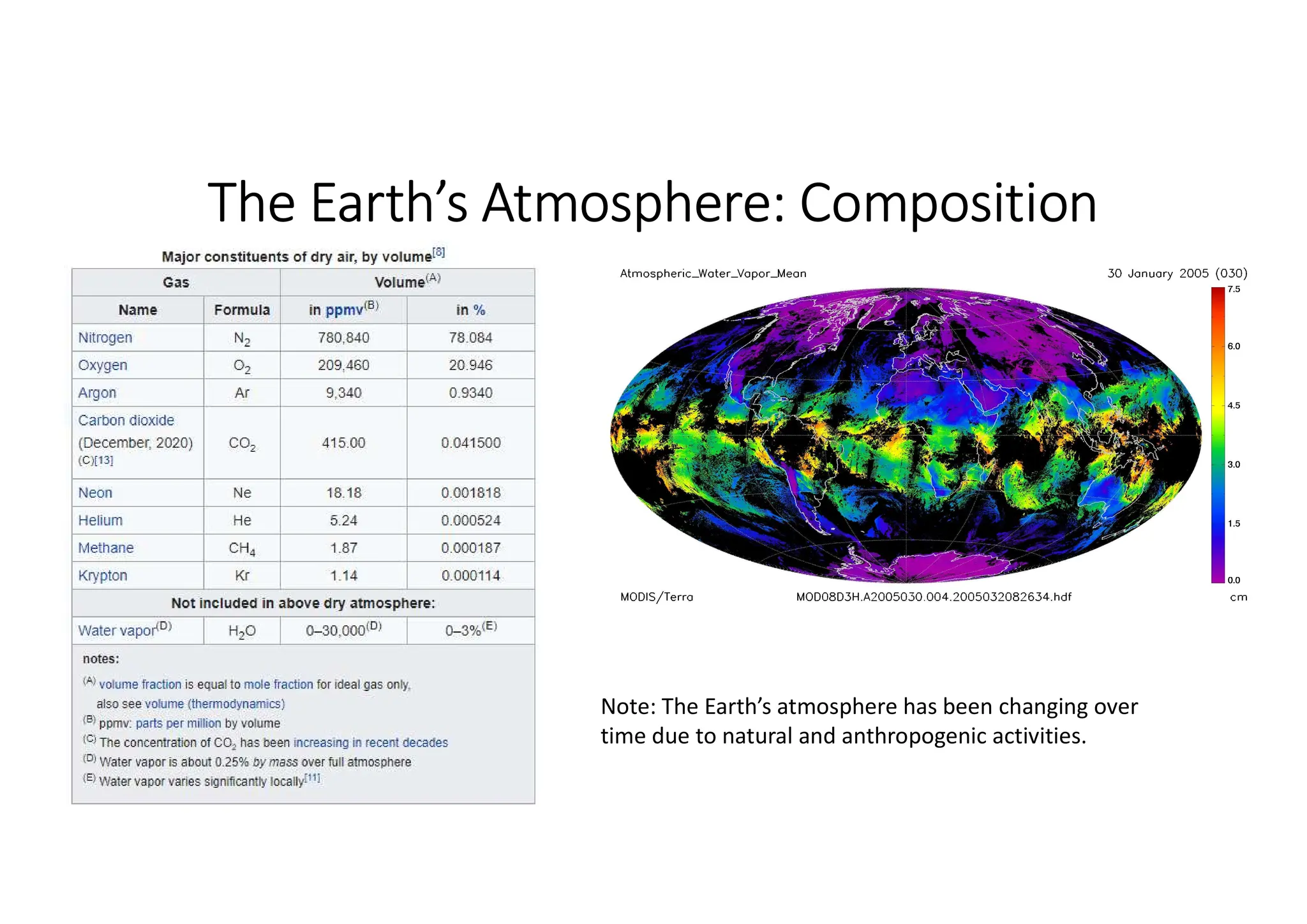
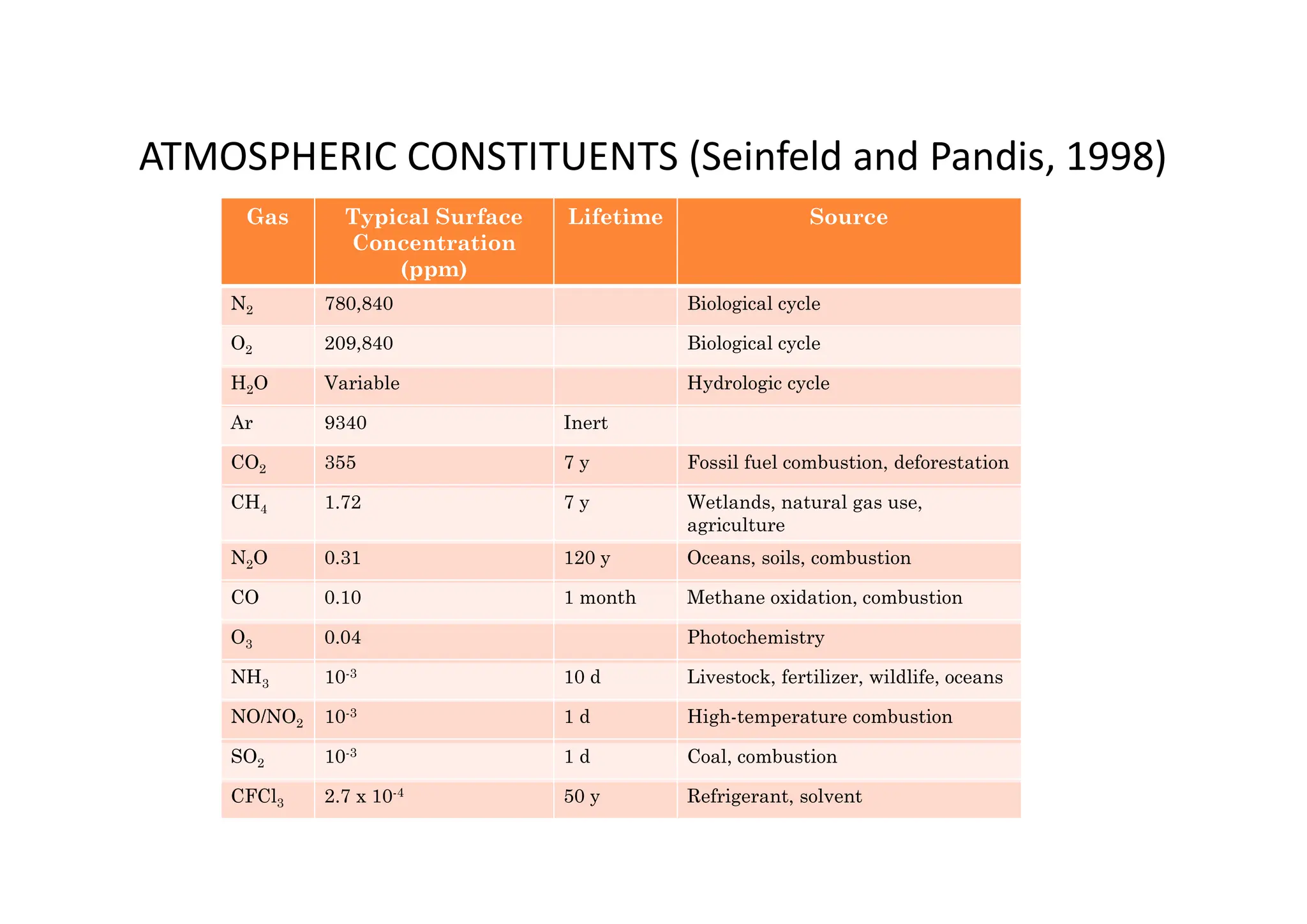
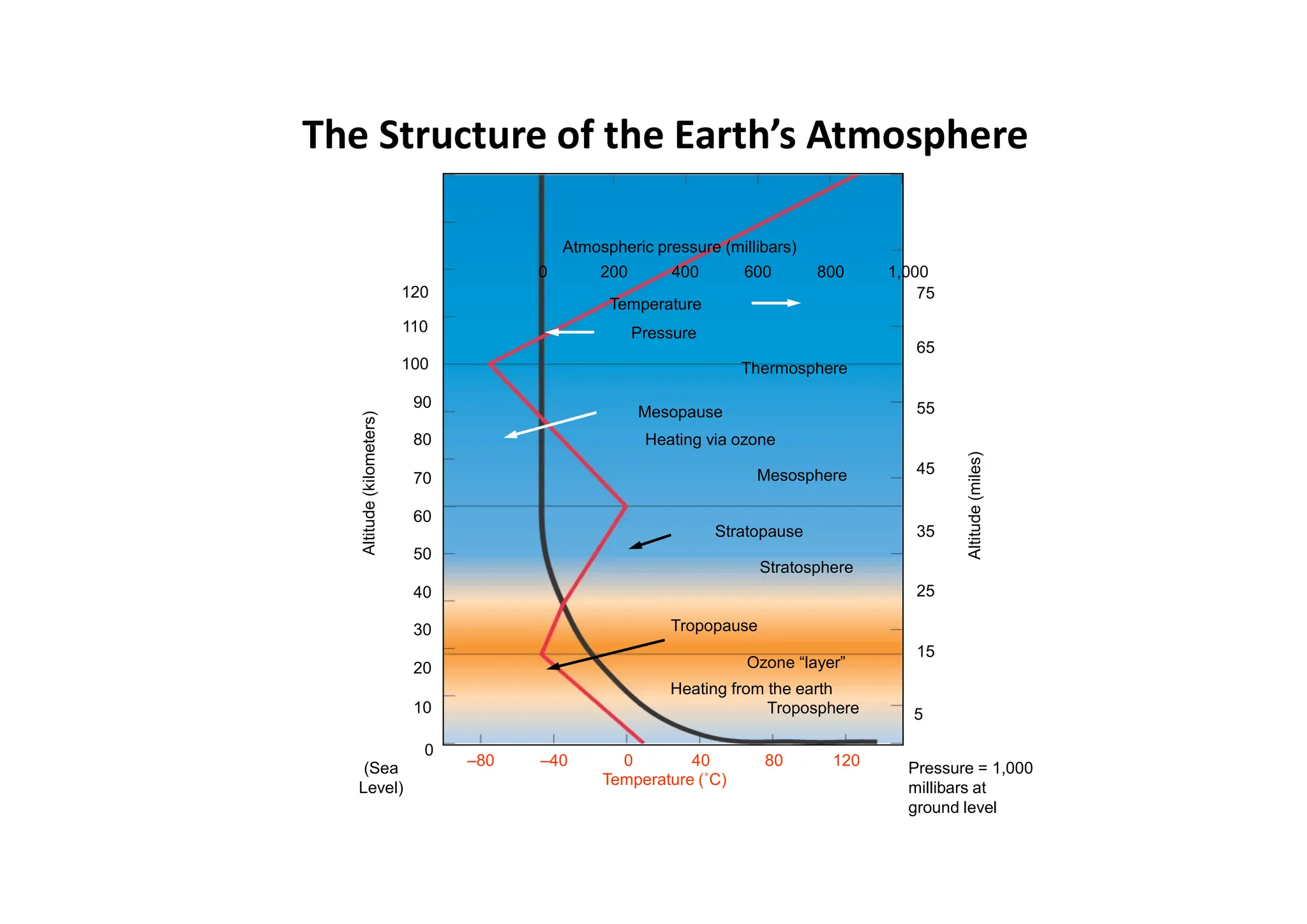
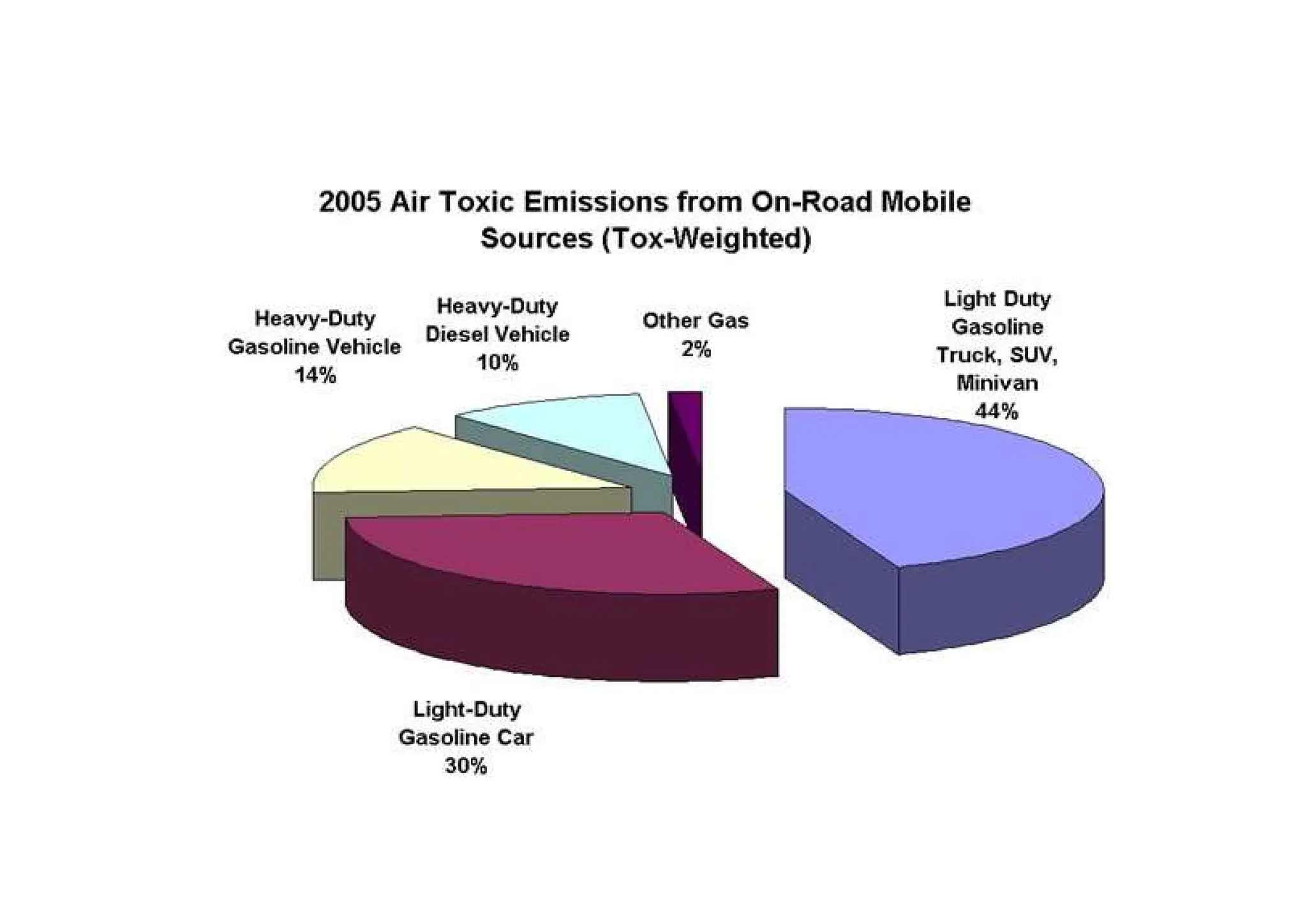
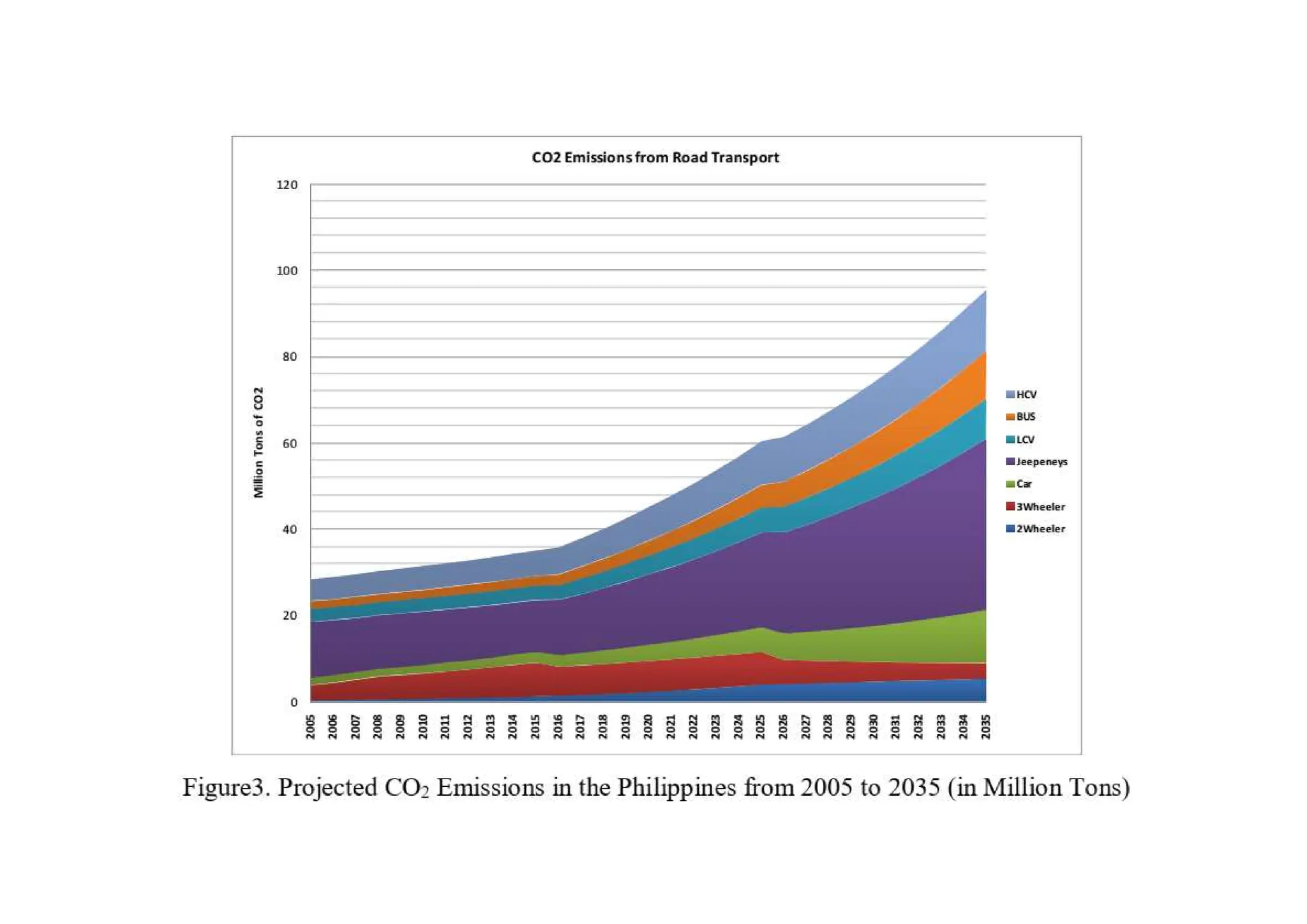
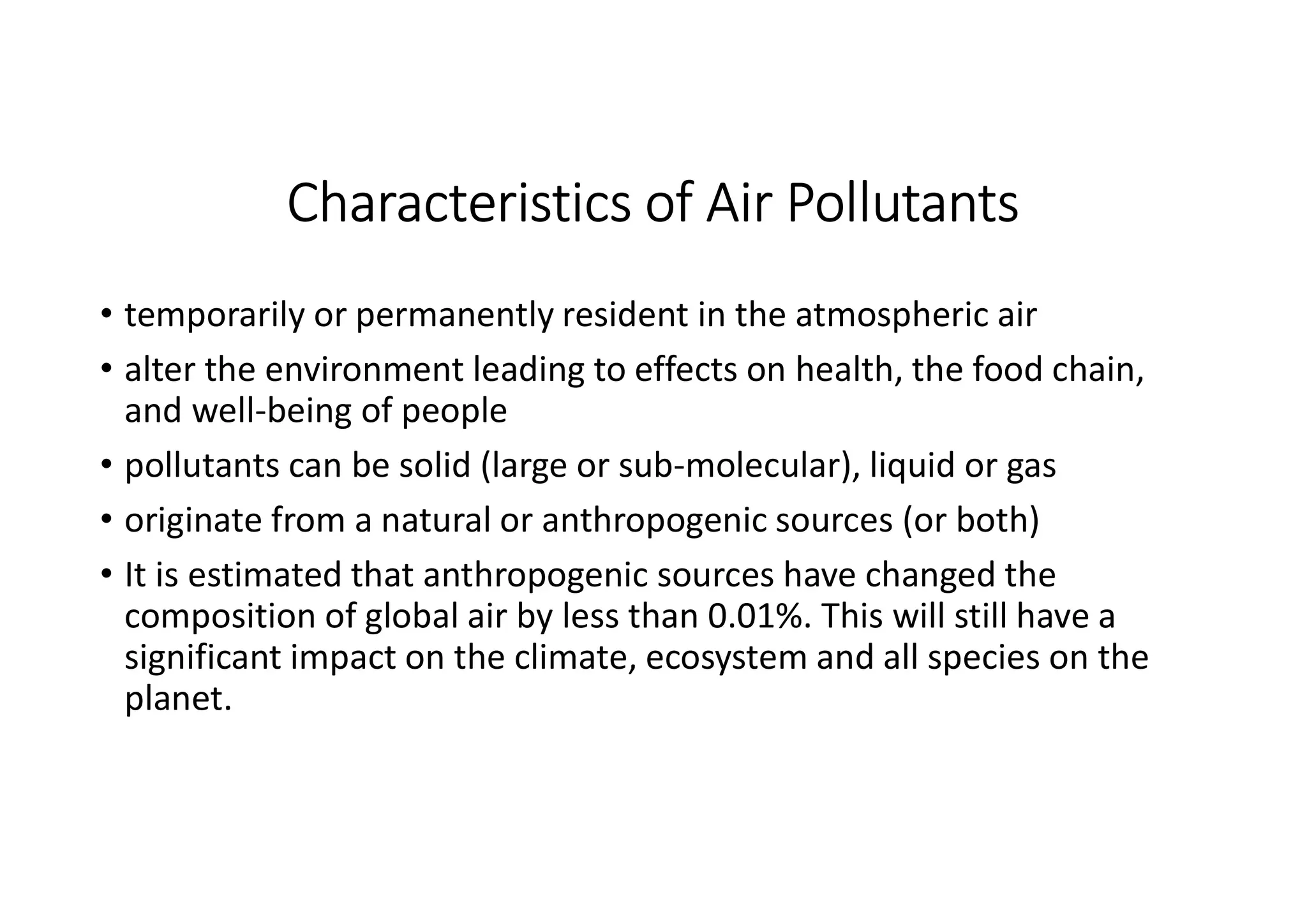
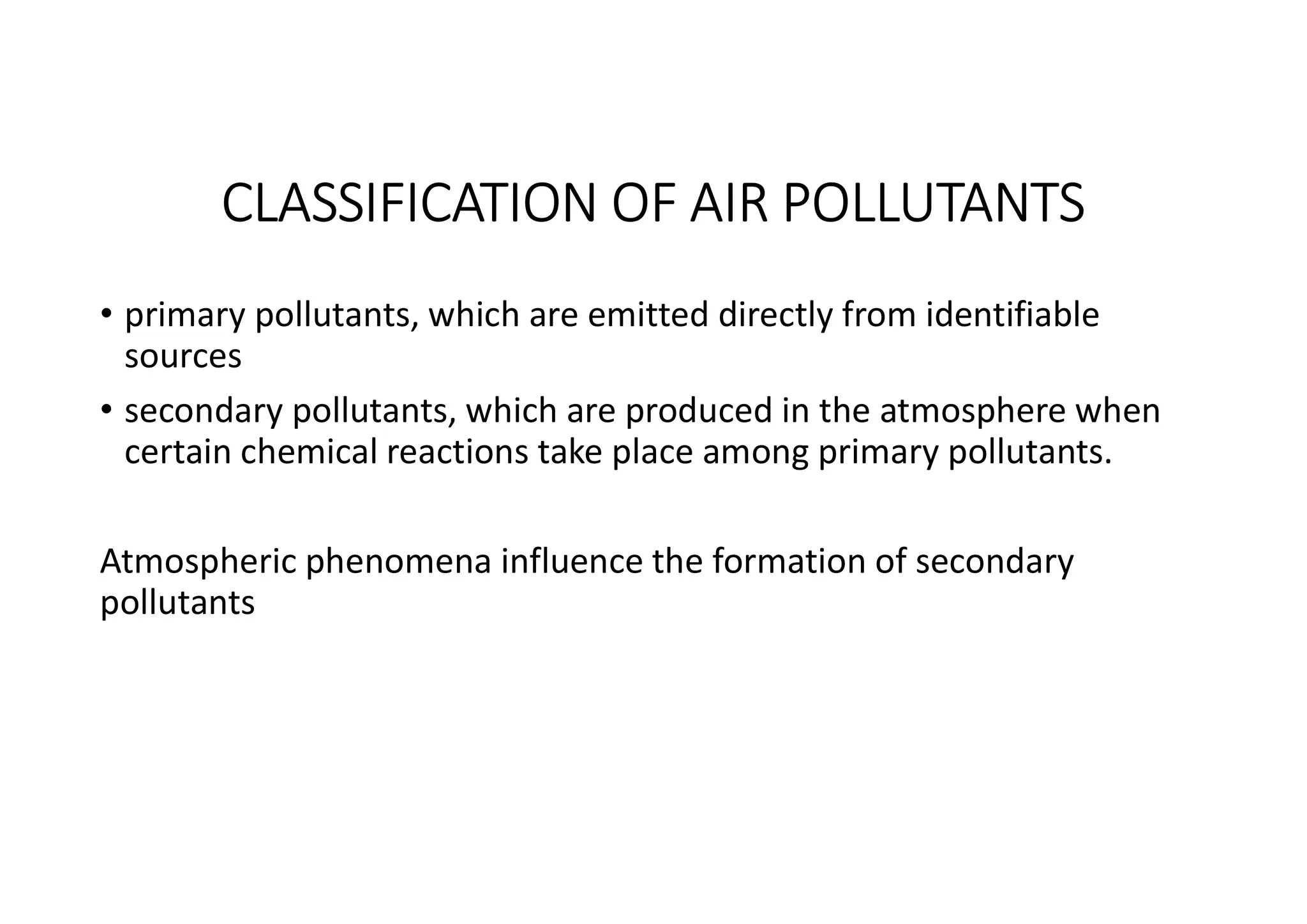
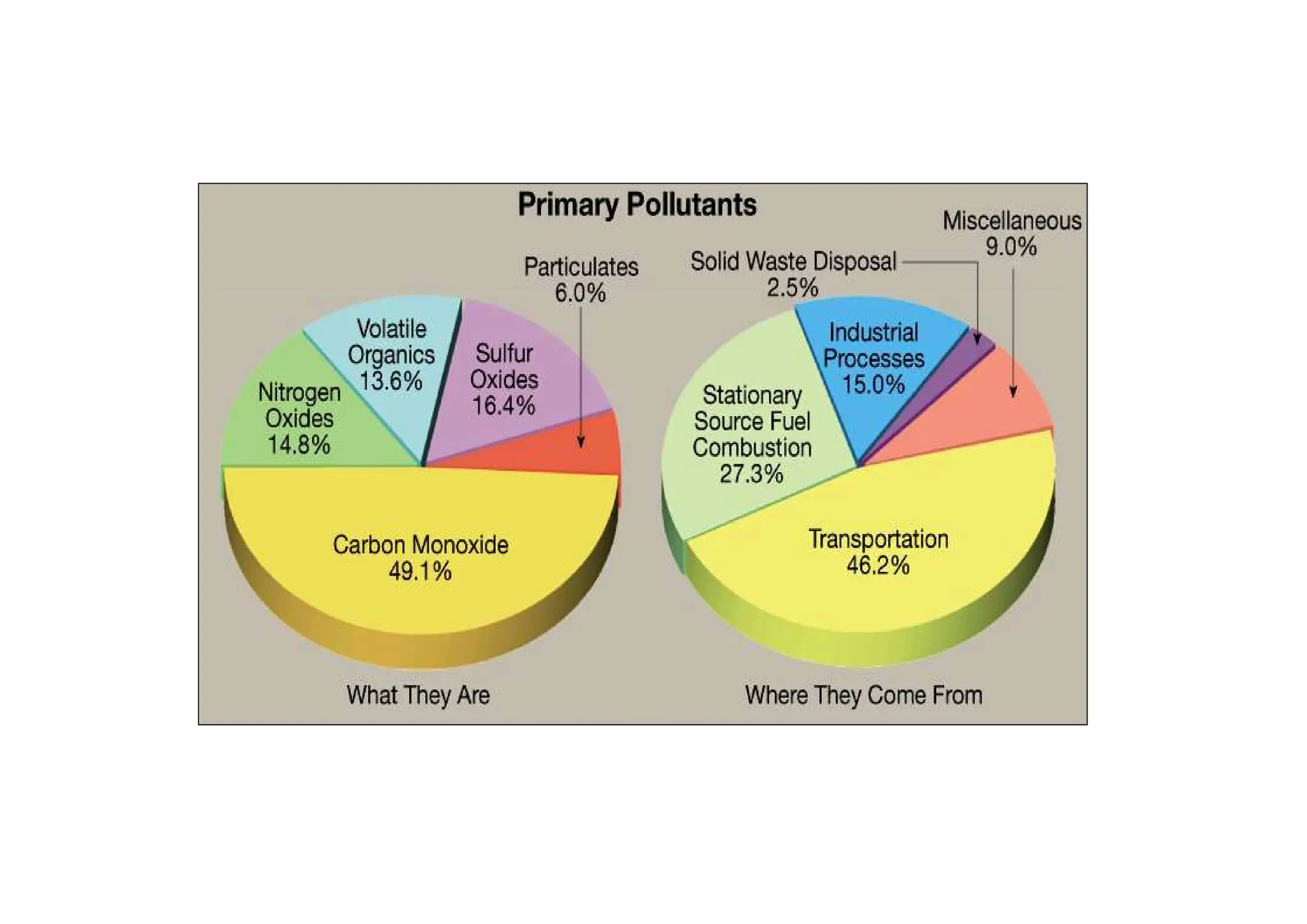
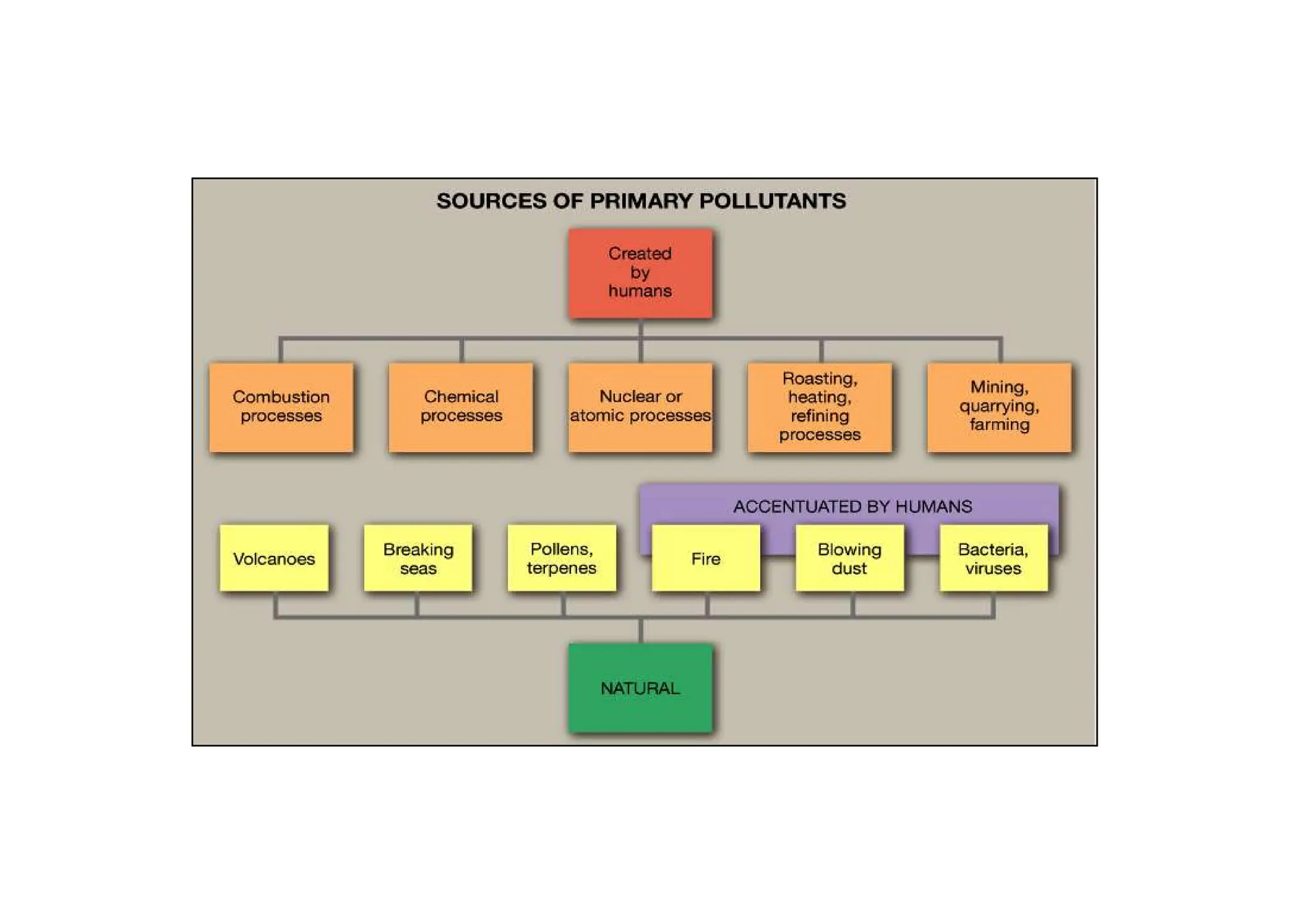
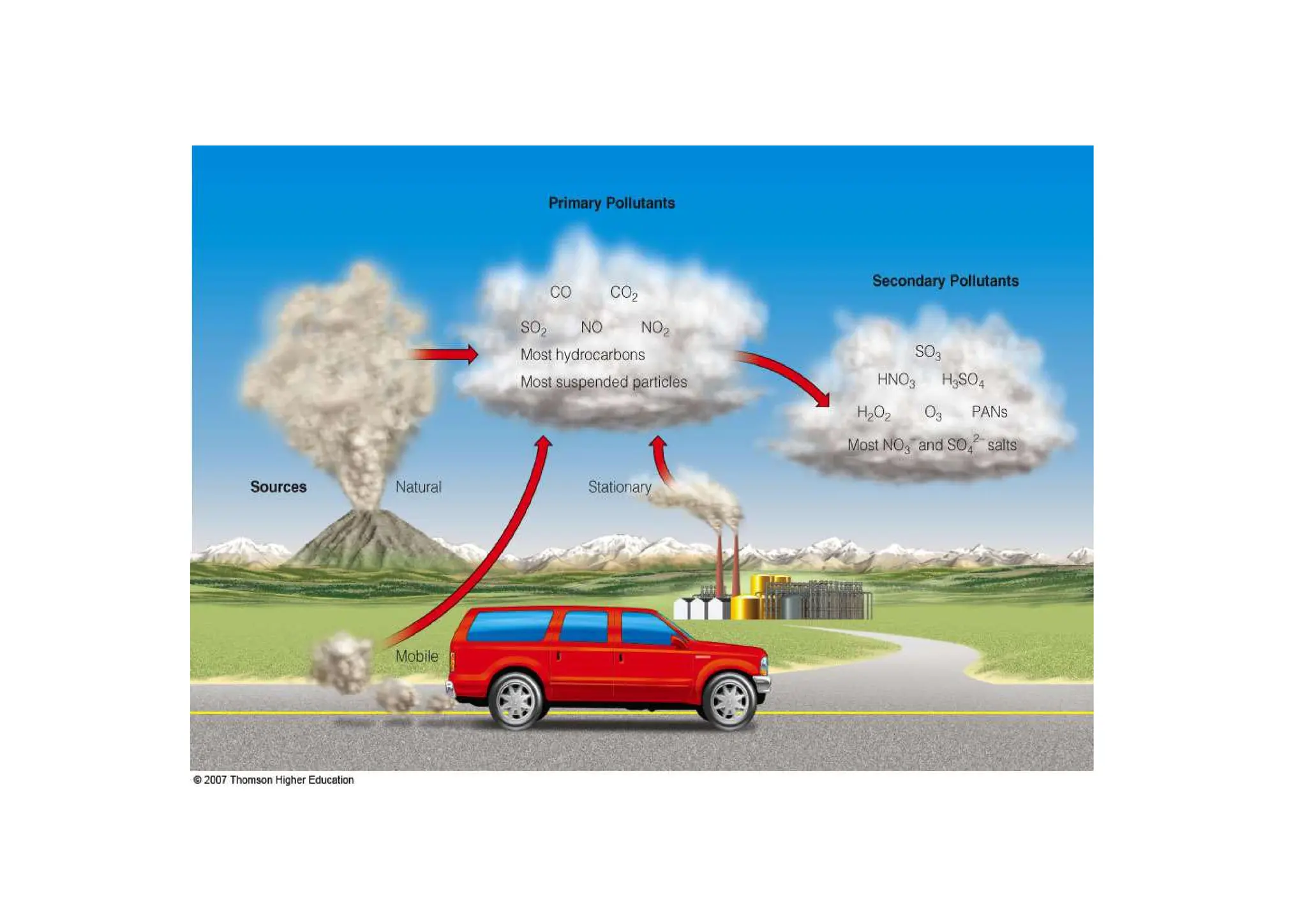
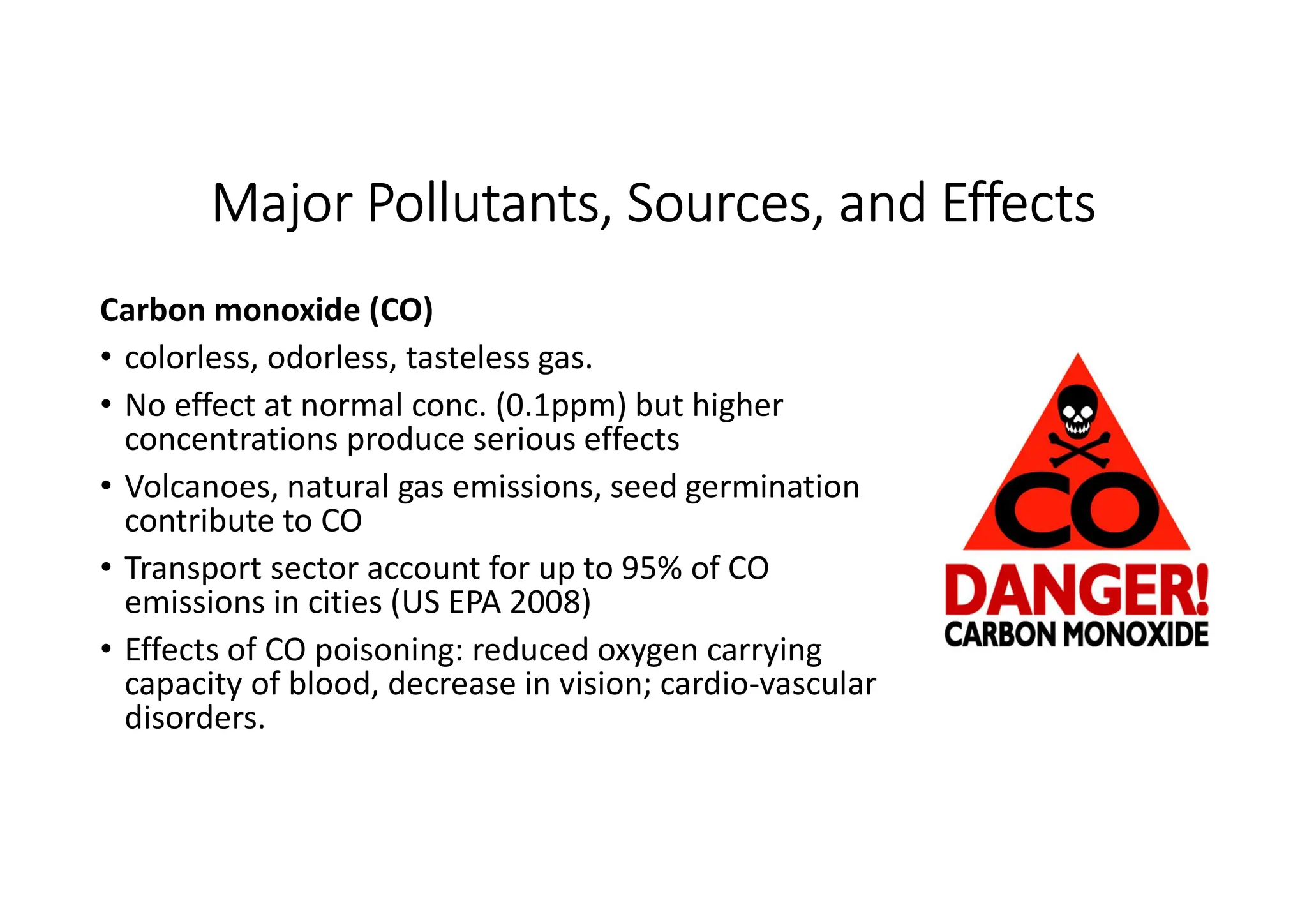
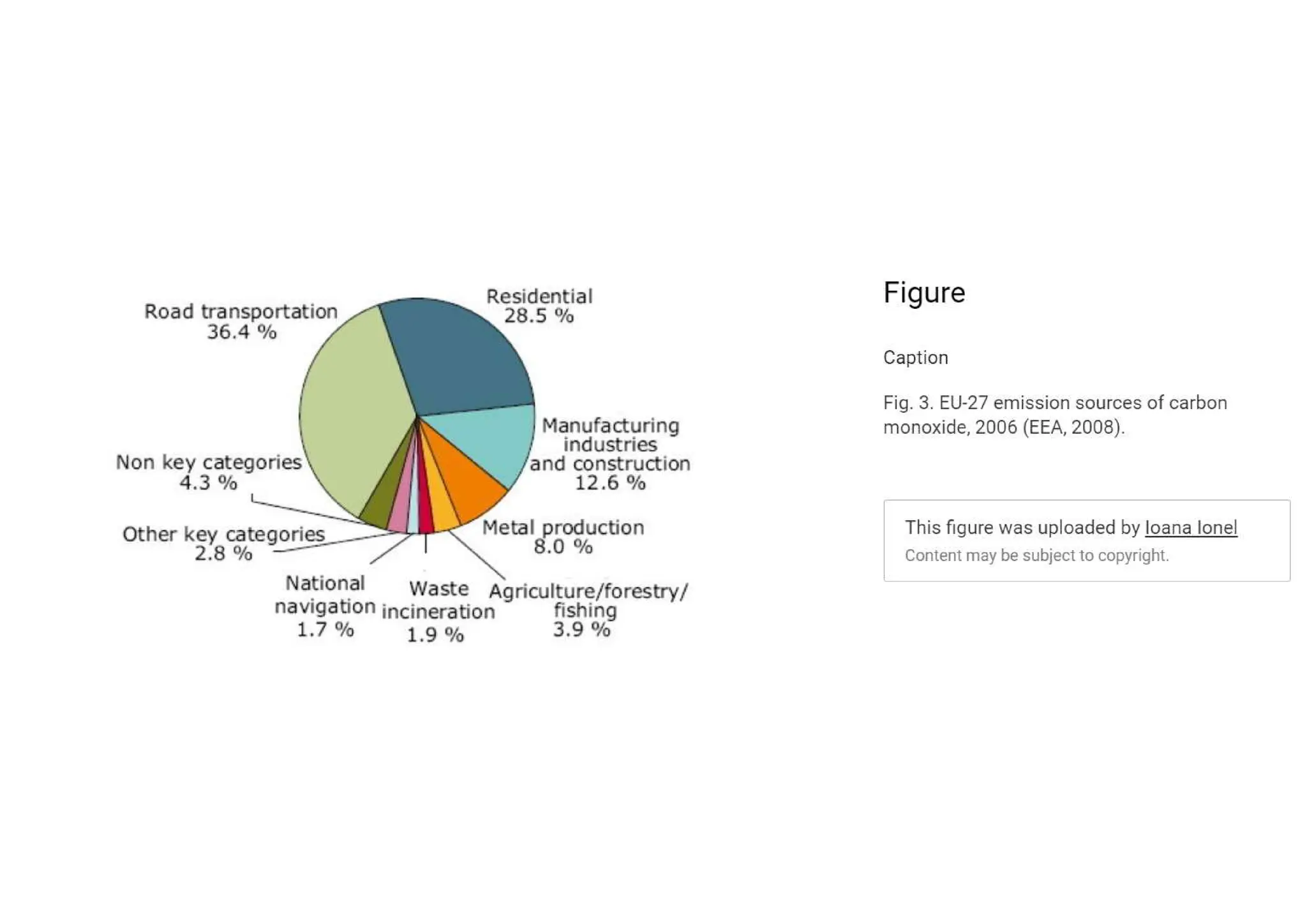
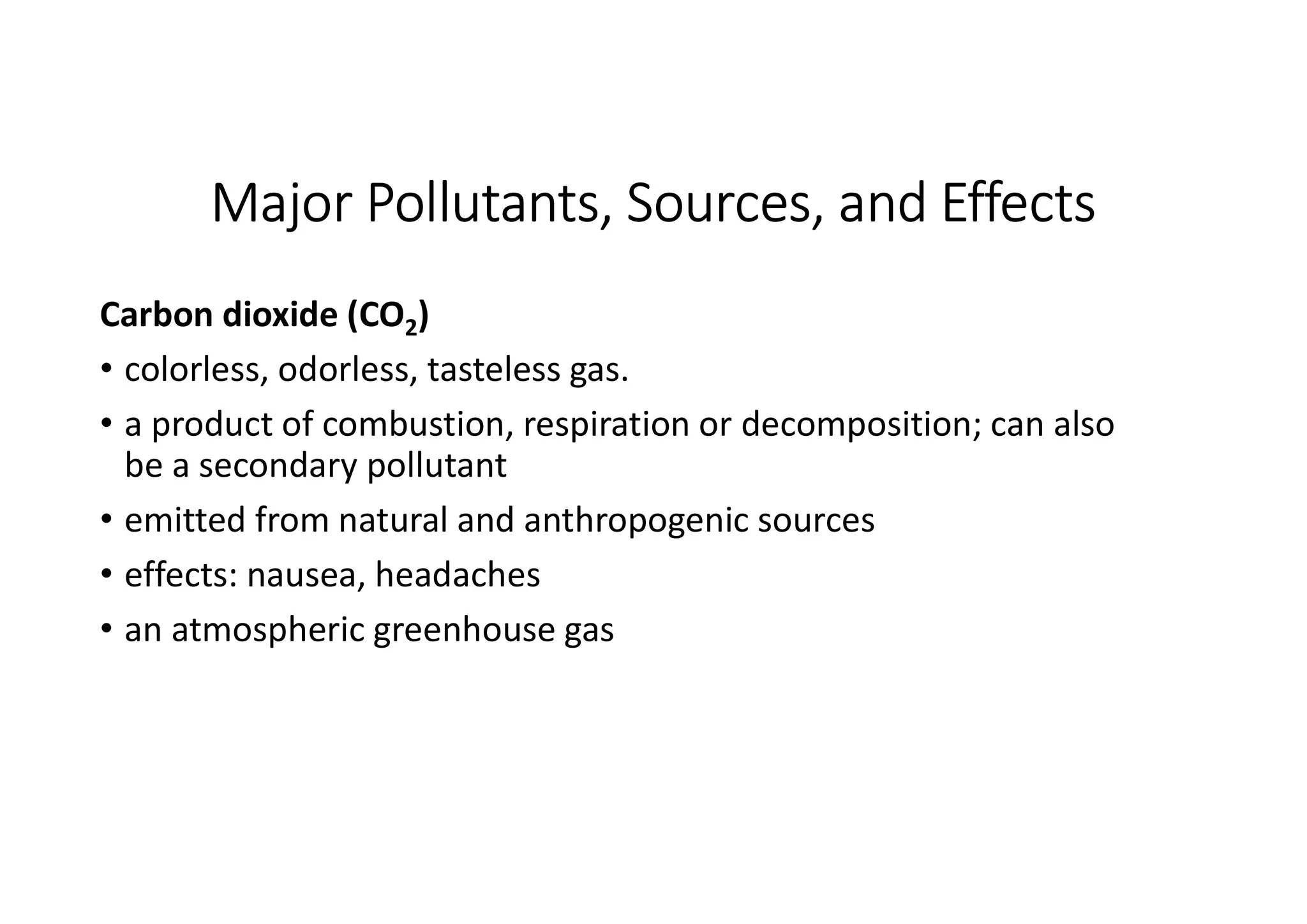
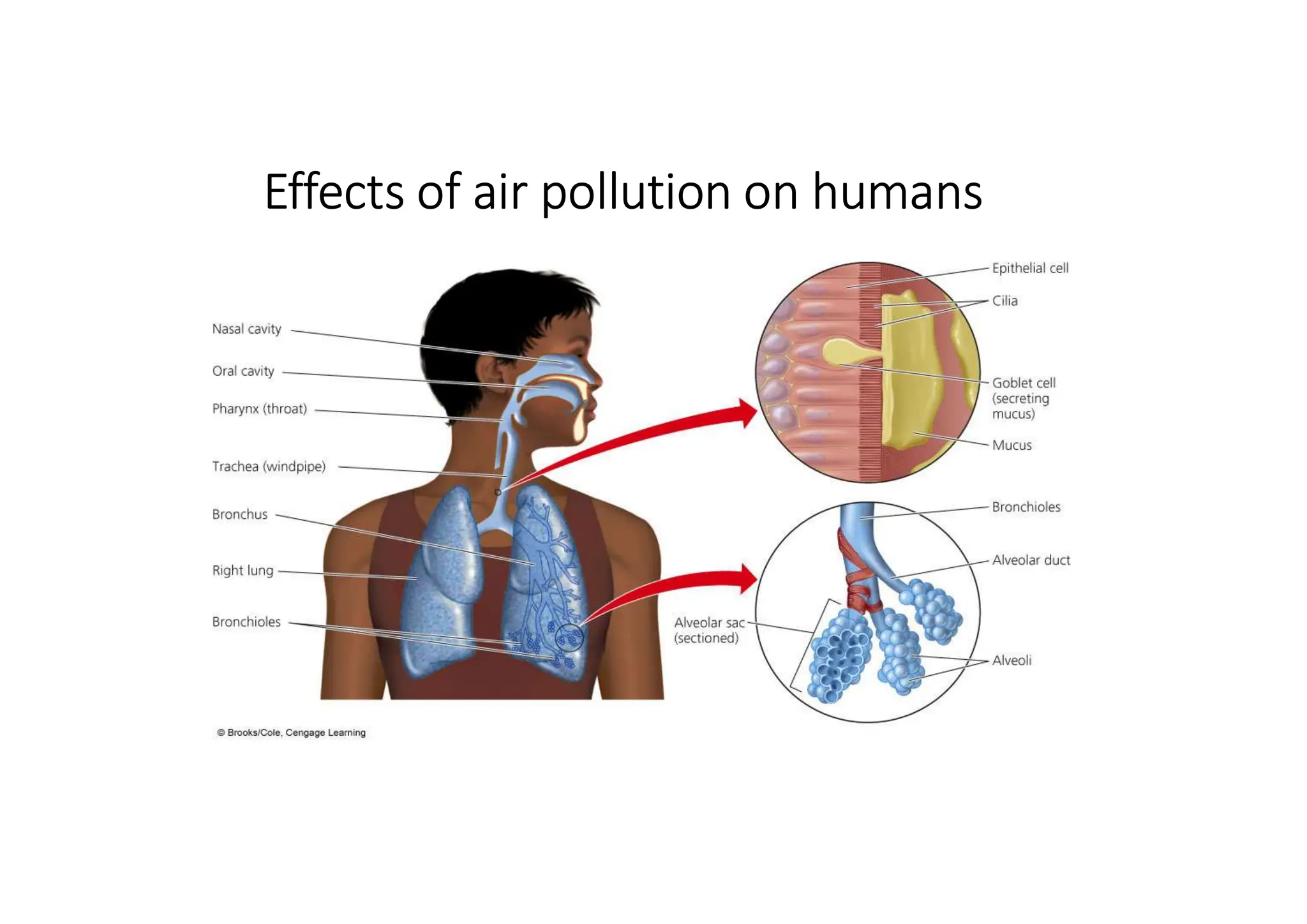
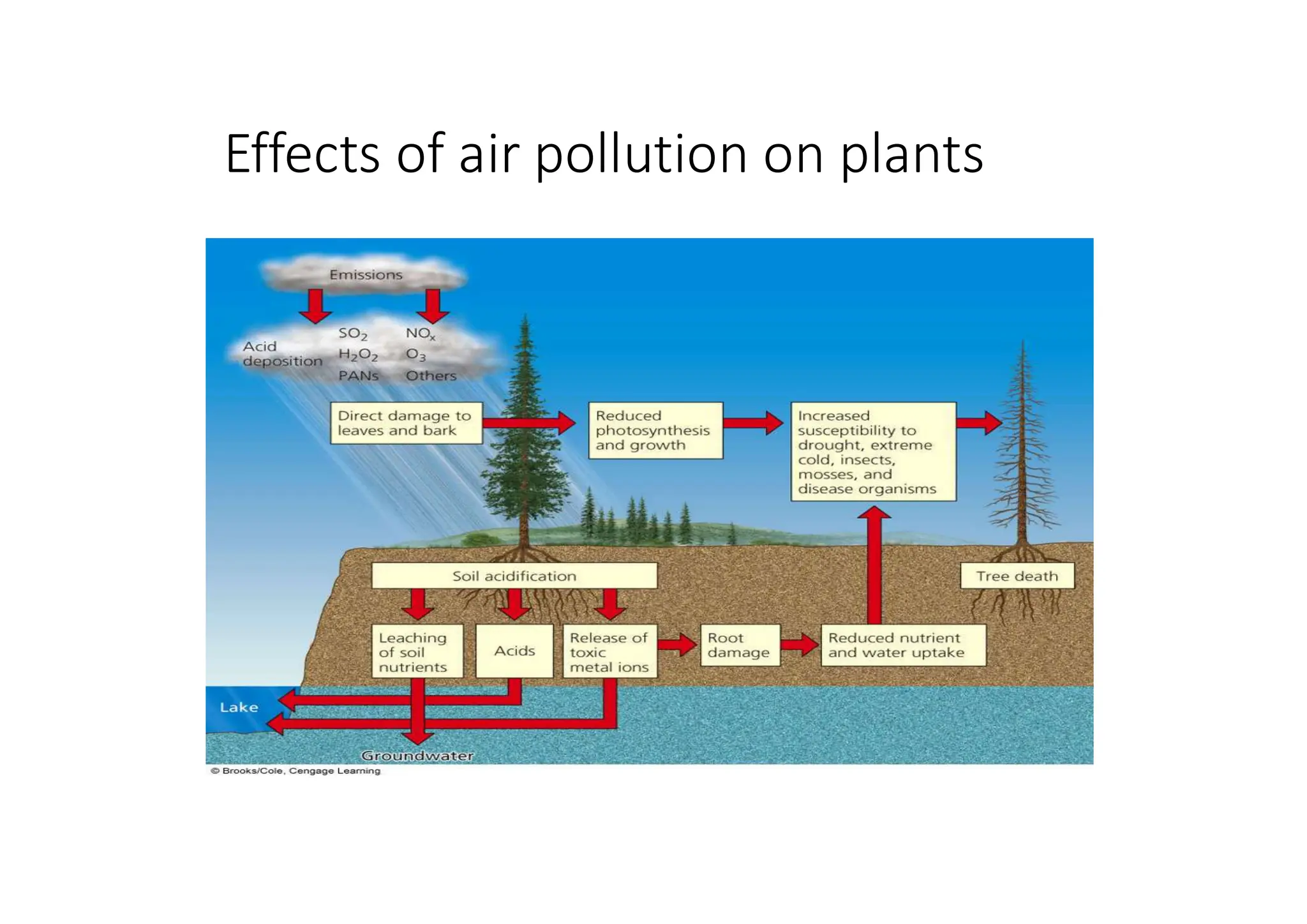
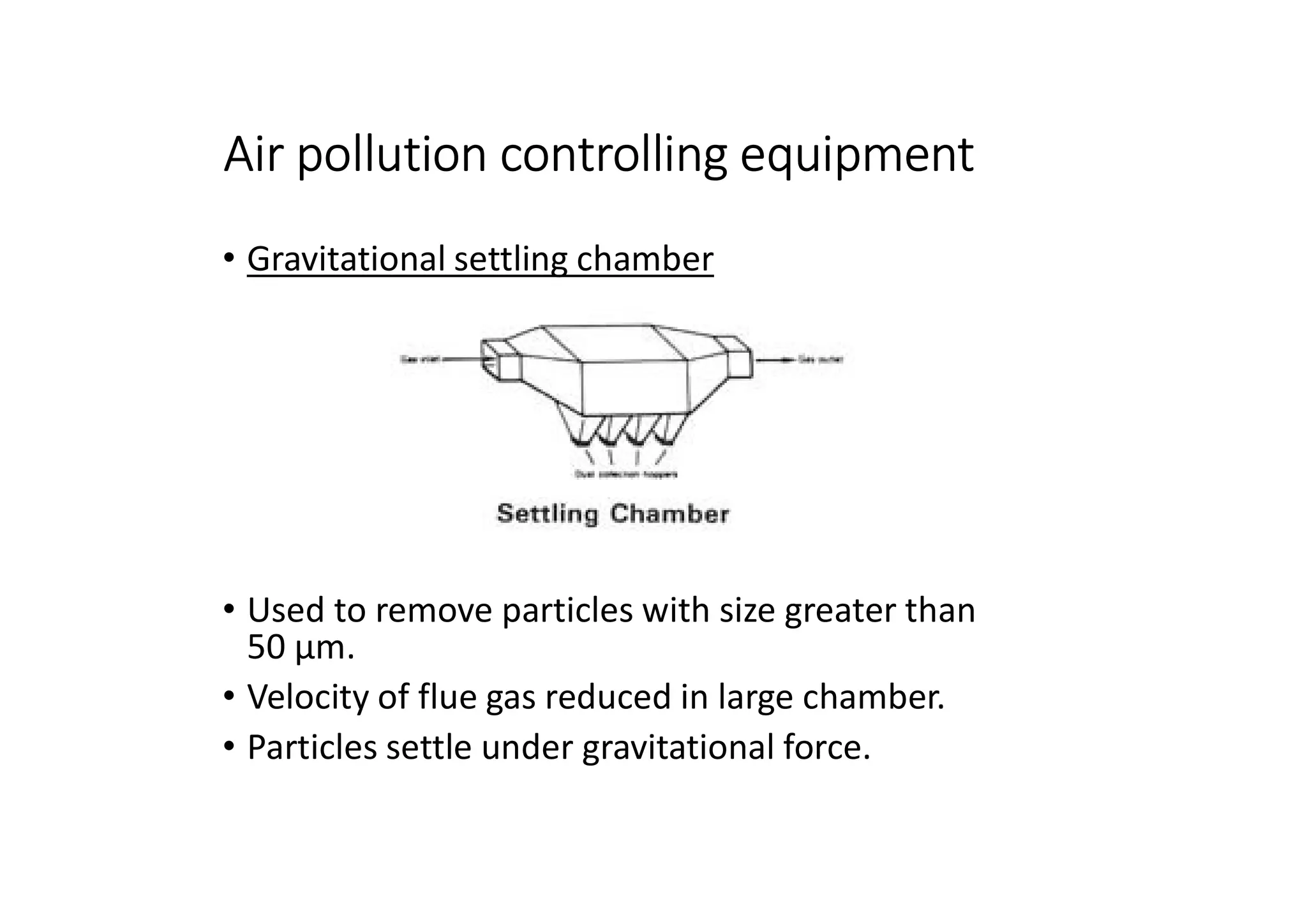
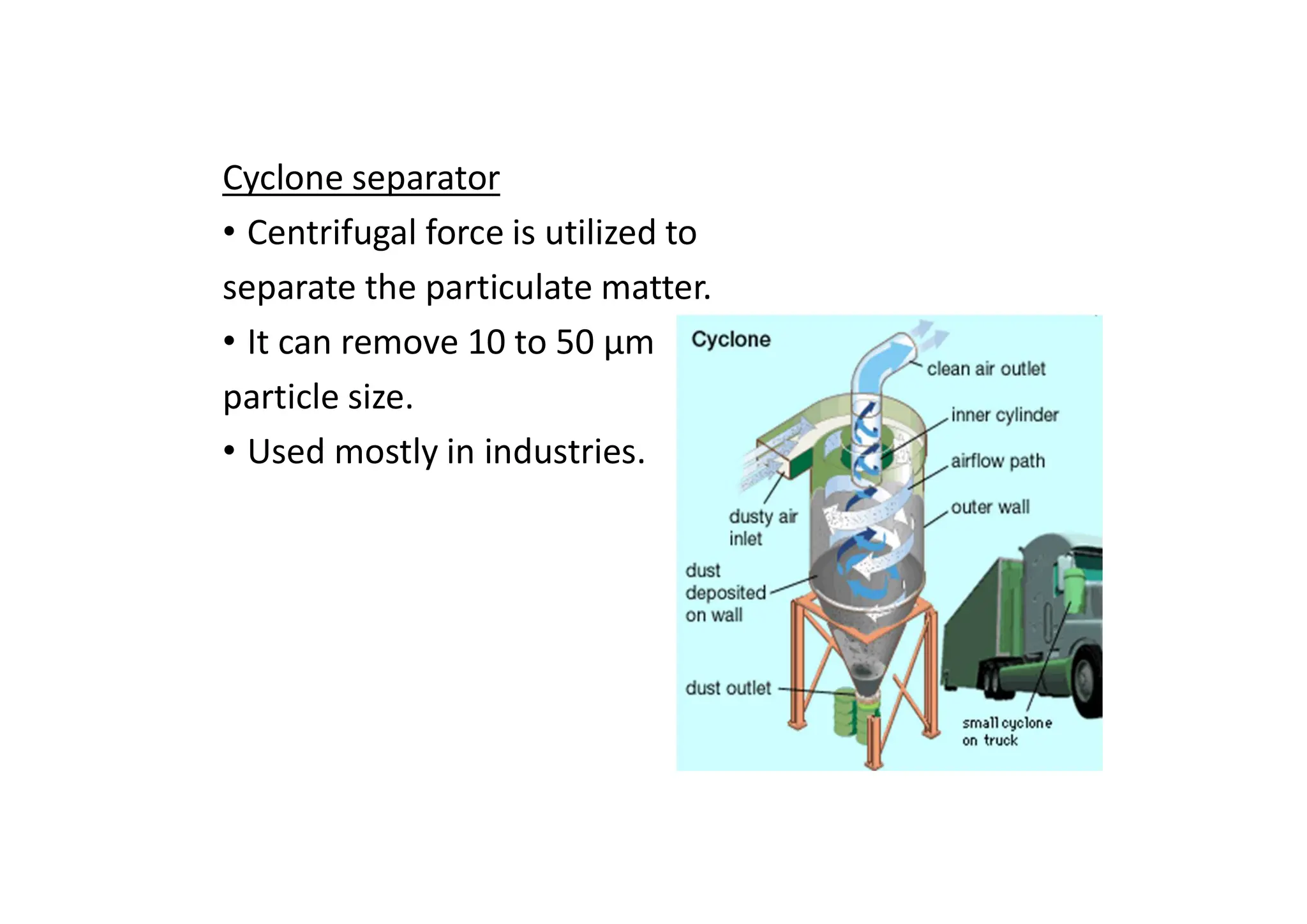
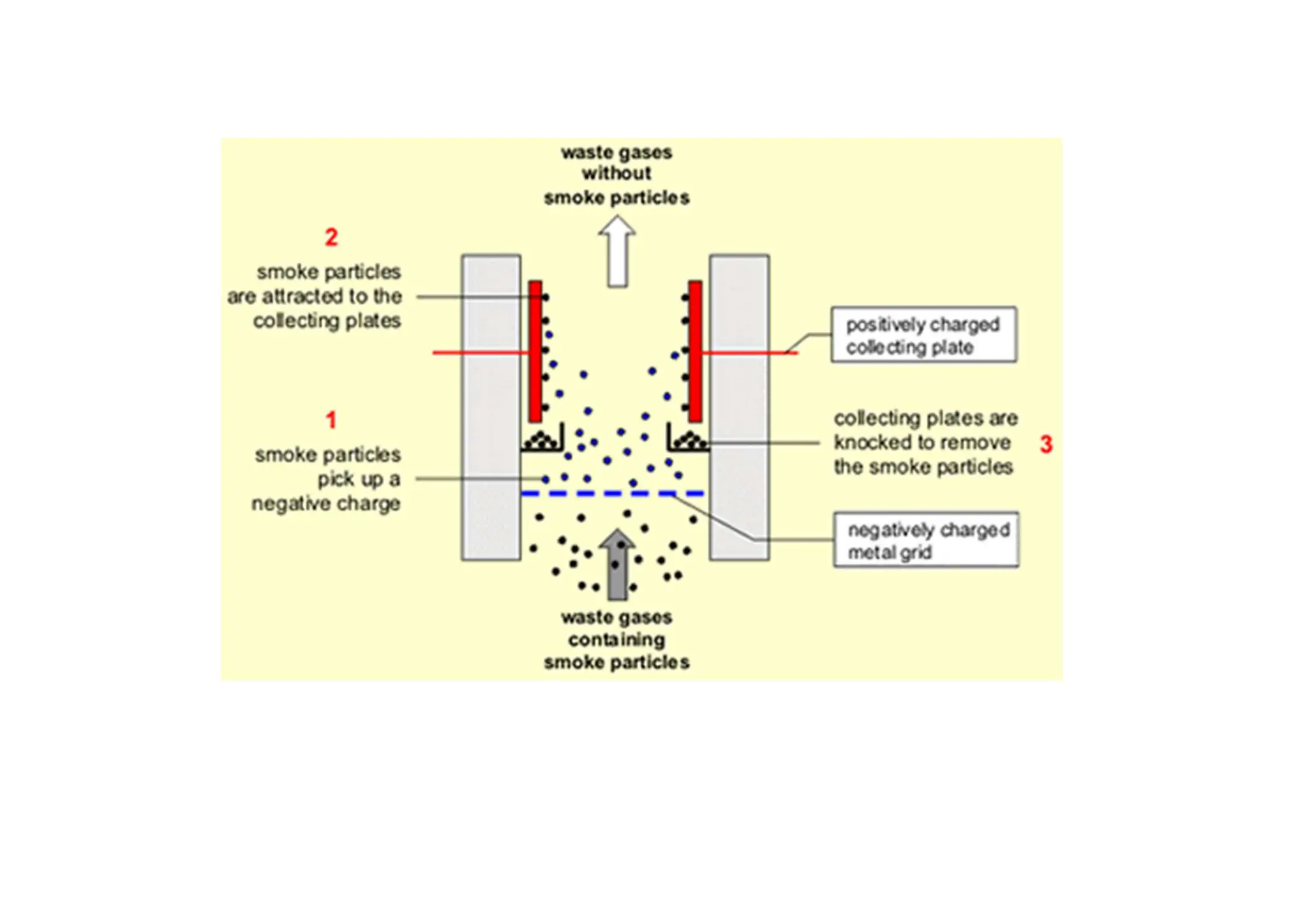
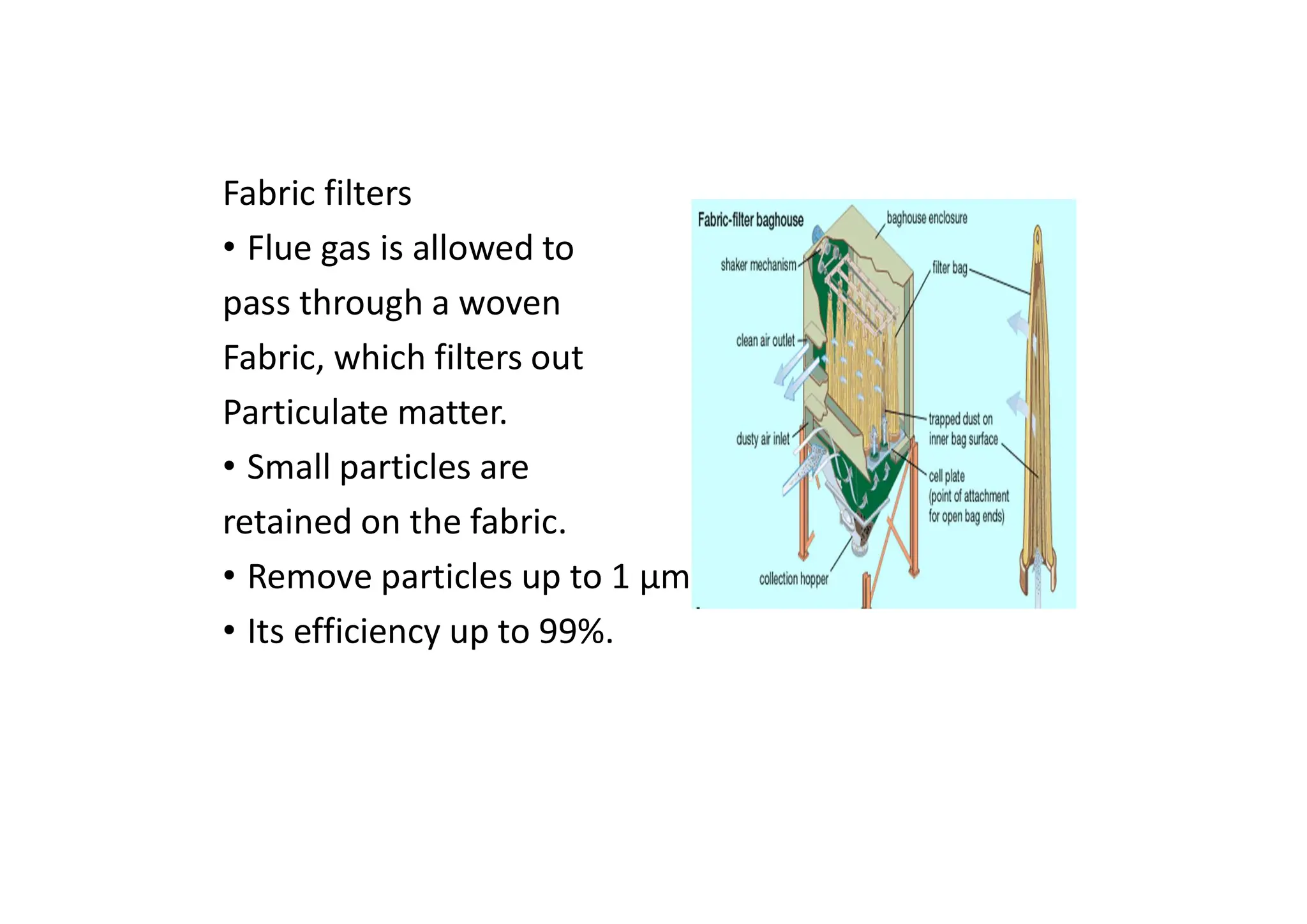
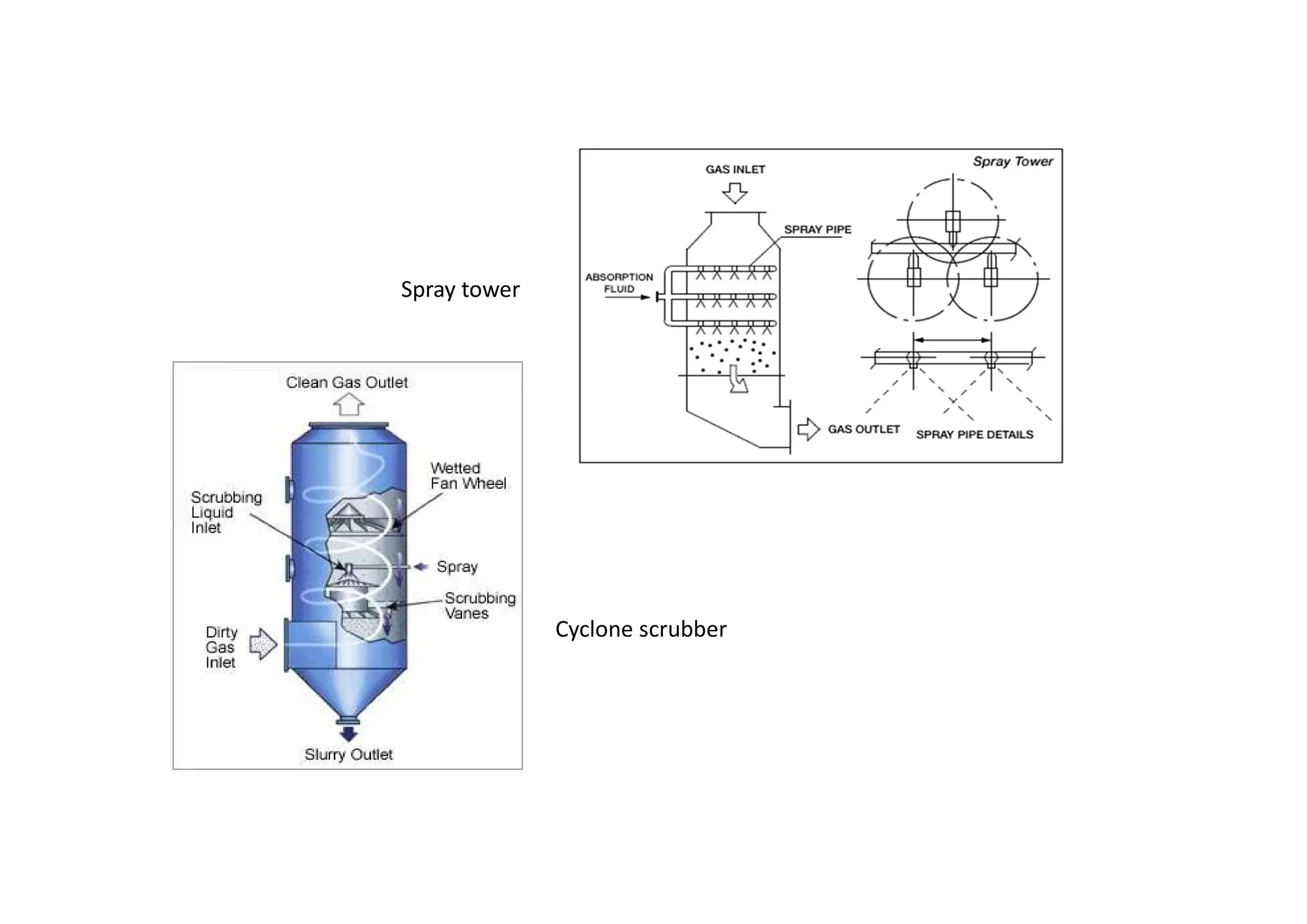
The document outlines air pollution, defining it as harmful contaminants in the atmosphere, addressing its composition, sources, and effects on health and the environment. It differentiates between natural and anthropogenic sources and categorizes pollutants as primary and secondary, highlighting major pollutants and their impacts. The lecture also discusses air pollution control methods, emphasizing preventive measures and various equipment used for pollution reduction.