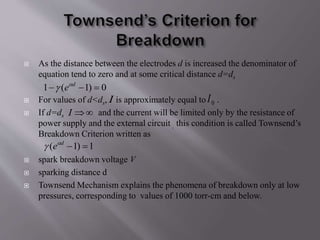
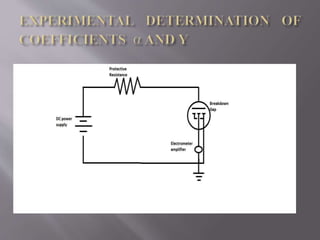
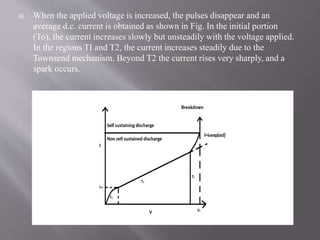
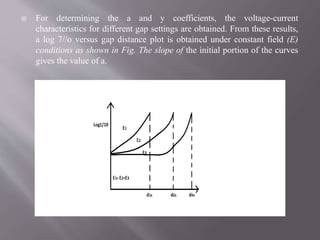
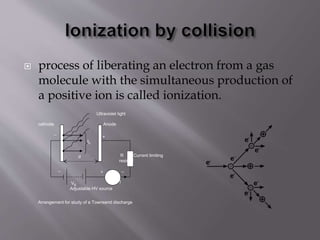
Townsend's theory addresses ionization in gases and outlines the mechanisms of electron emission, including primary and secondary ionization processes. It describes Townsend's breakdown criterion, emphasizing the role of gas pressure and electron attachment, particularly in electronegative gases. Experimental arrangements for studying Townsend discharges are also detailed, highlighting the method for measuring current characteristics and breakdown voltage in a controlled environment.




1(1
0
d
d
e
en
n
)1(1
0
d
d
e
eI
I
](https://image.slidesharecdn.com/townsendstheory-160722183015/85/Townsend-s-theory-6-320.jpg)