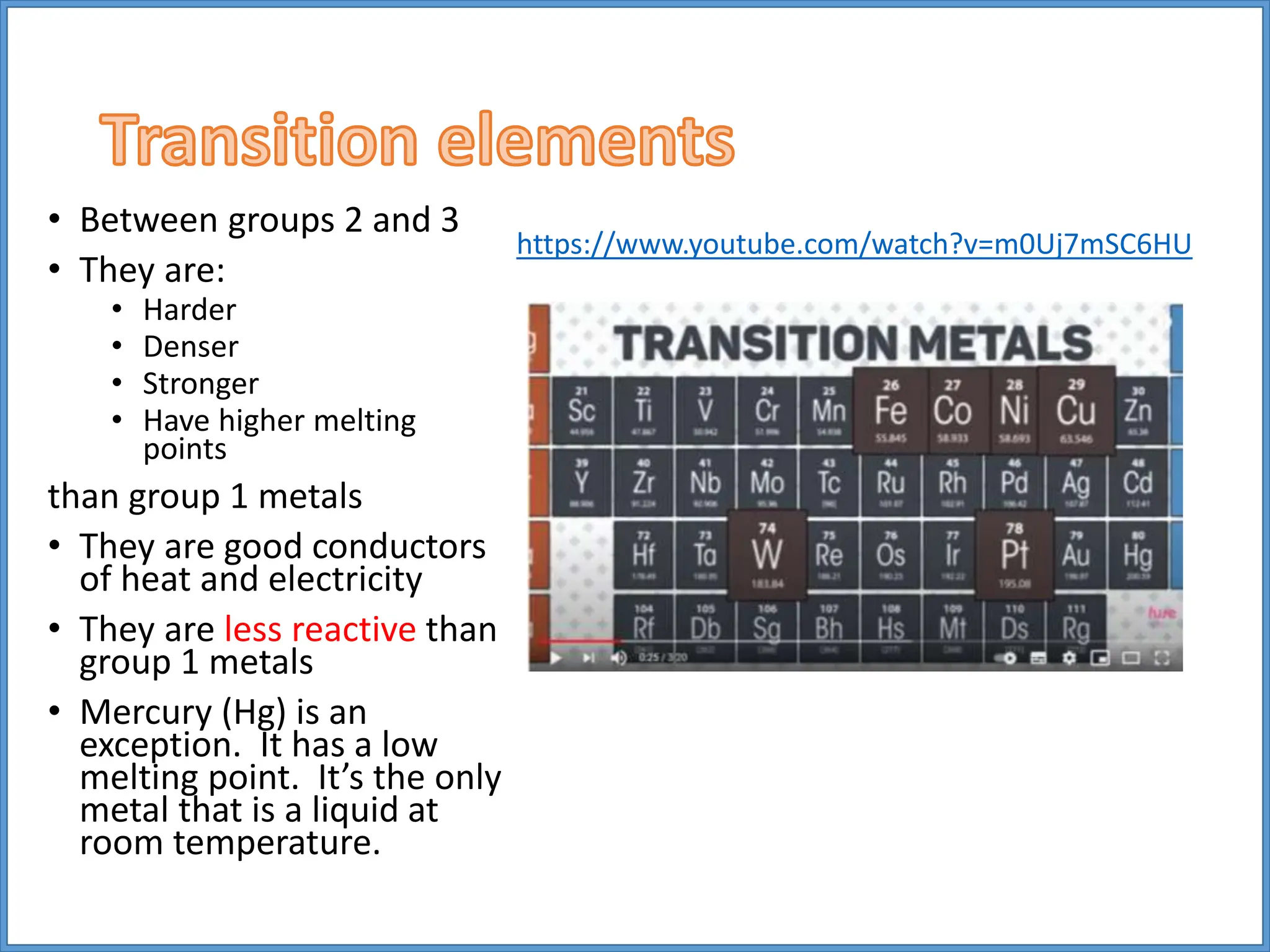
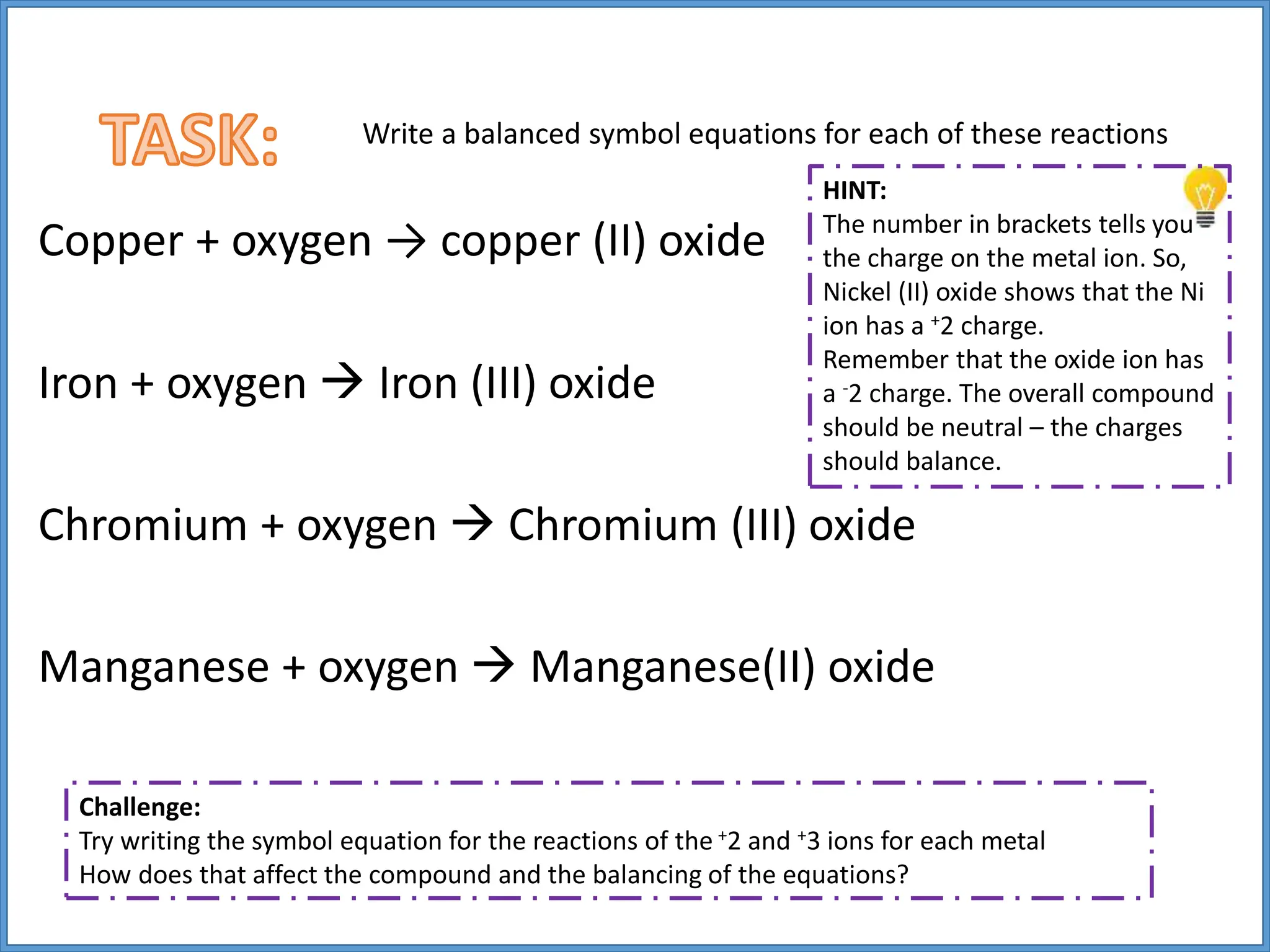
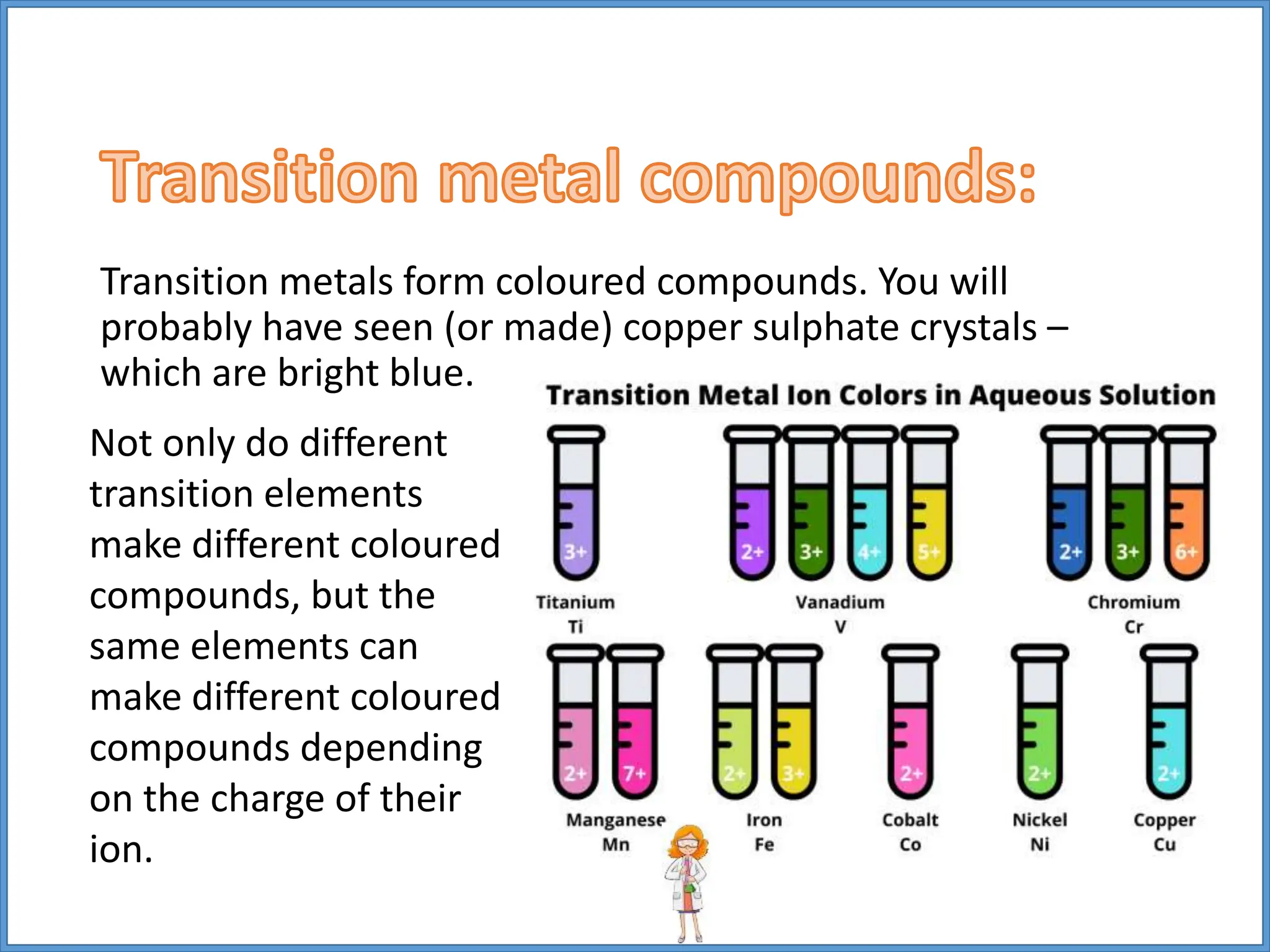
Transition metals have higher melting points and densities than group 1 metals, and are stronger yet still good conductors of heat and electricity. They can form positive ions with different charges and form colored compounds. Common transition metals include iron, copper, chromium, and manganese, which can react with oxygen to form metal oxide compounds.