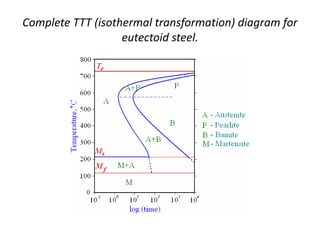
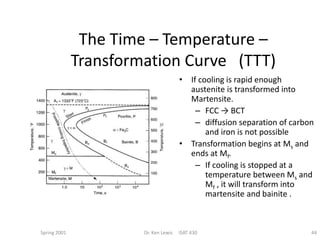
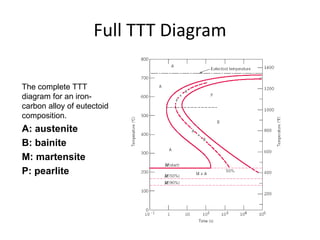
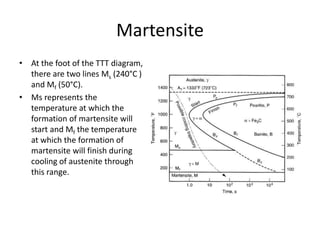
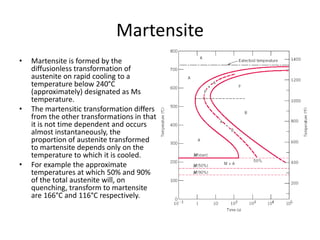
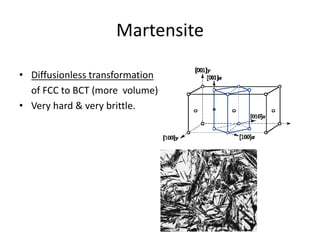
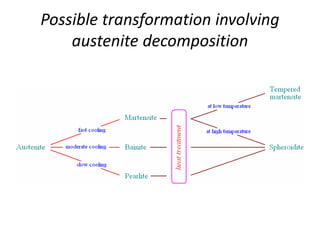
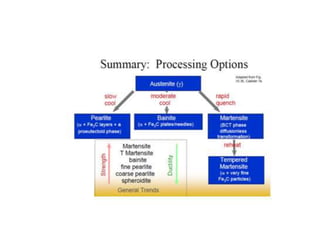
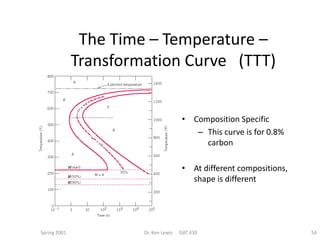
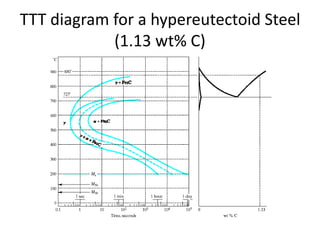
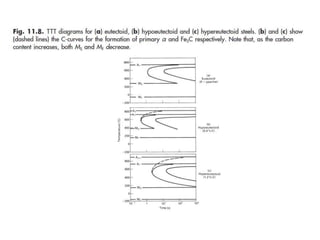
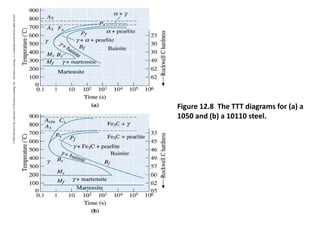
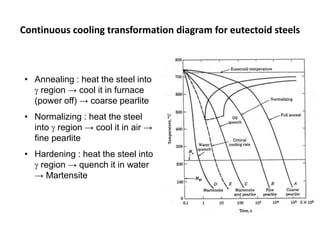
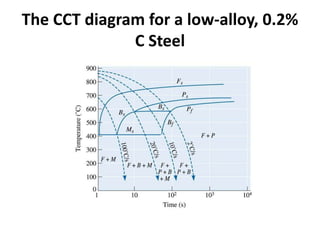
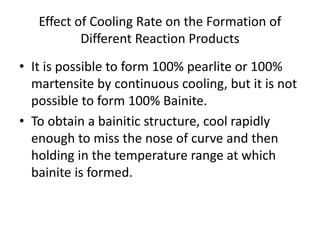
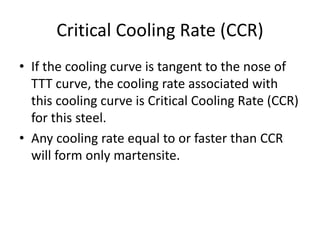
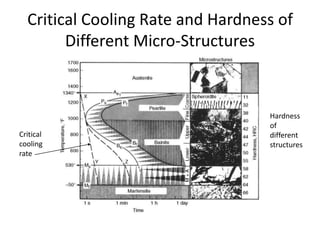
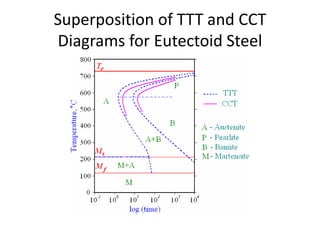
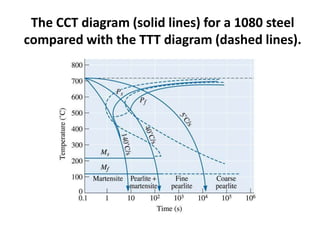
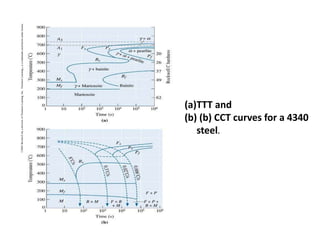
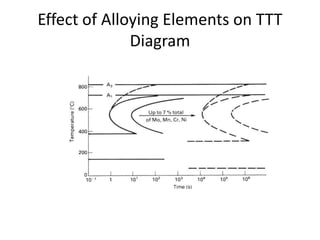
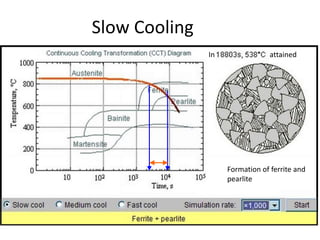
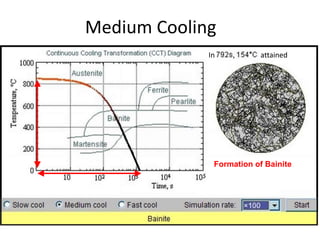
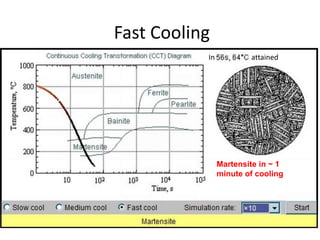
The document discusses time-temperature-transformation (TTT) diagrams, which show the microstructural phases that form in steels at different temperatures over time. In contrast to equilibrium phase diagrams, TTT diagrams account for nonequilibrium cooling rates. They indicate that at higher temperatures, pearlite forms with slow cooling, while faster cooling produces bainite or martensite. The document includes an example TTT diagram for eutectoid steel and explains how isothermal experiments at different temperatures are used to construct these diagrams.