This document discusses pharmaceutical aerosols, including their definition, advantages, components, propellants, classification of propellants, and formulation systems. Key points include:
- Pharmaceutical aerosols contain therapeutically active ingredients intended for administration via various routes, including respiratory systems.
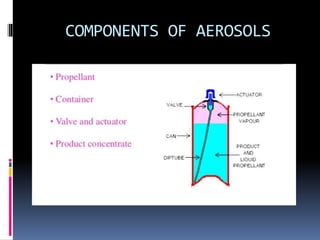
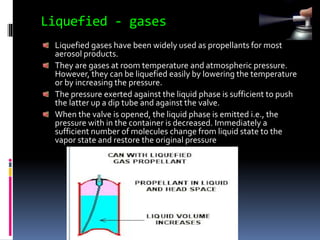
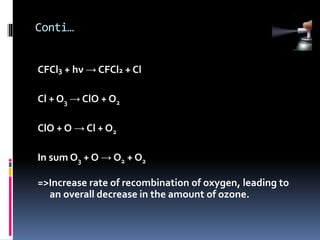
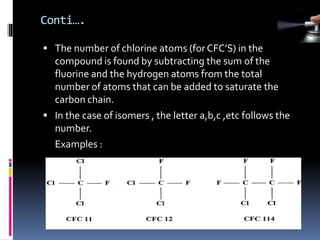
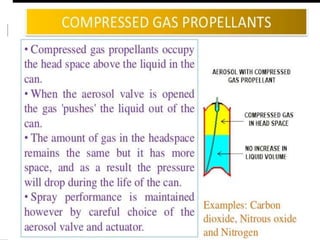
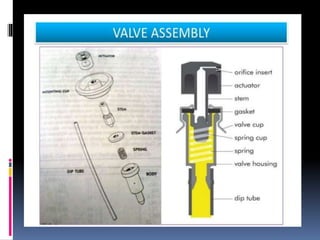
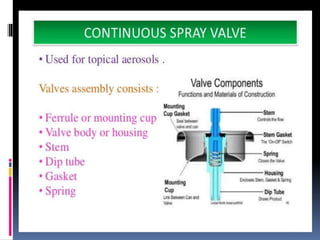
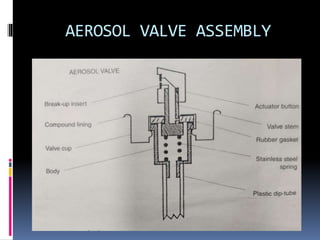
- Propellants are responsible for developing pressure to expel the product from the container. Common propellants include CFCs, HCFCs, HFCs, hydrocarbons, nitrogen, nitrous oxide, and carbon dioxide.
- Formulation systems include solution aerosols, suspension aerosols, emulsion aerosols, water-based systems, and aquasol systems. Solution systems are classified as space or surface sp