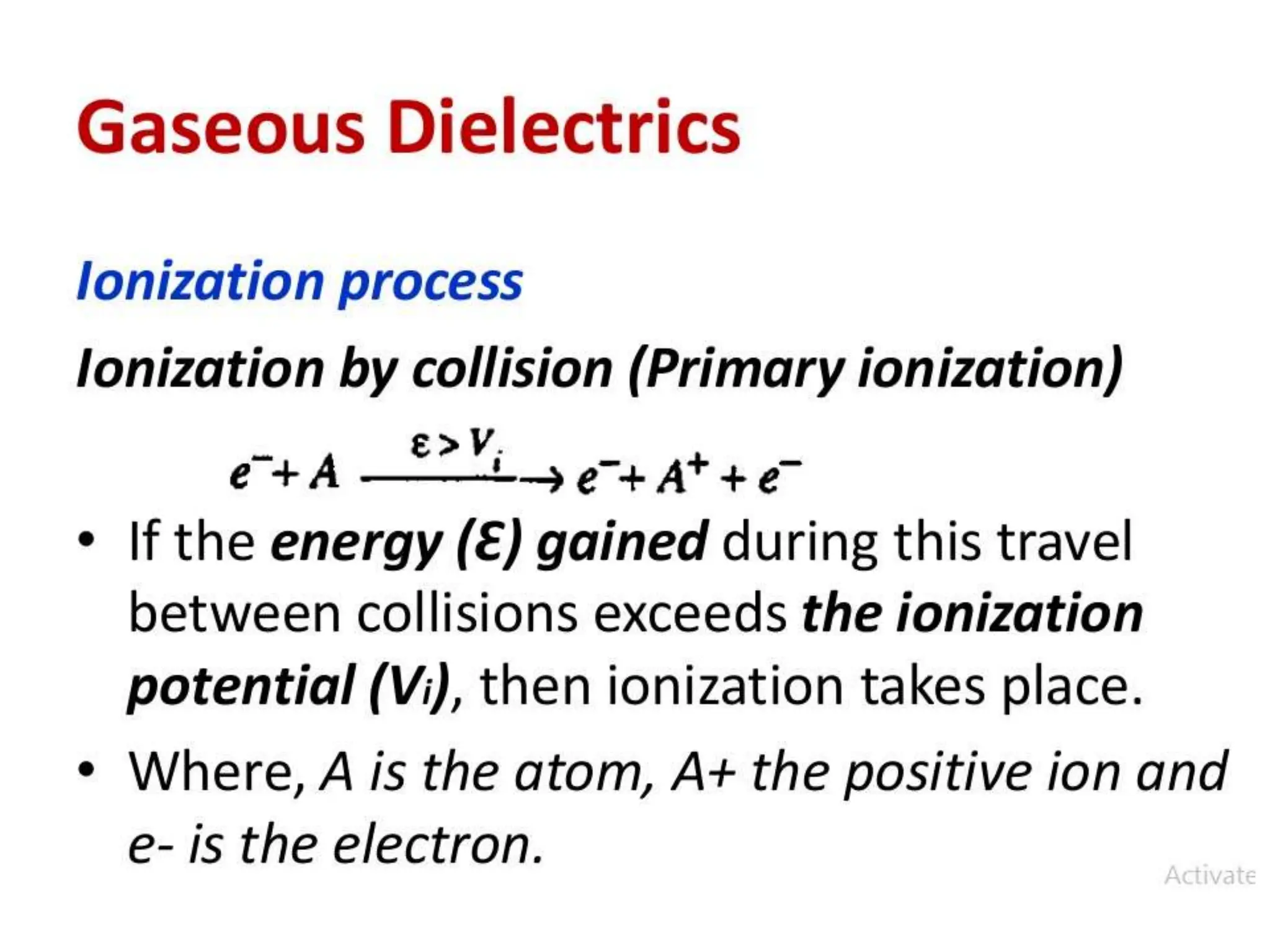
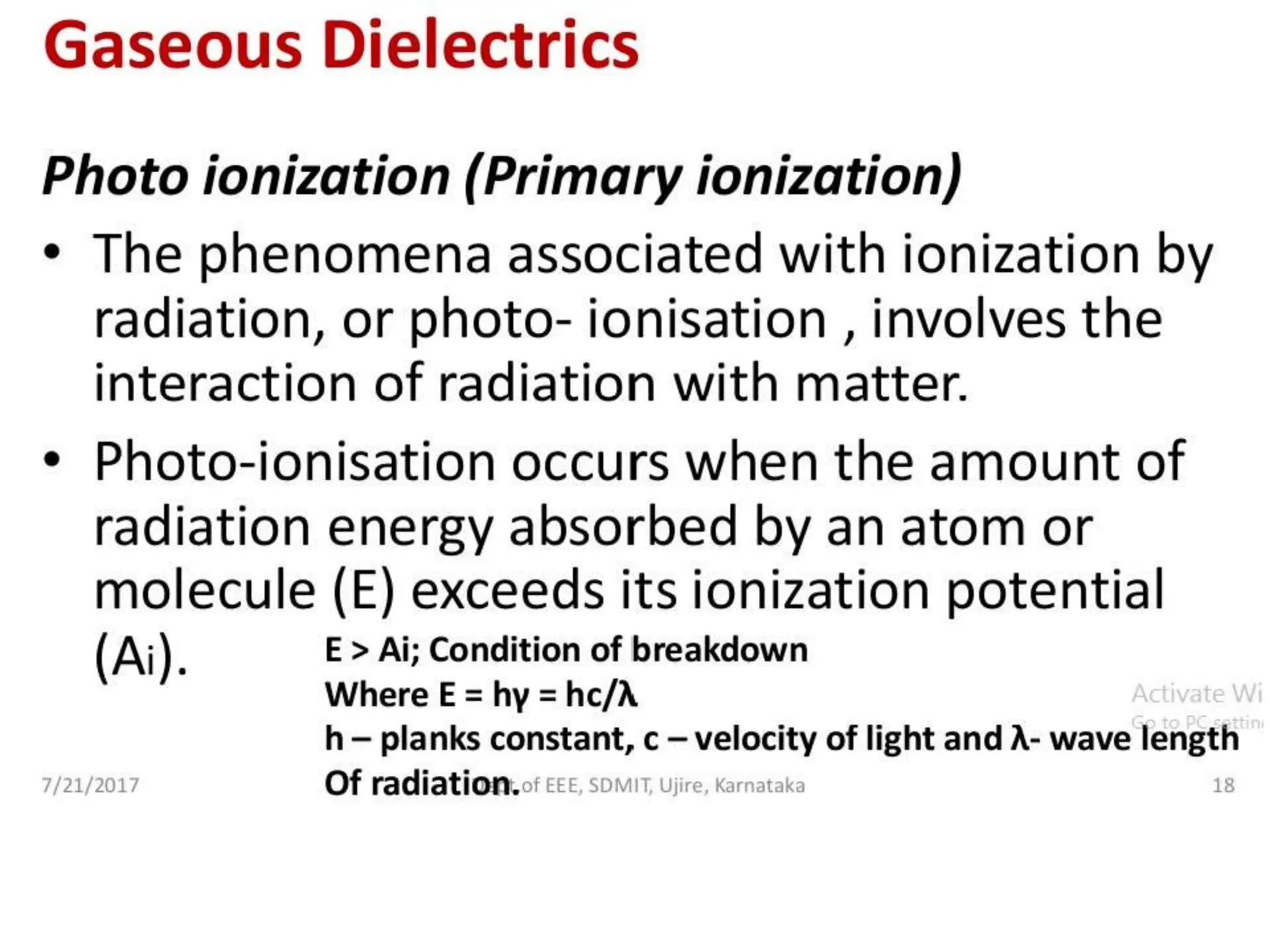
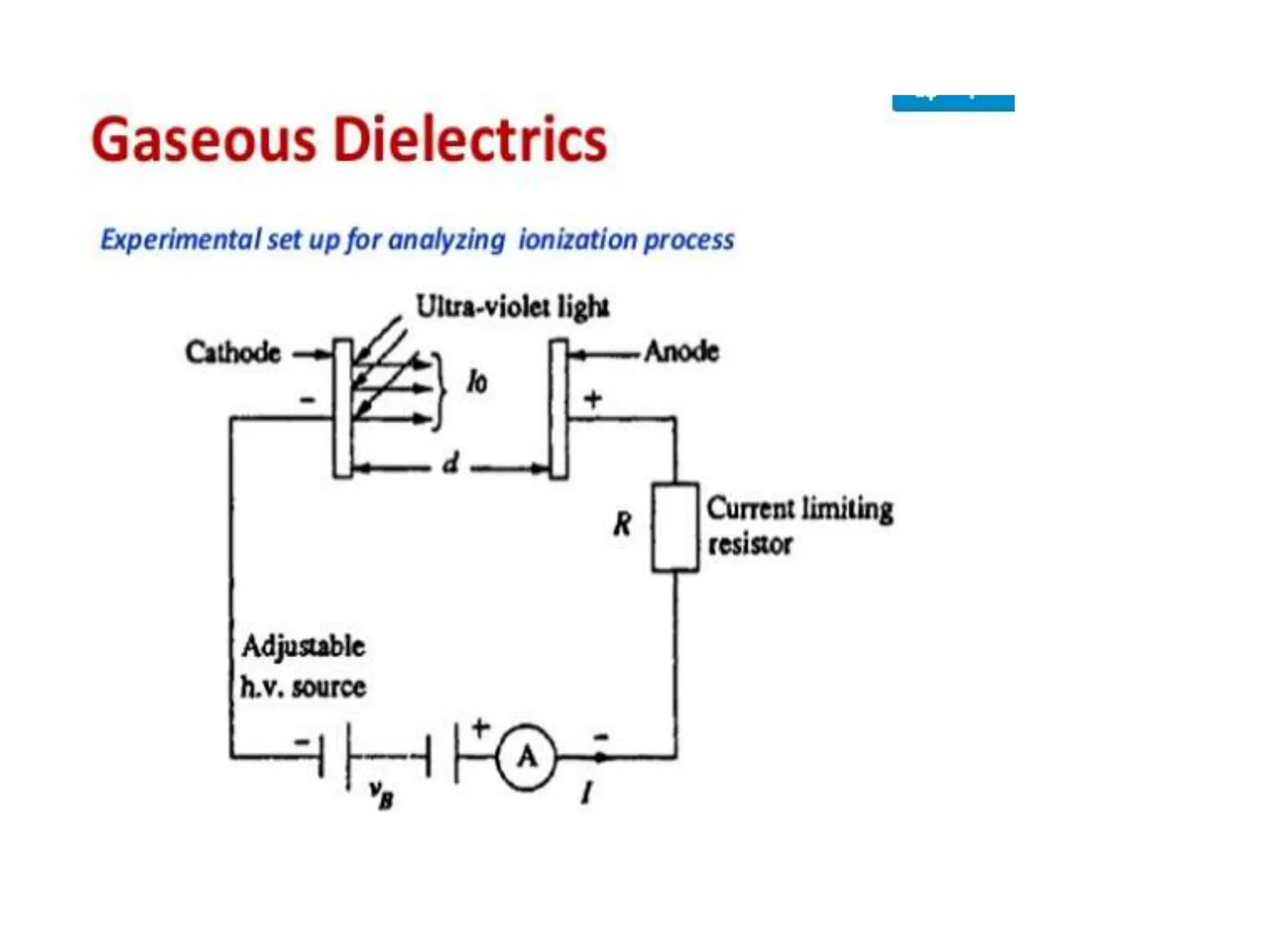
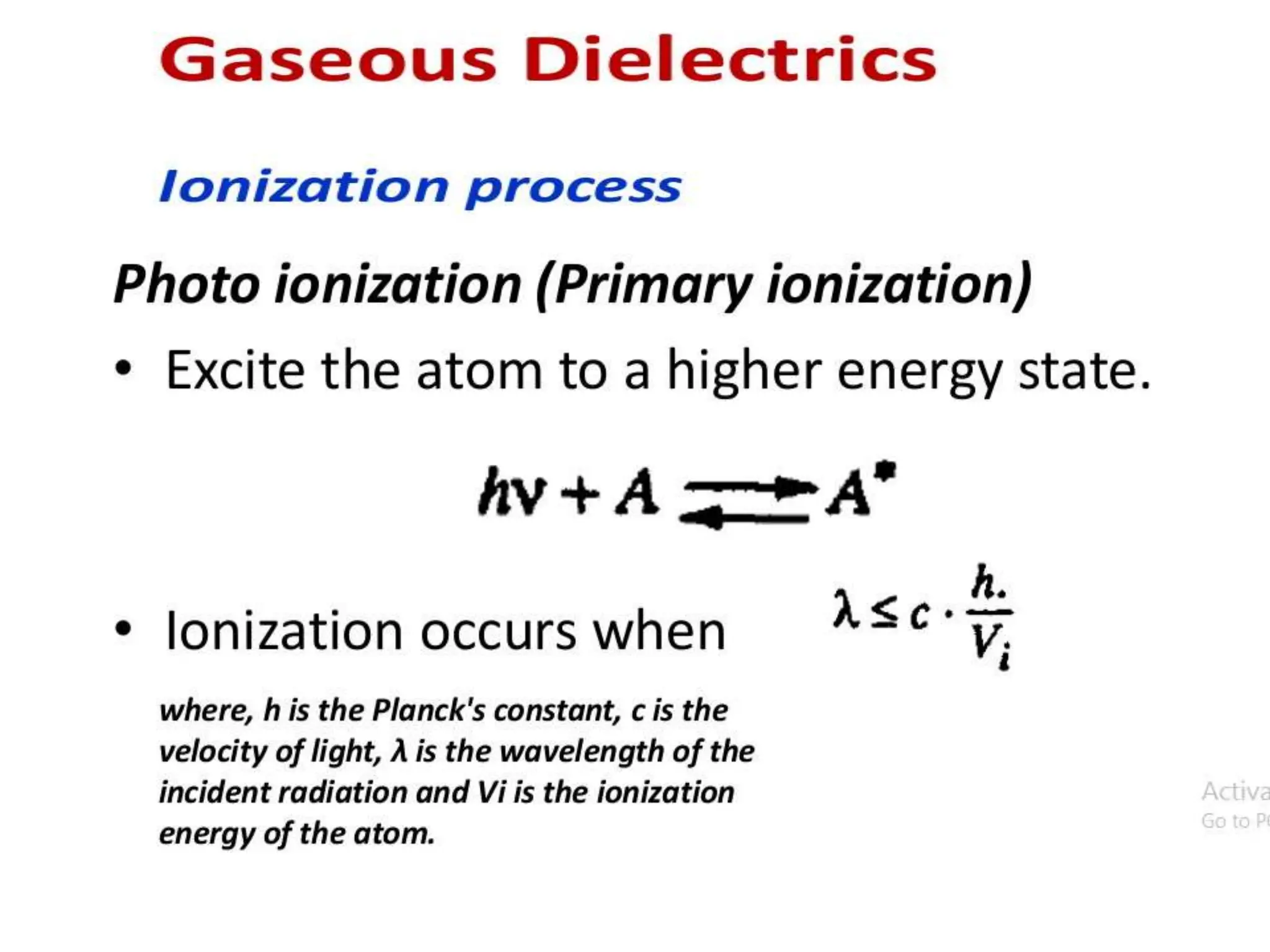
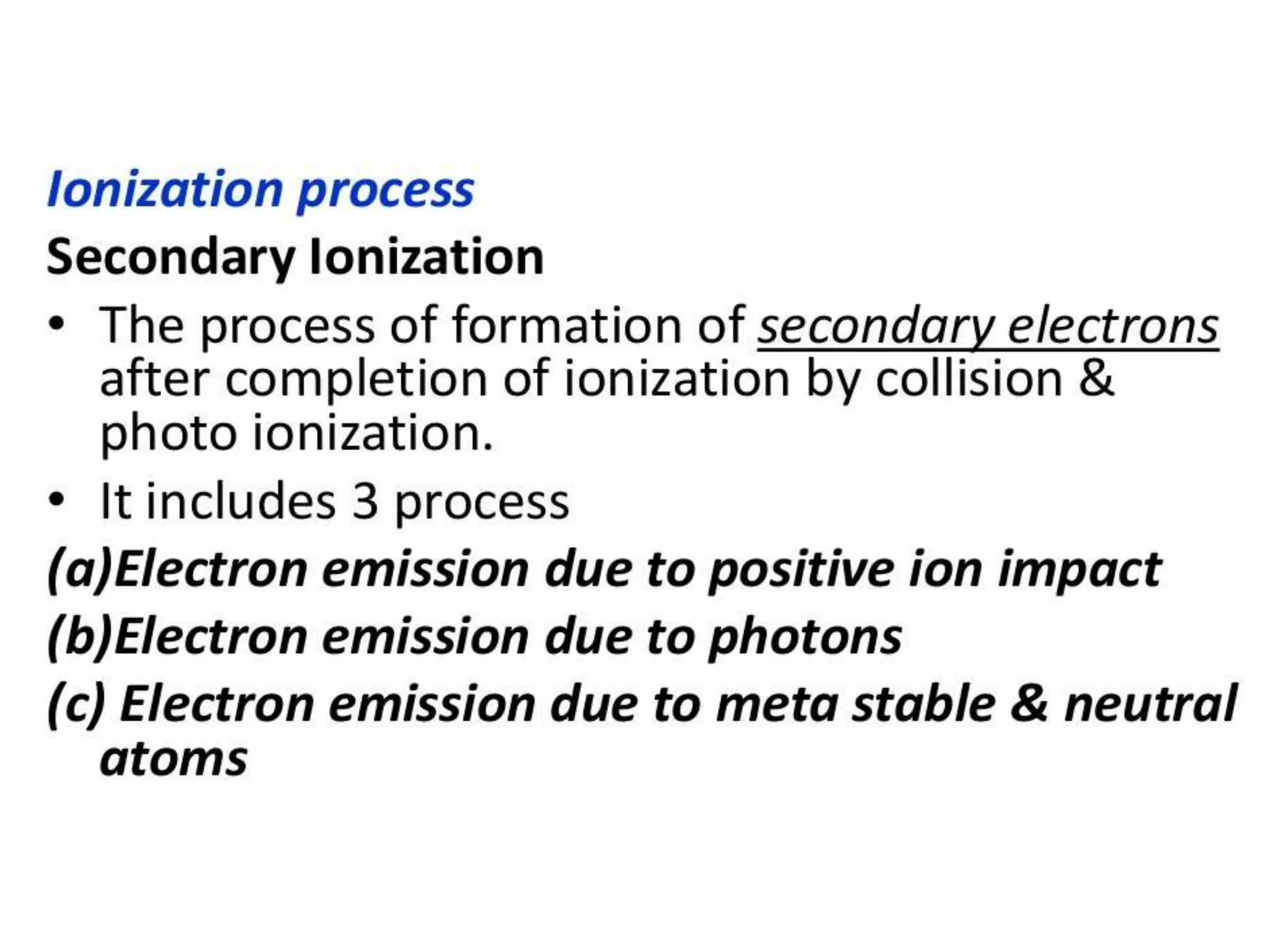
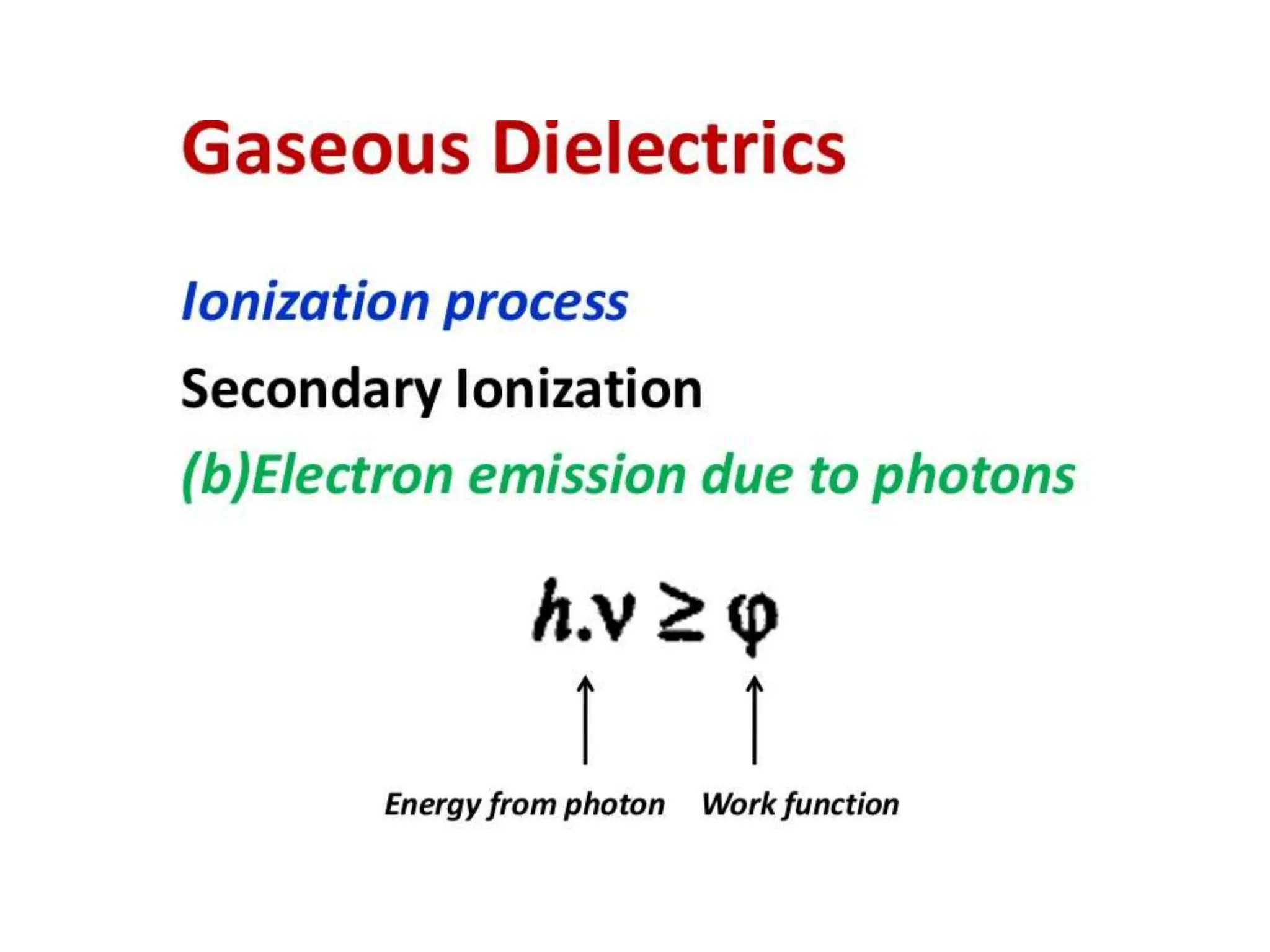
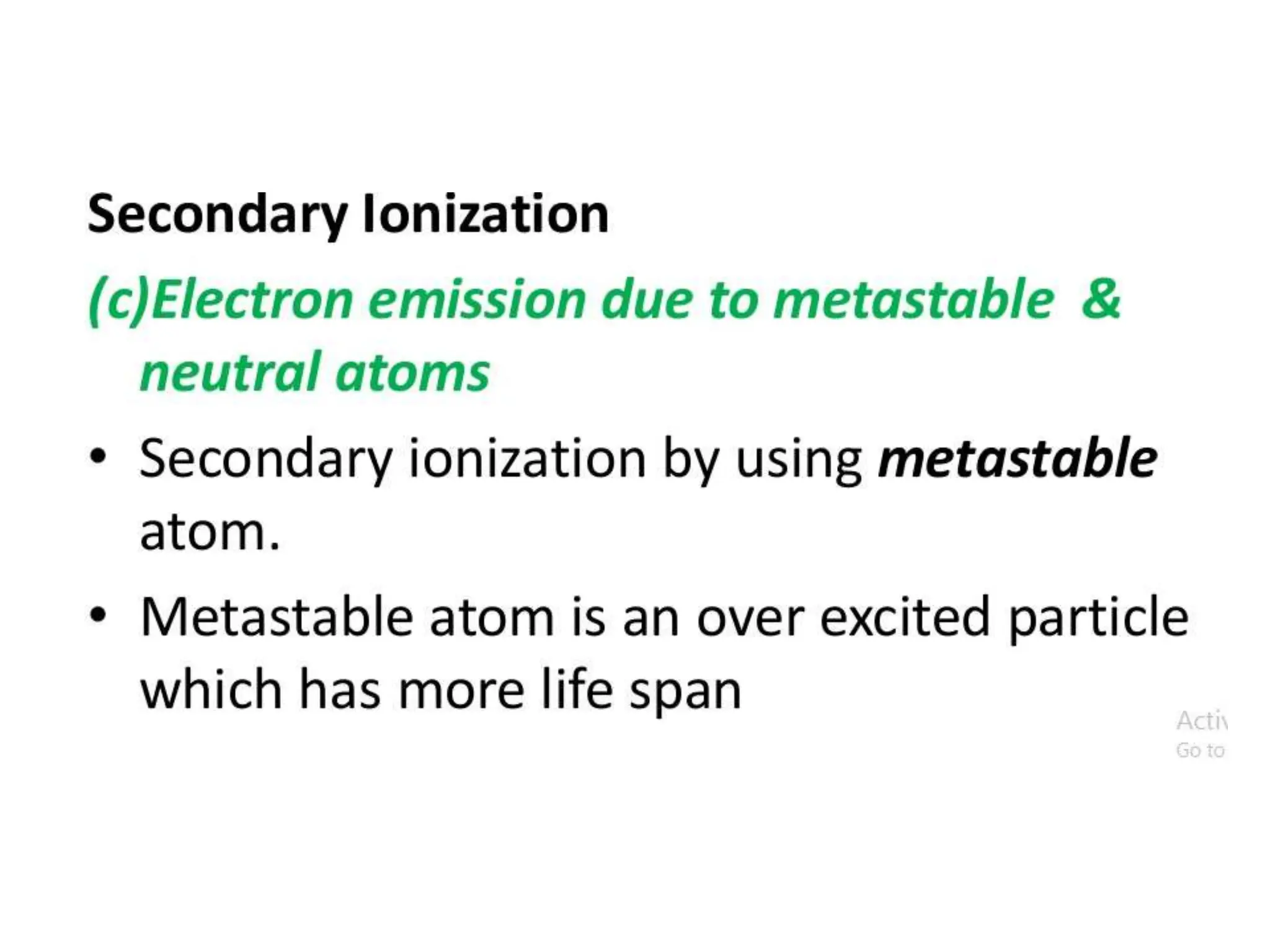
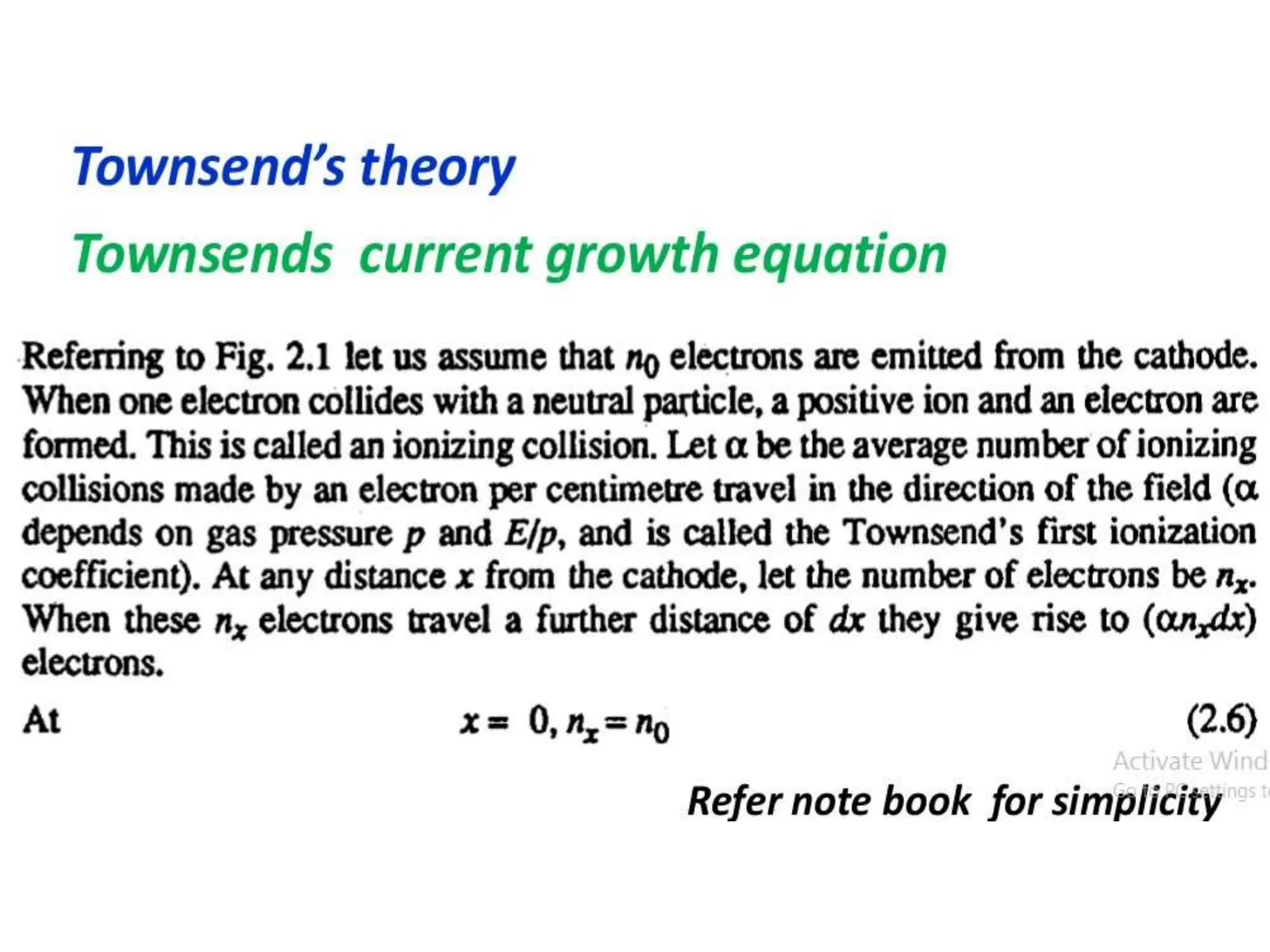
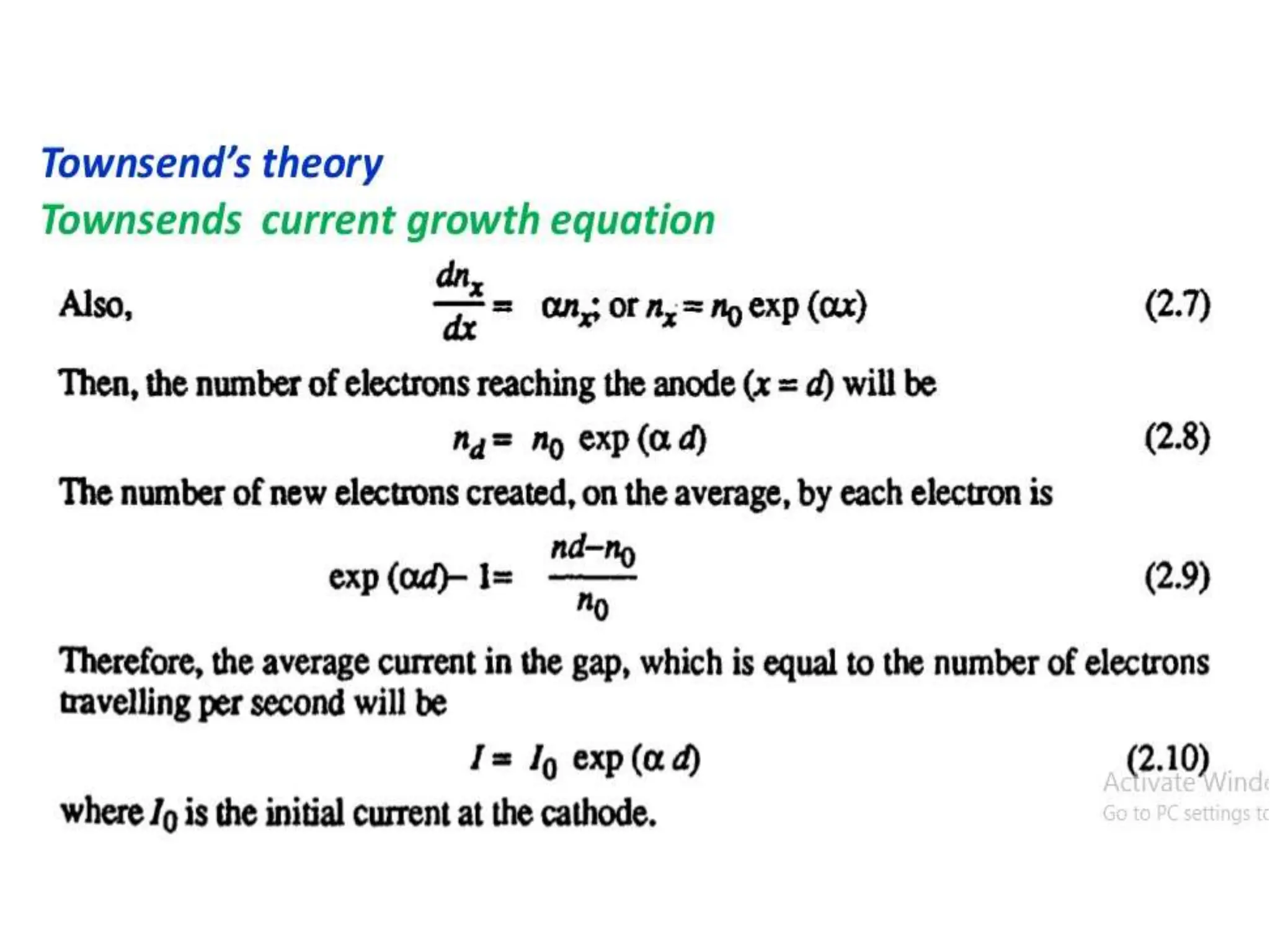
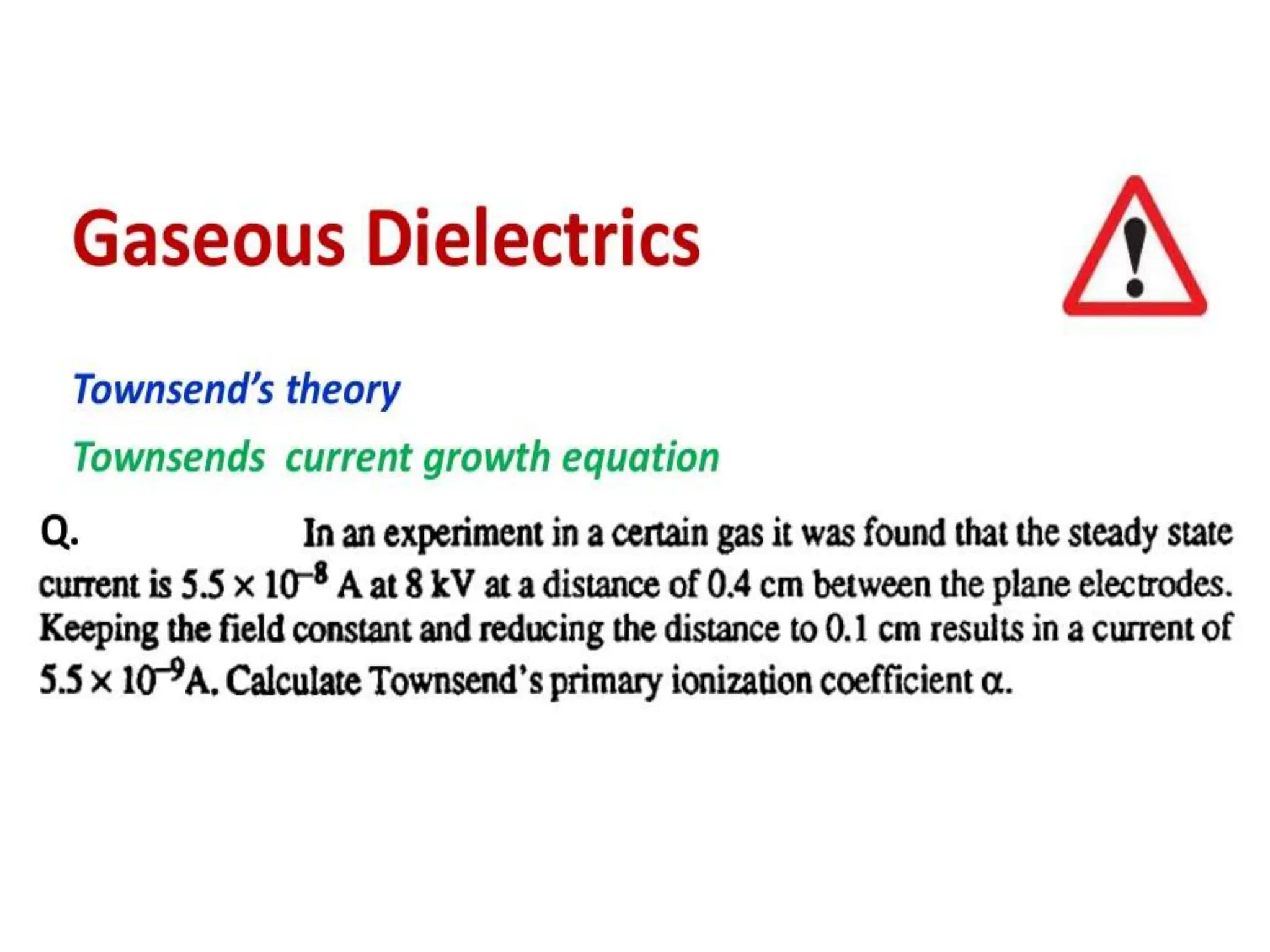
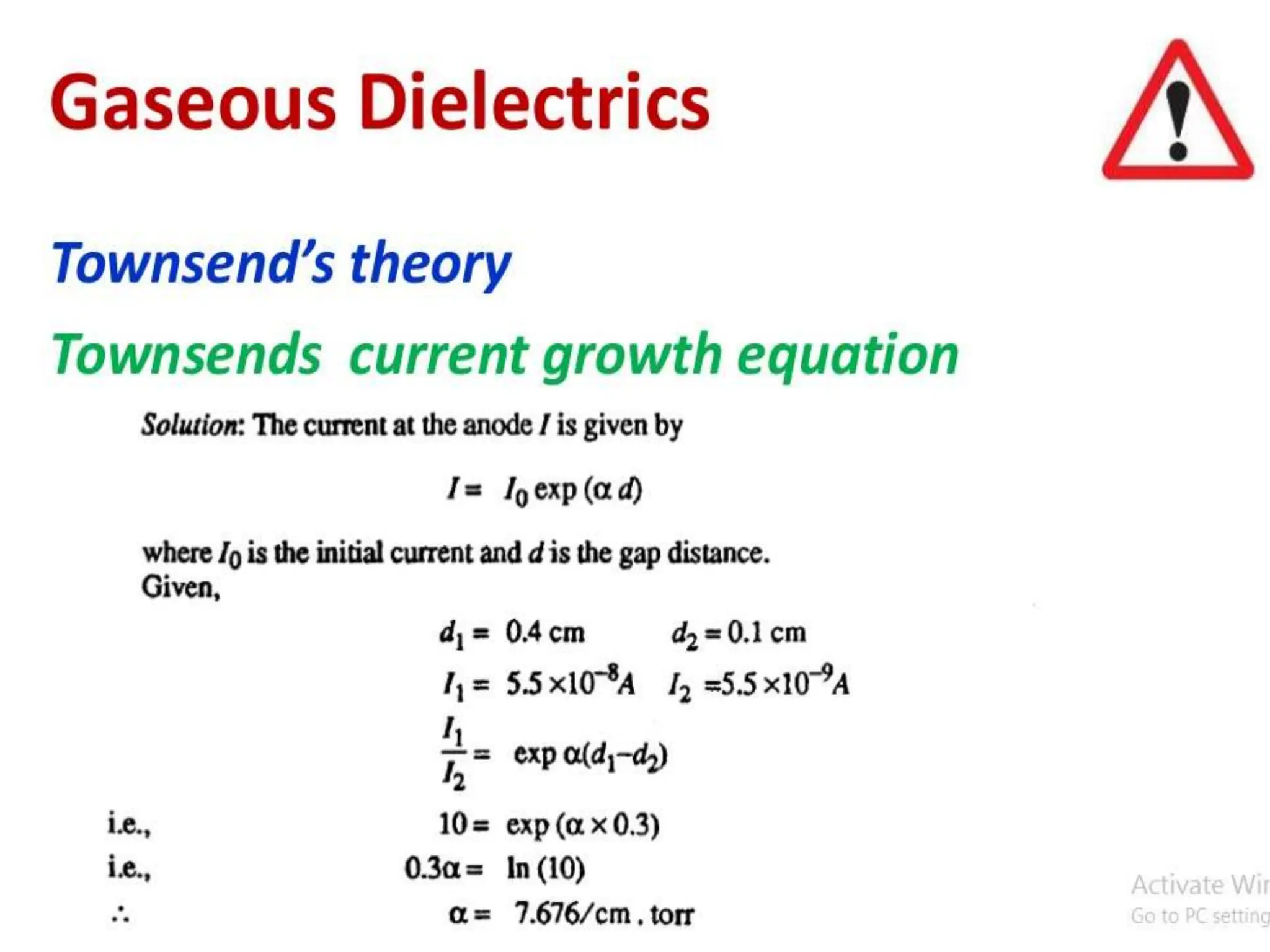
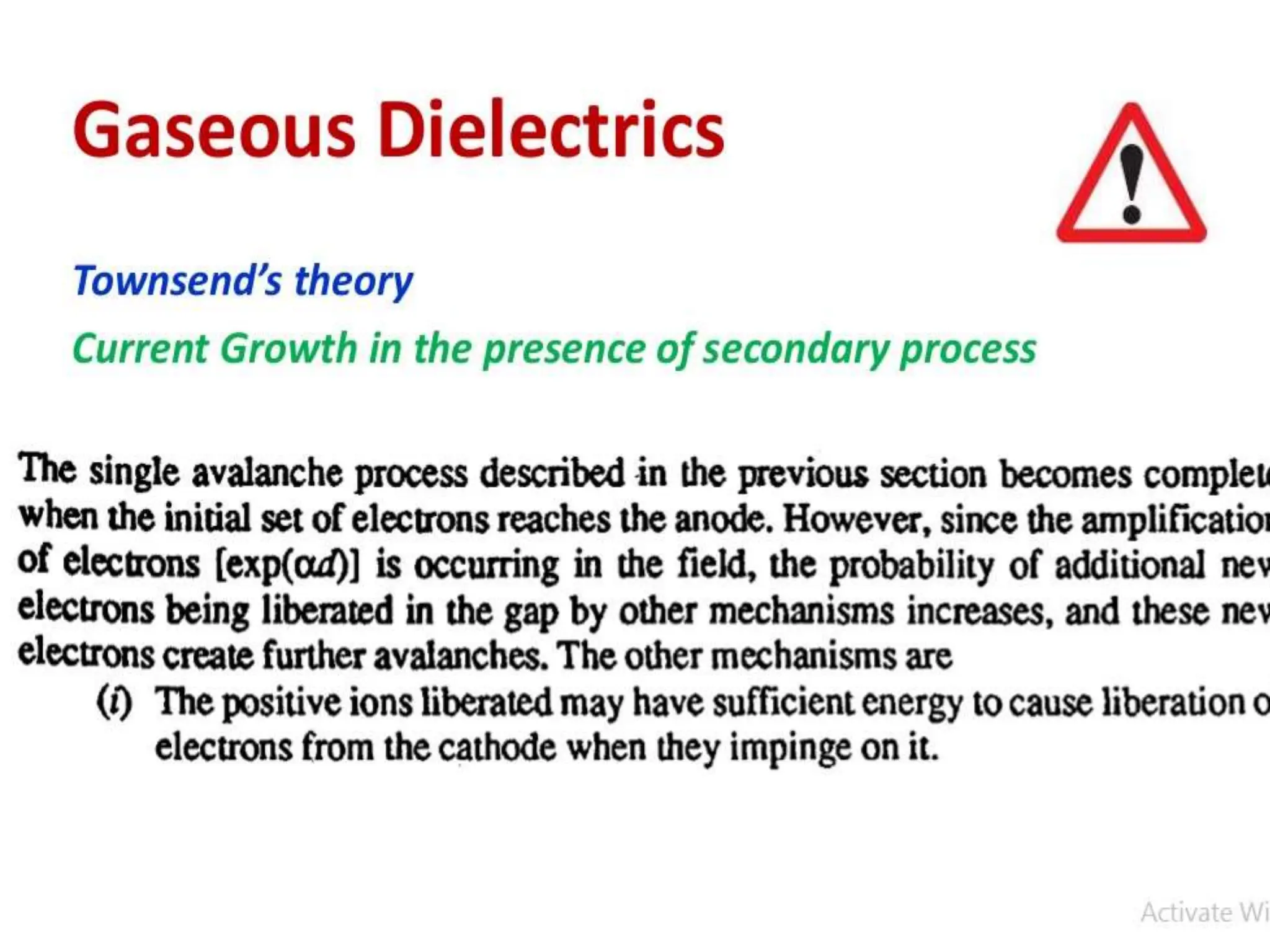
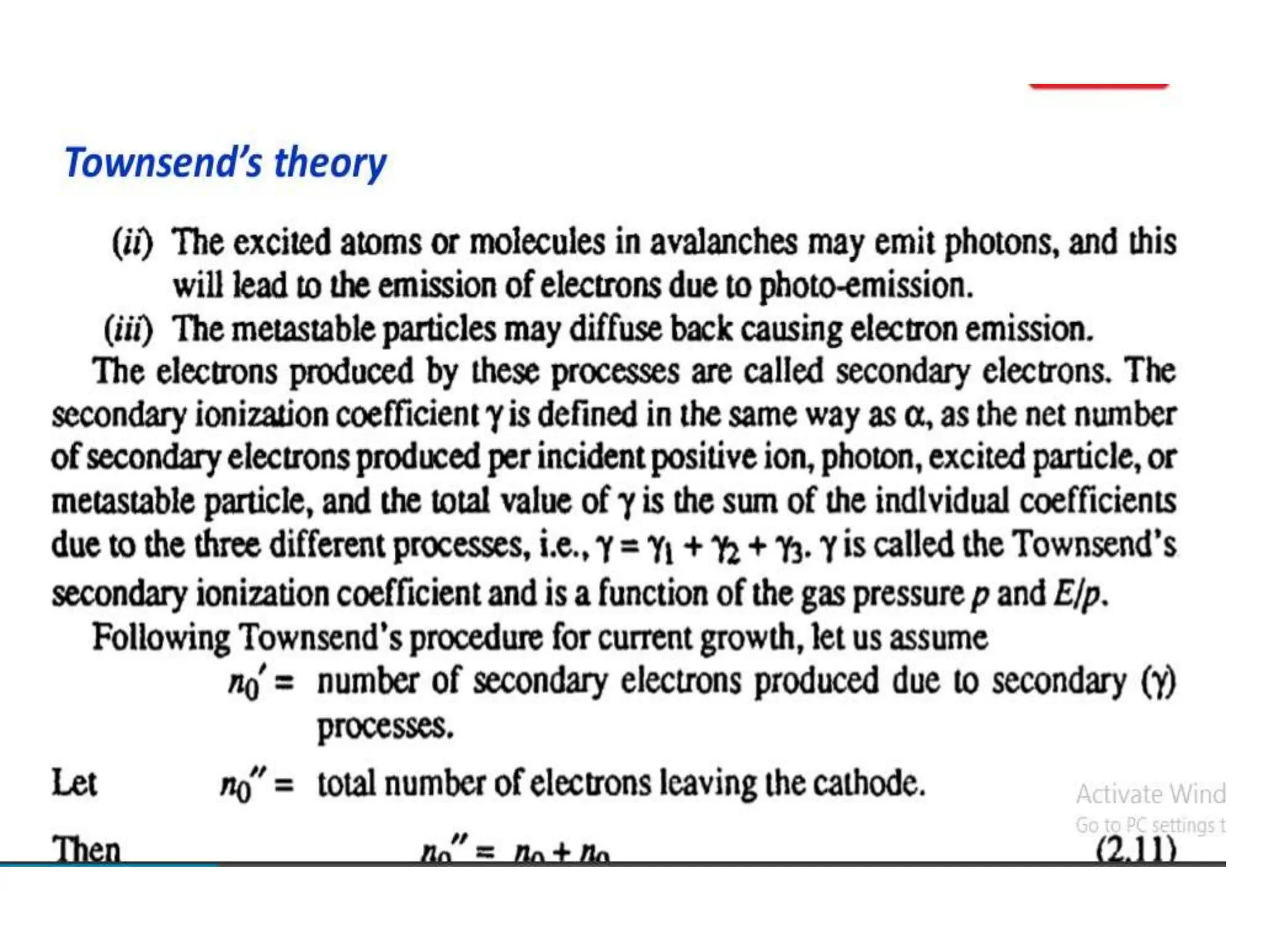
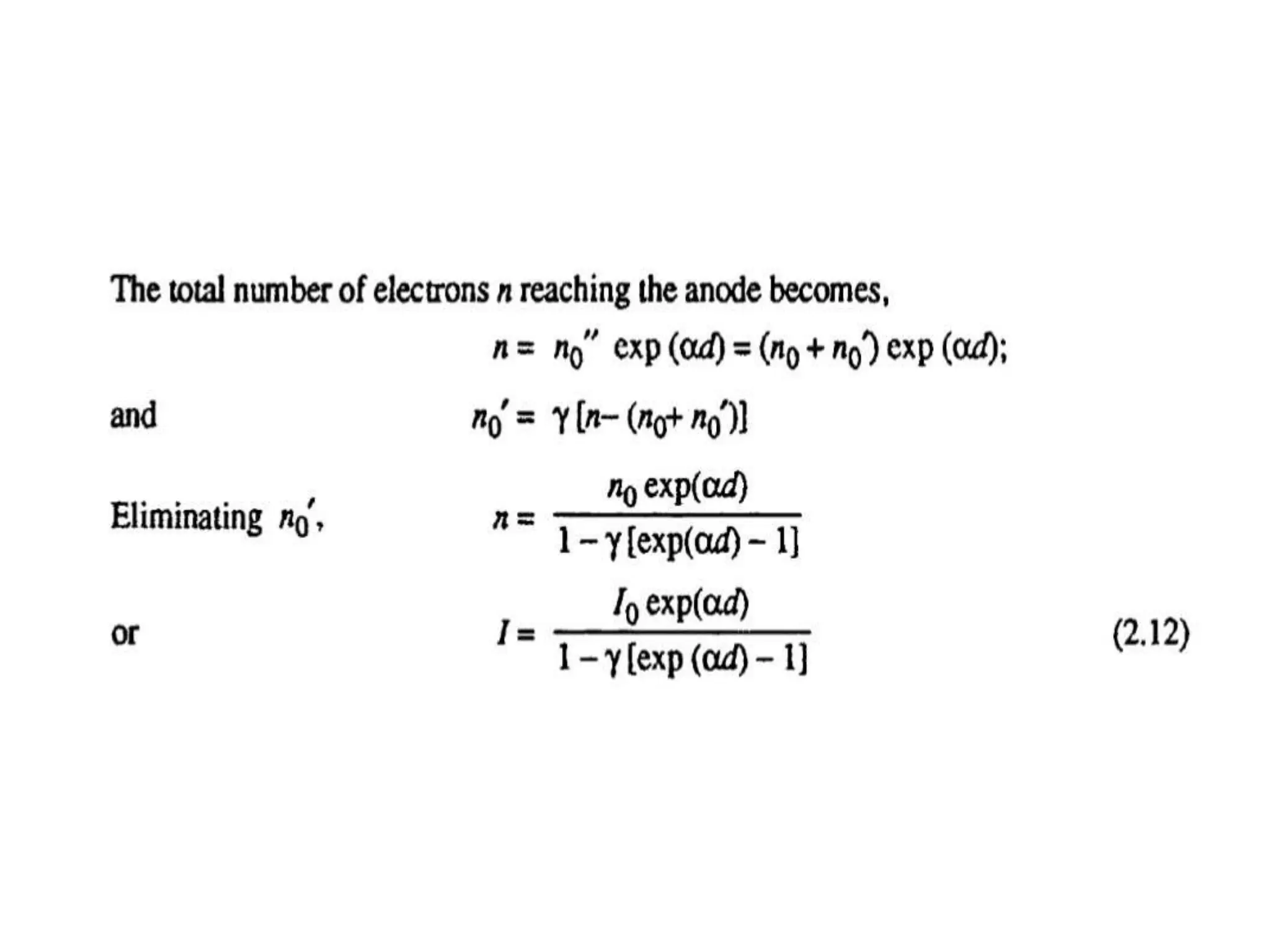
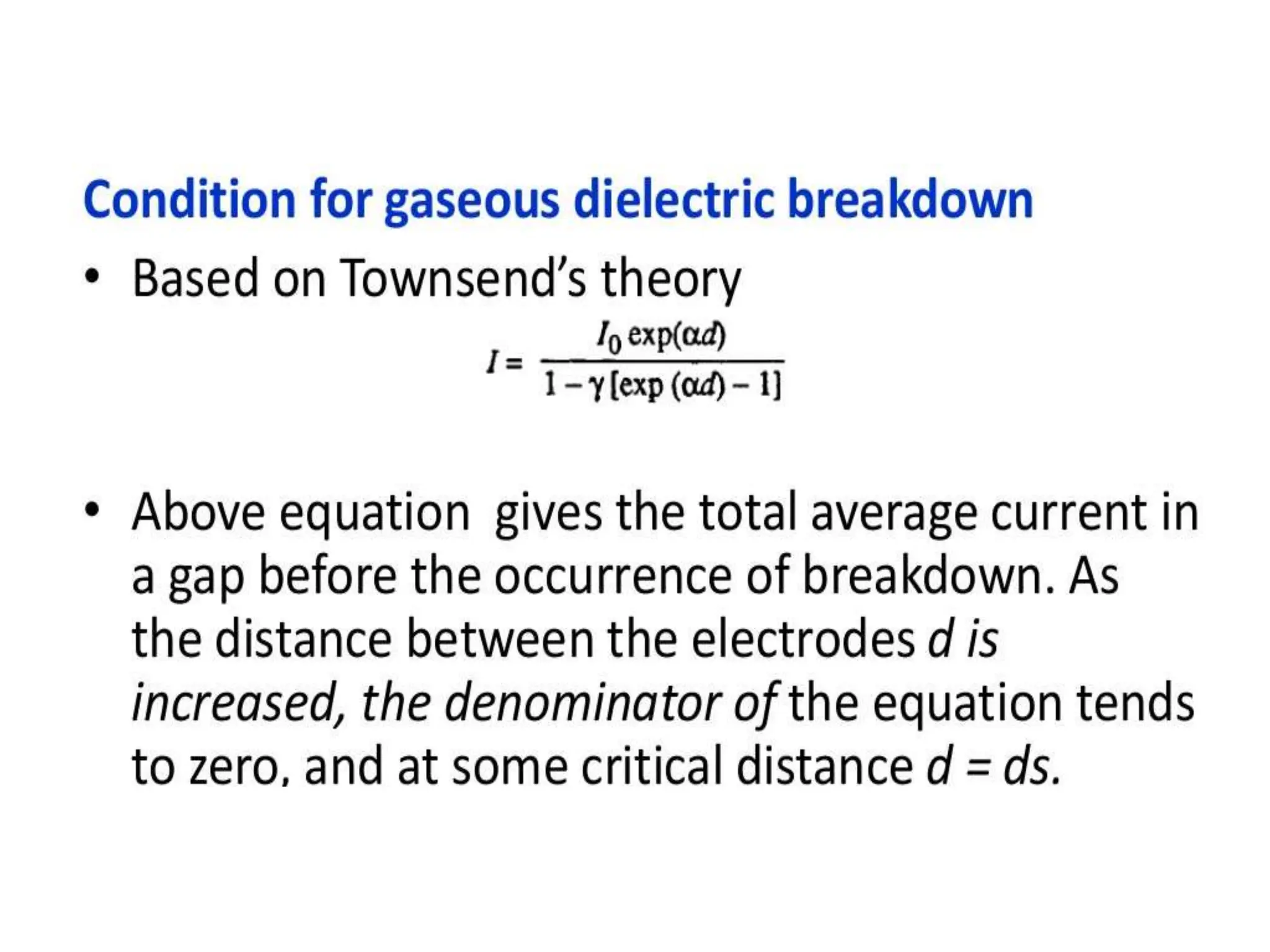
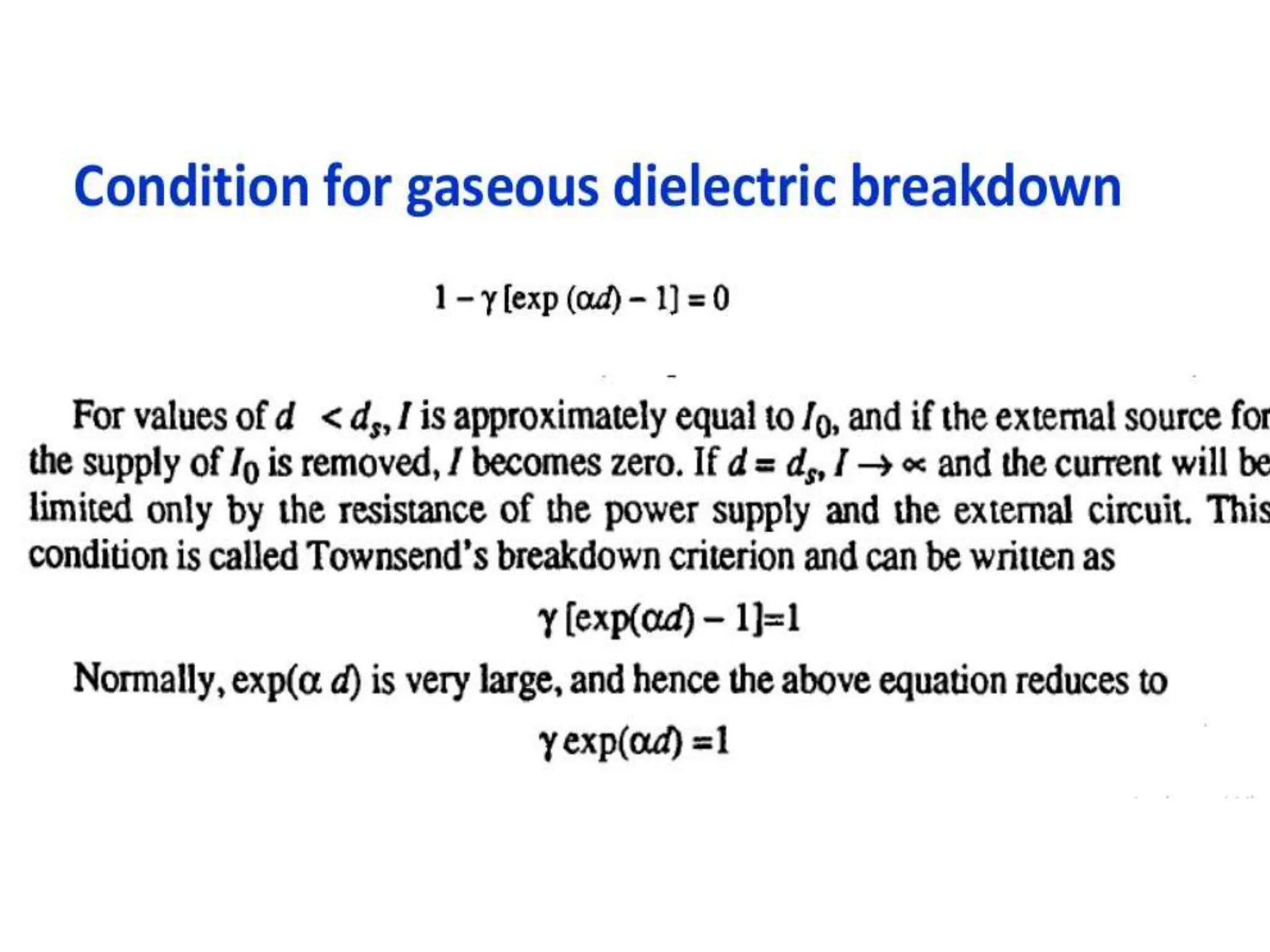
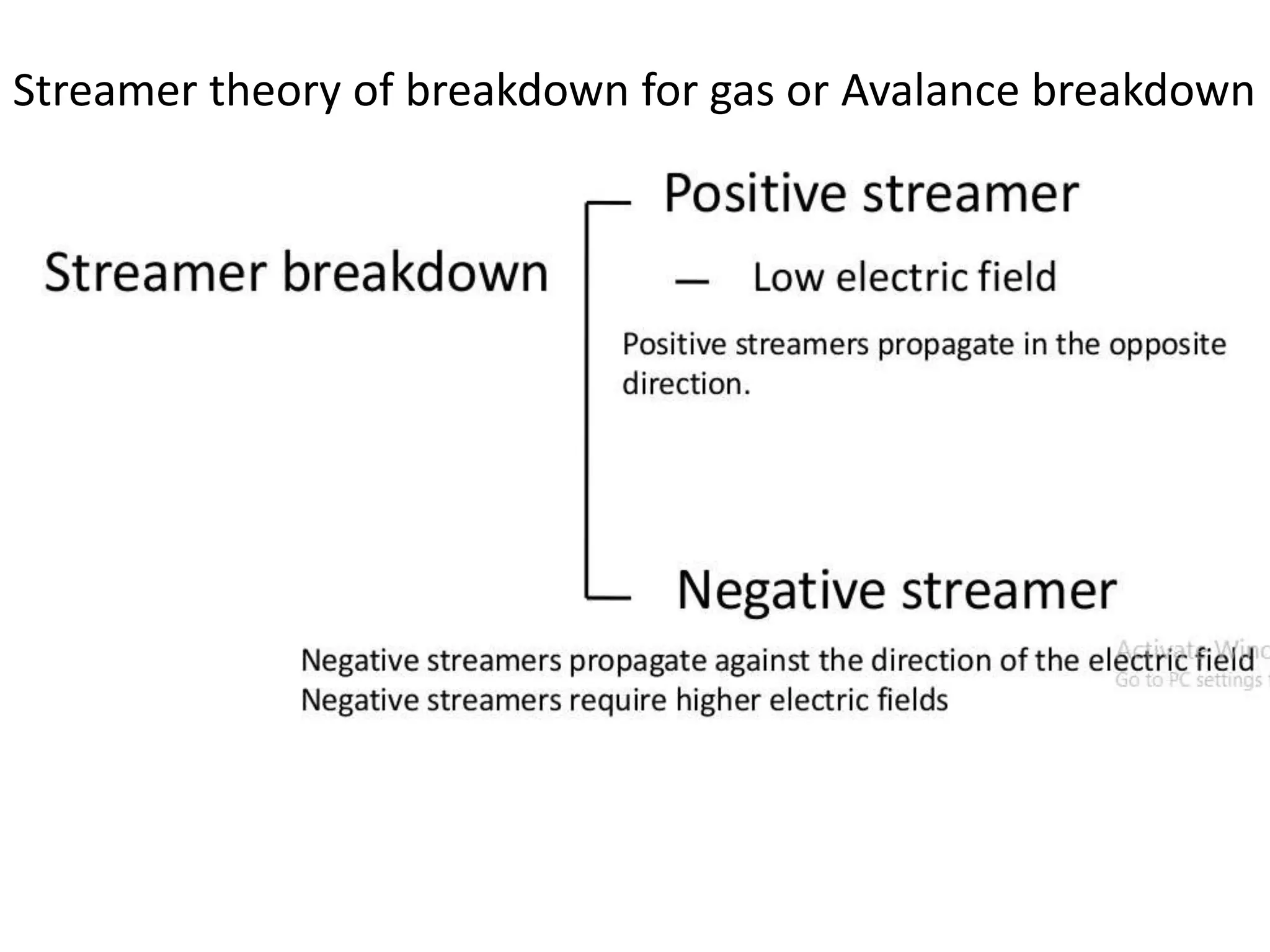
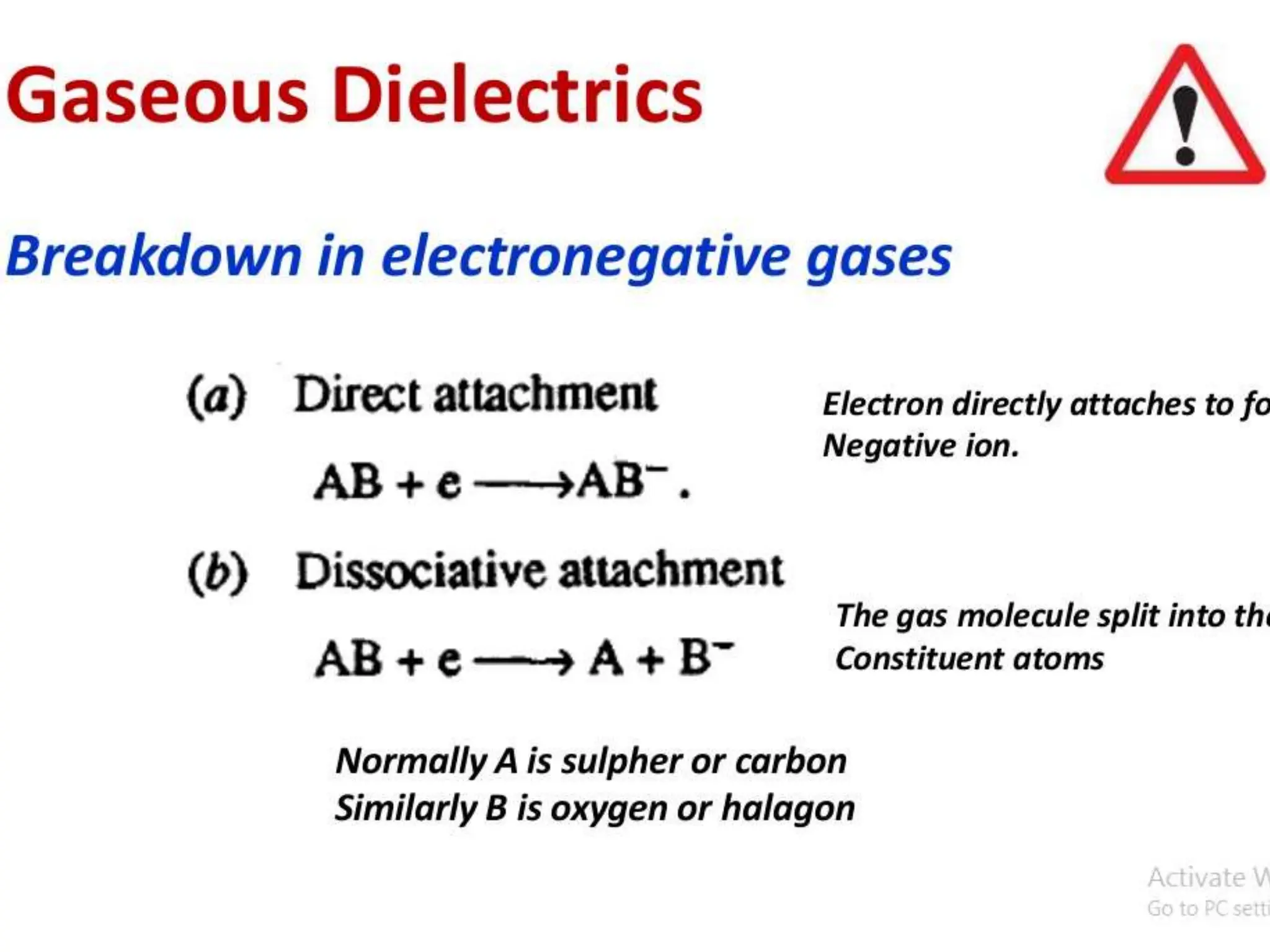
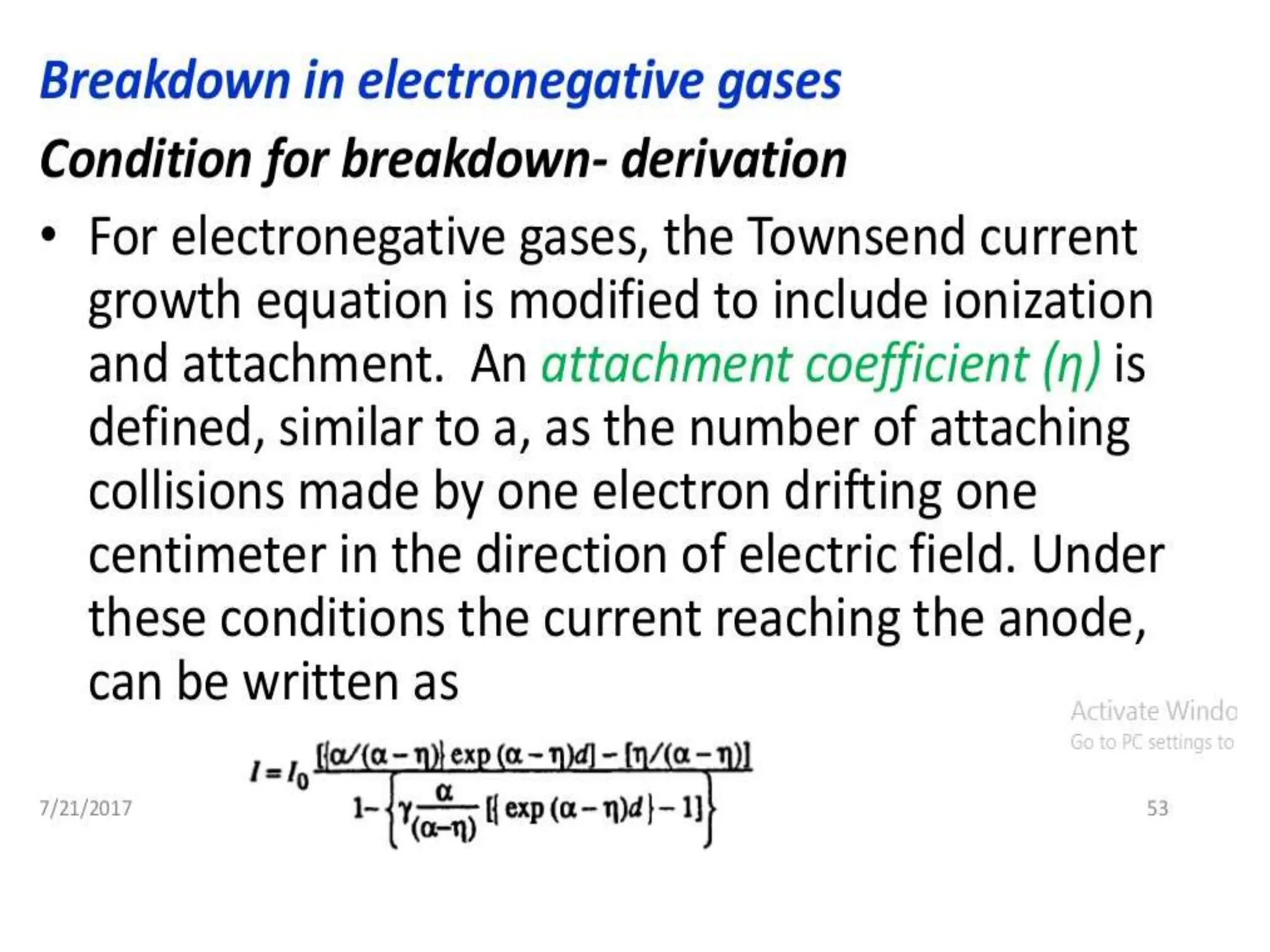
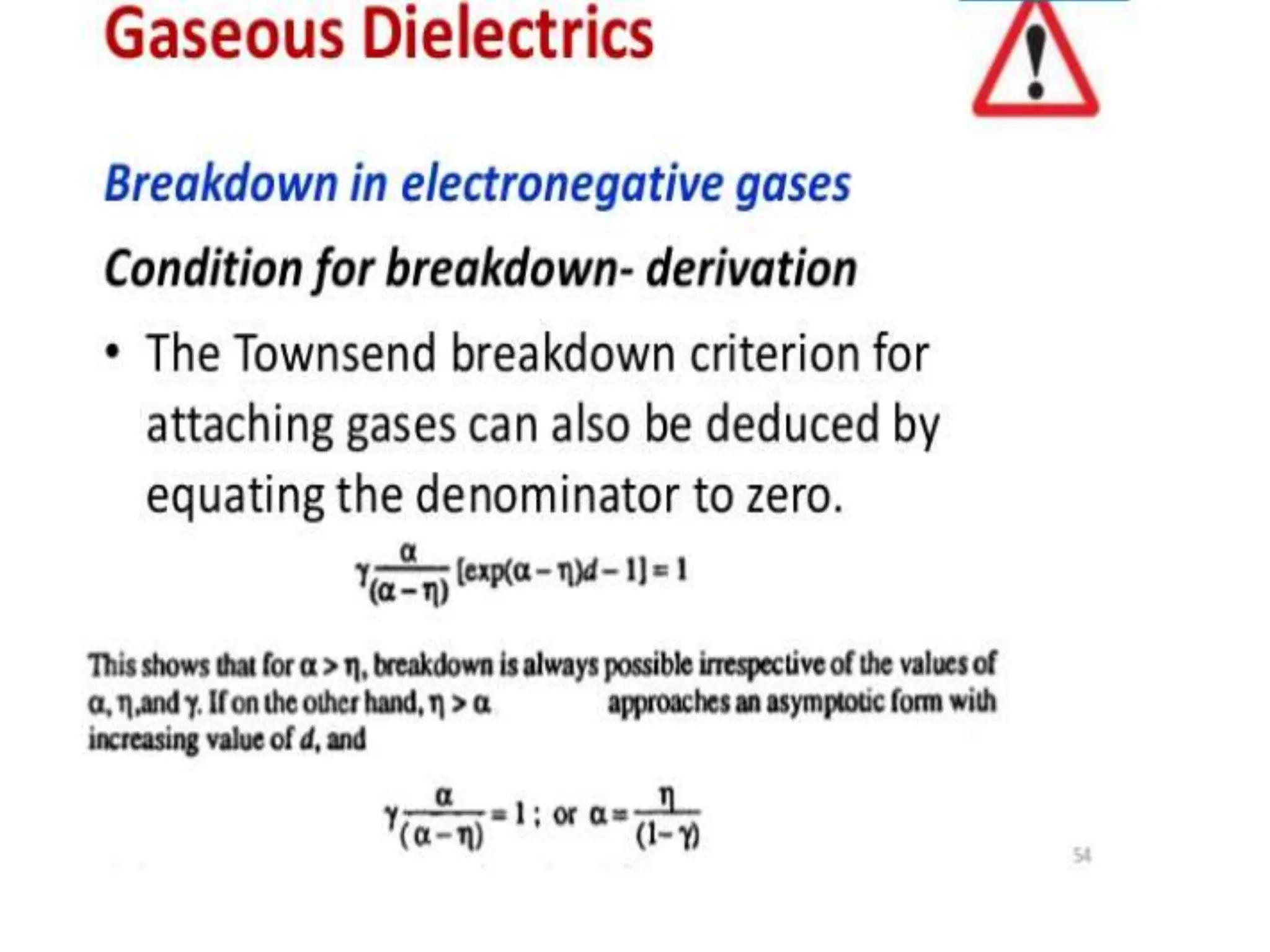
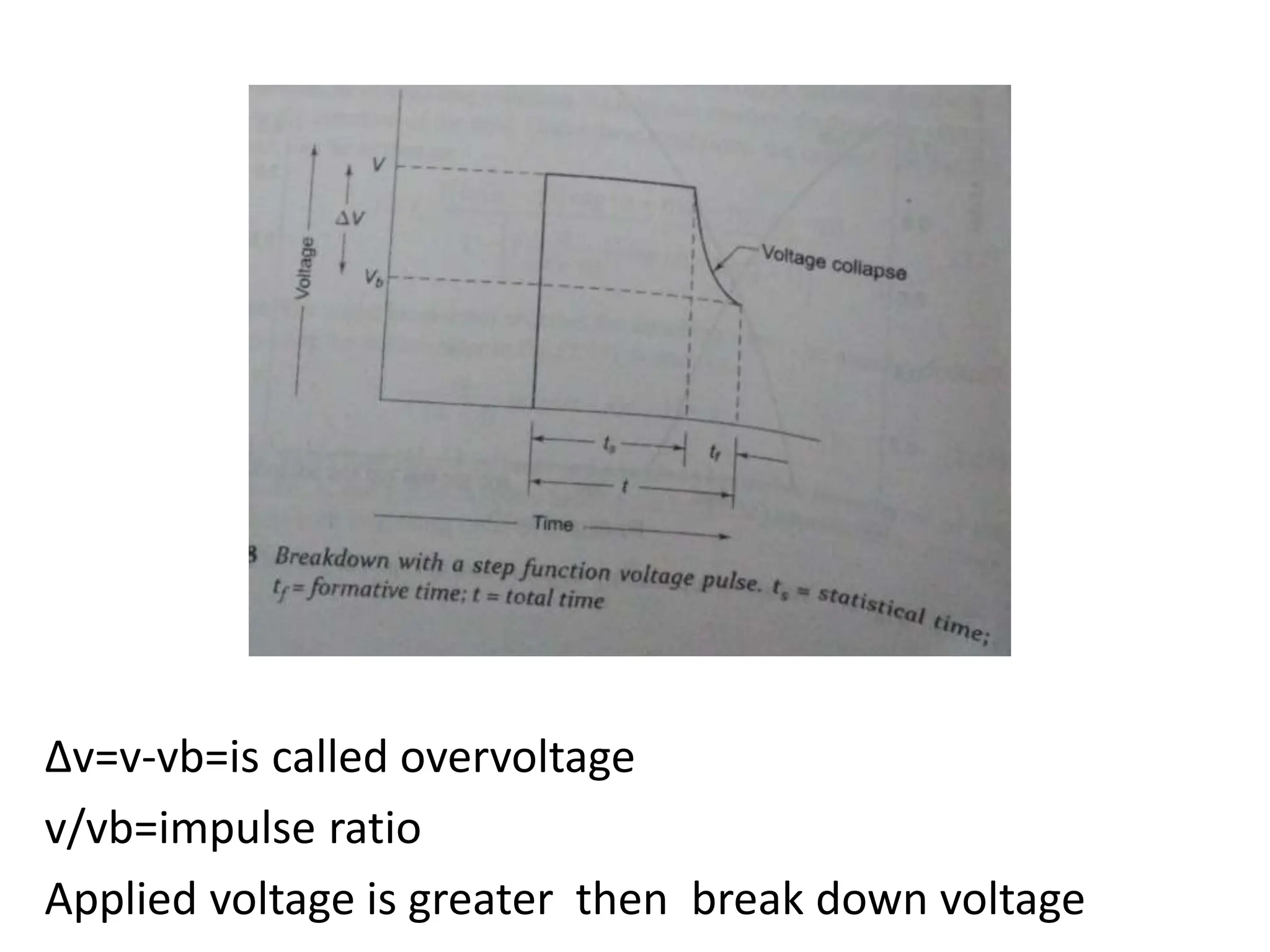
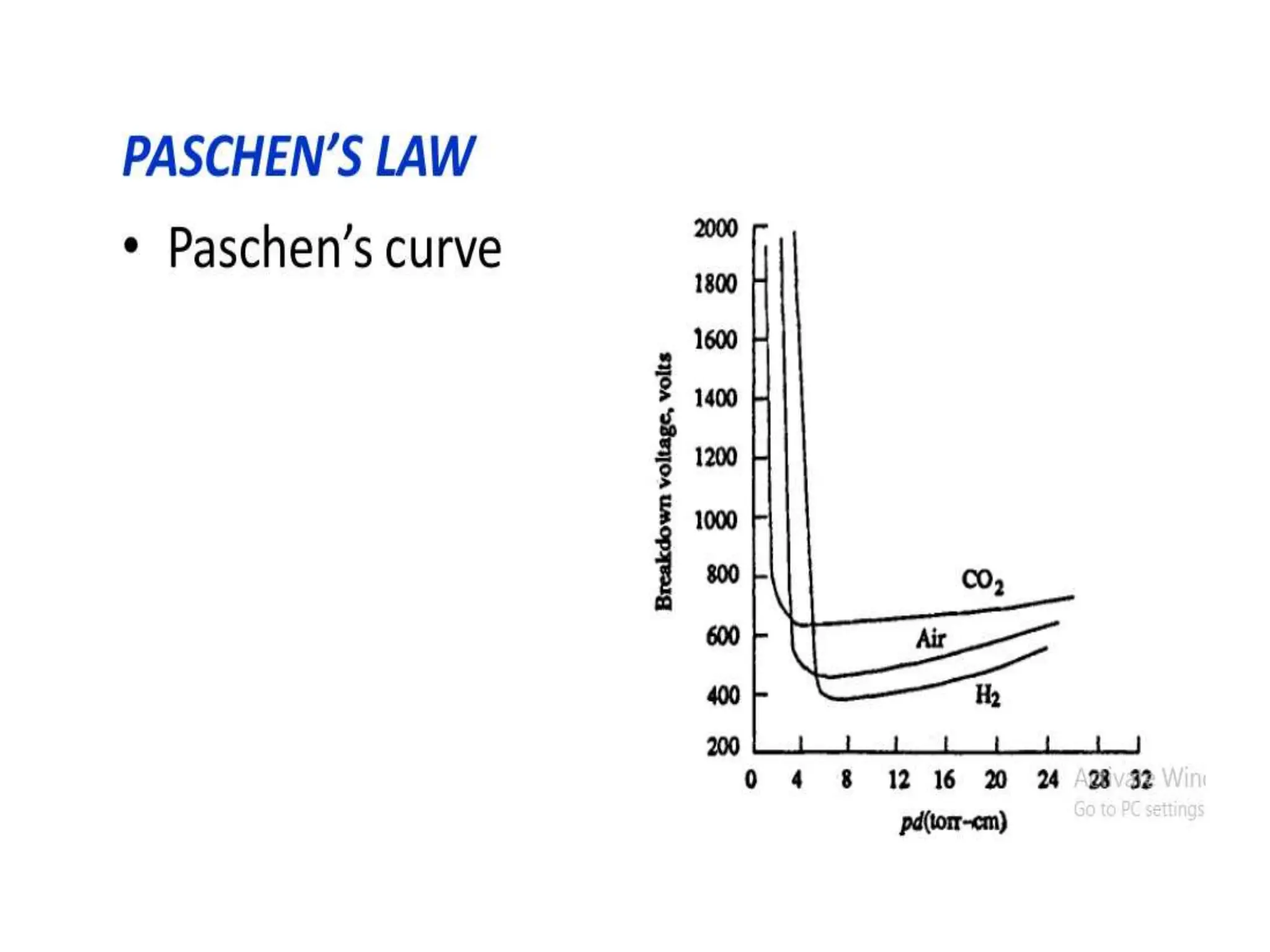
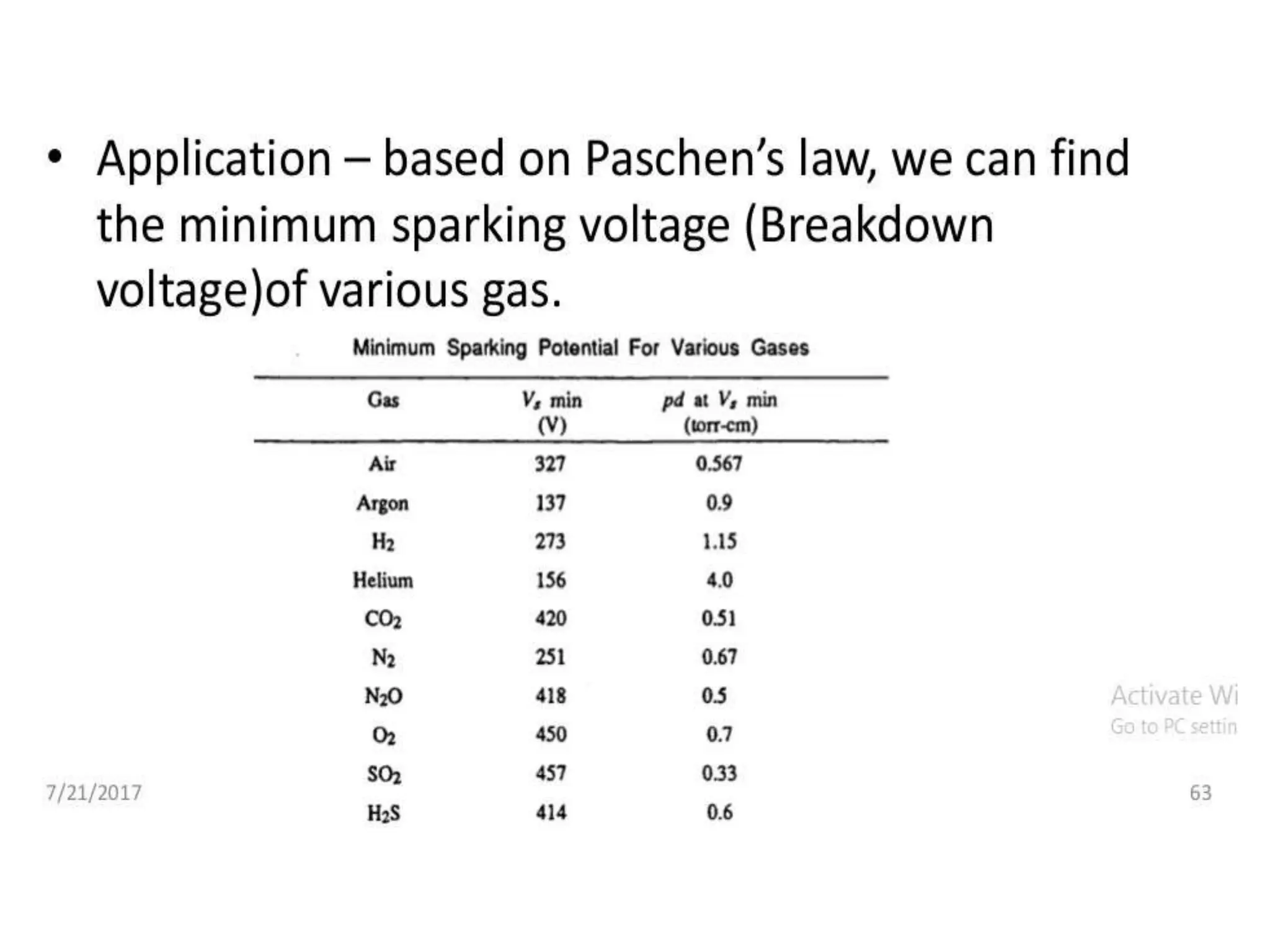
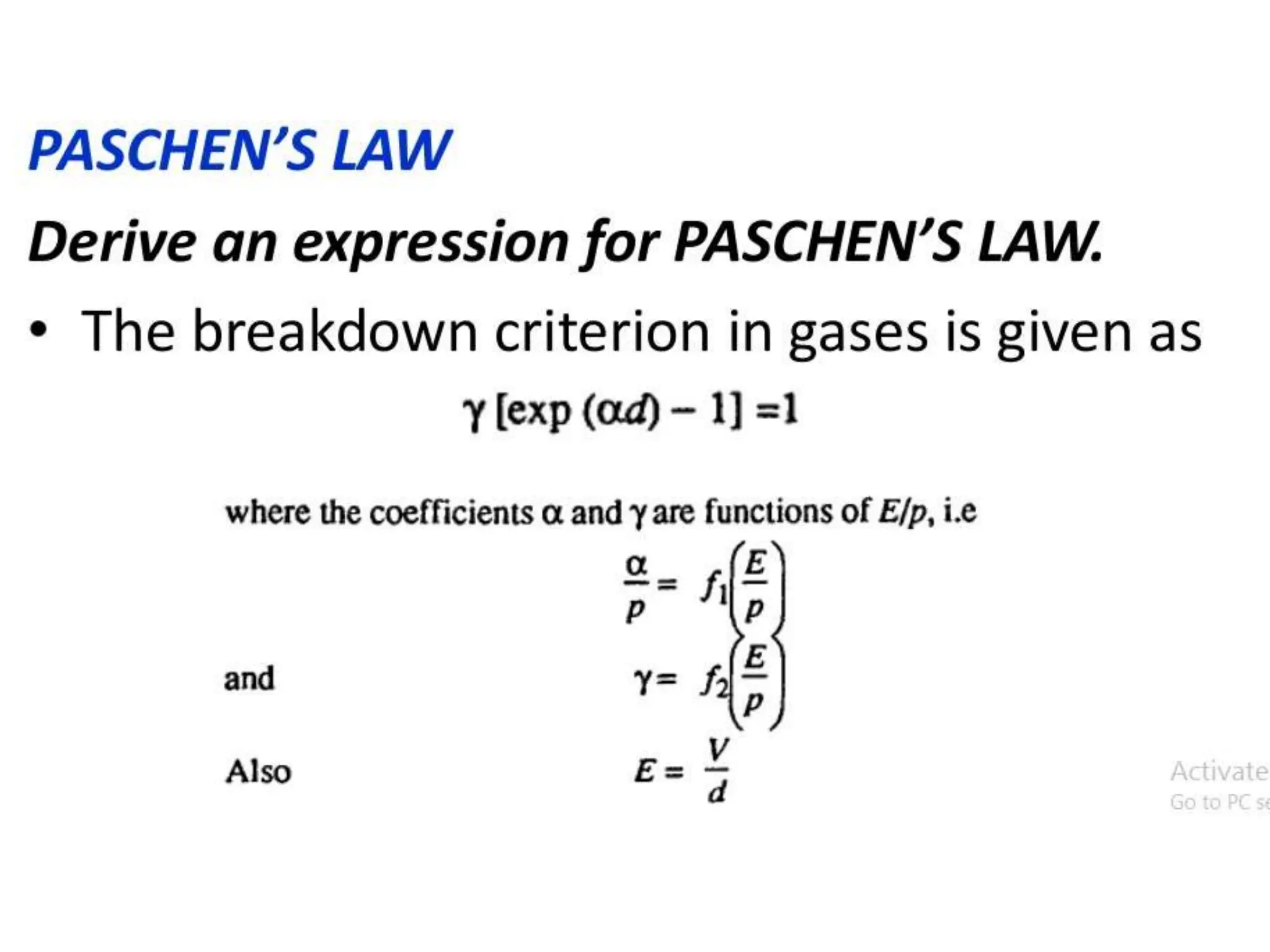
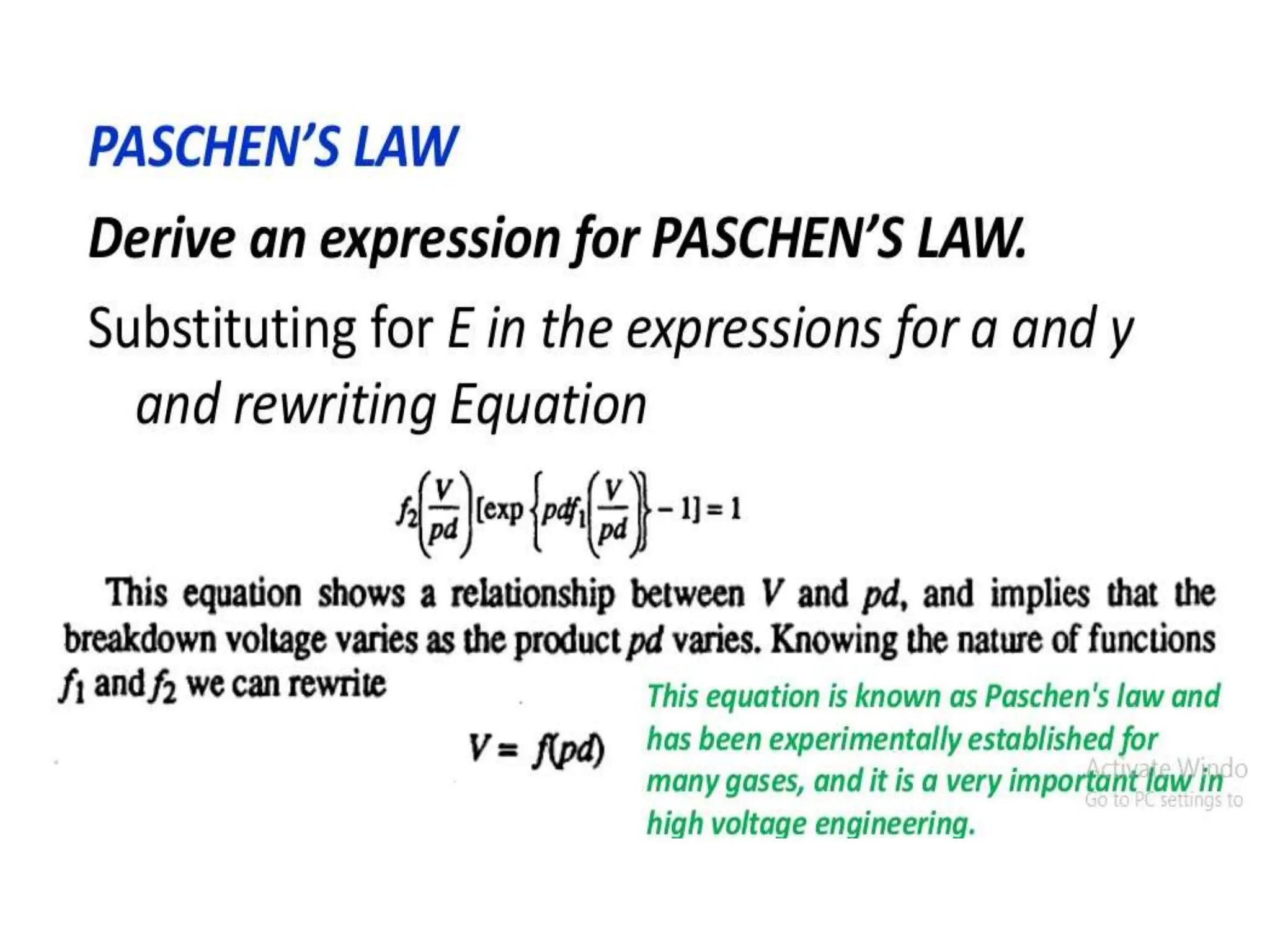
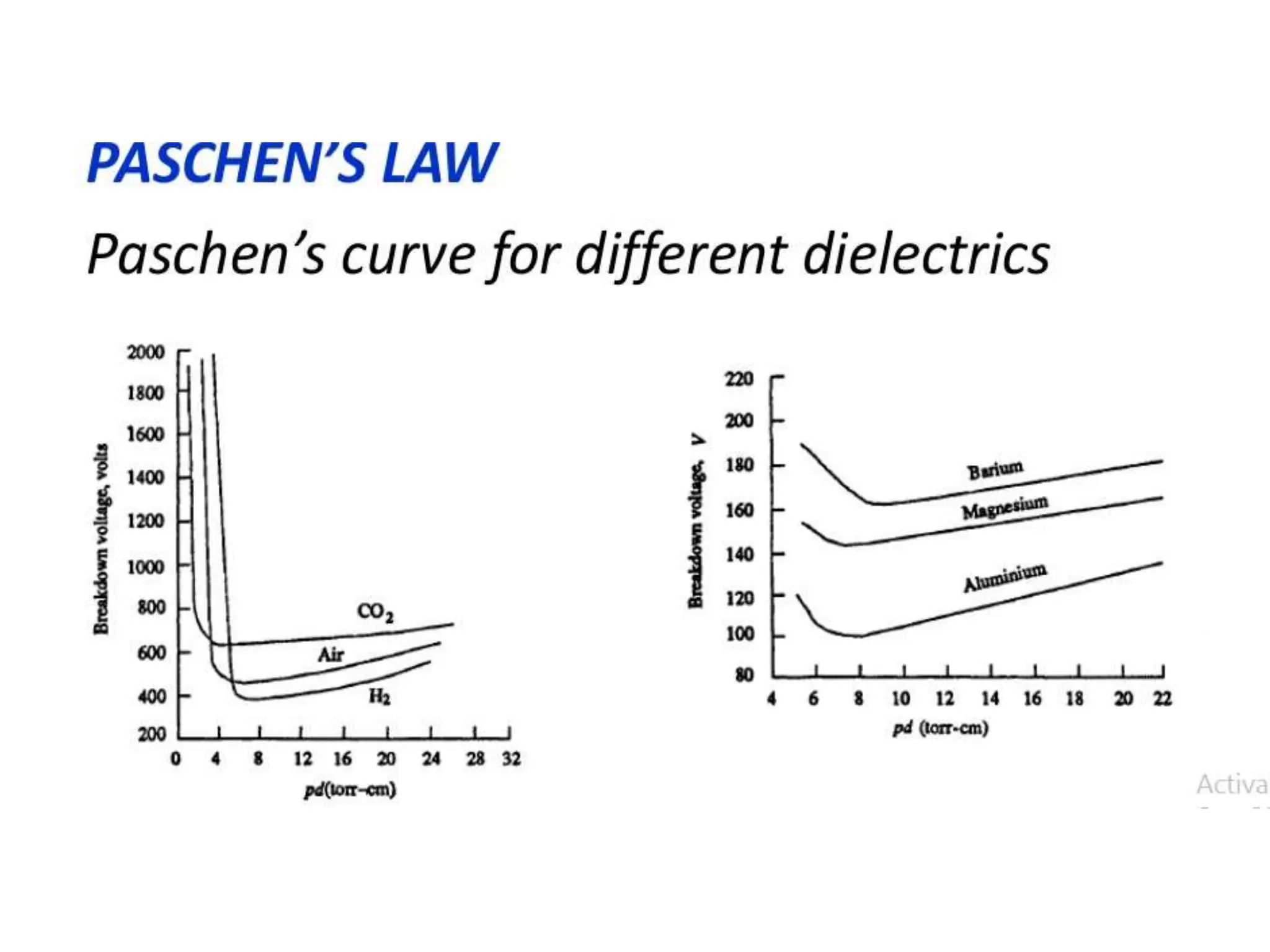
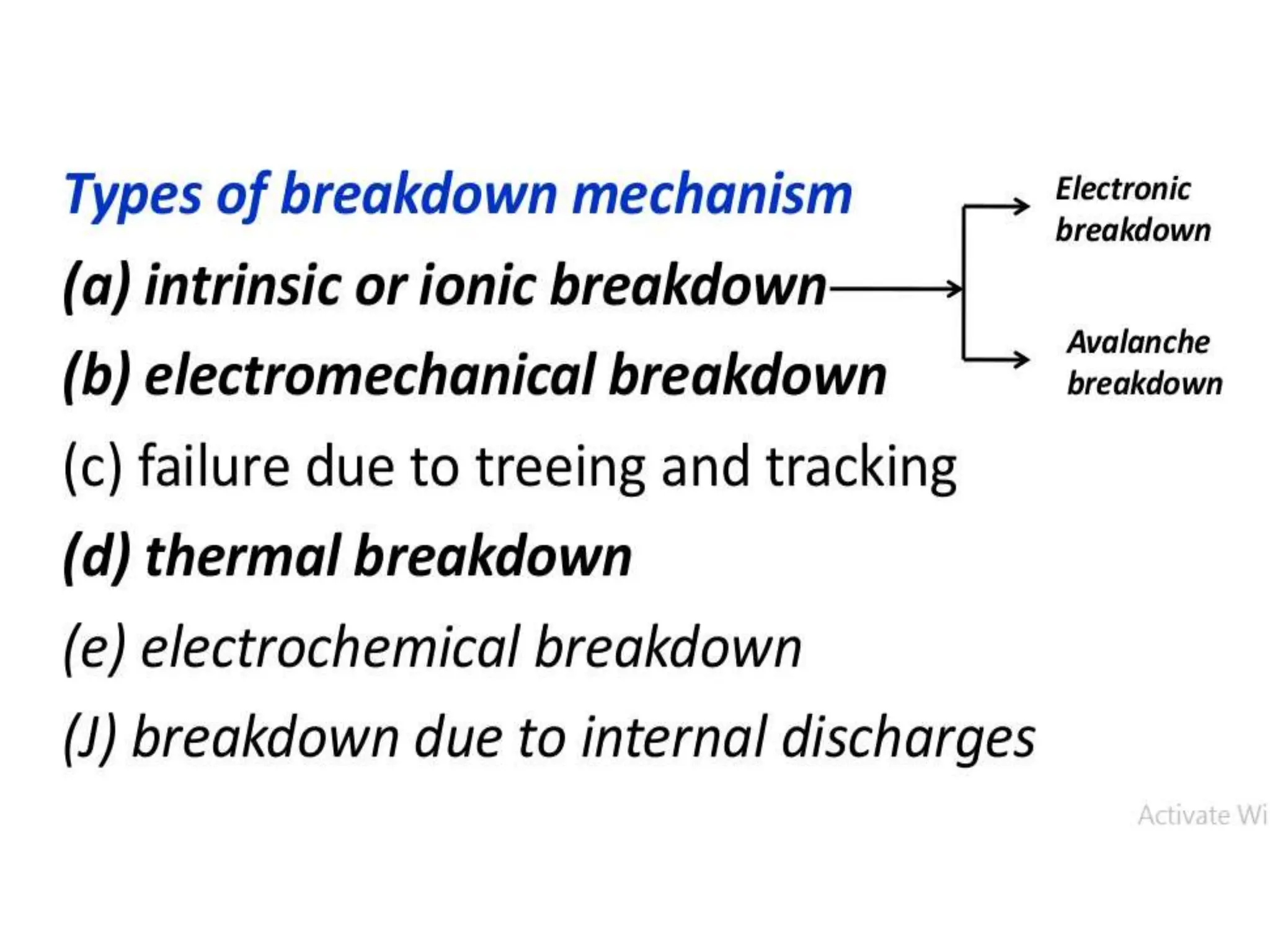
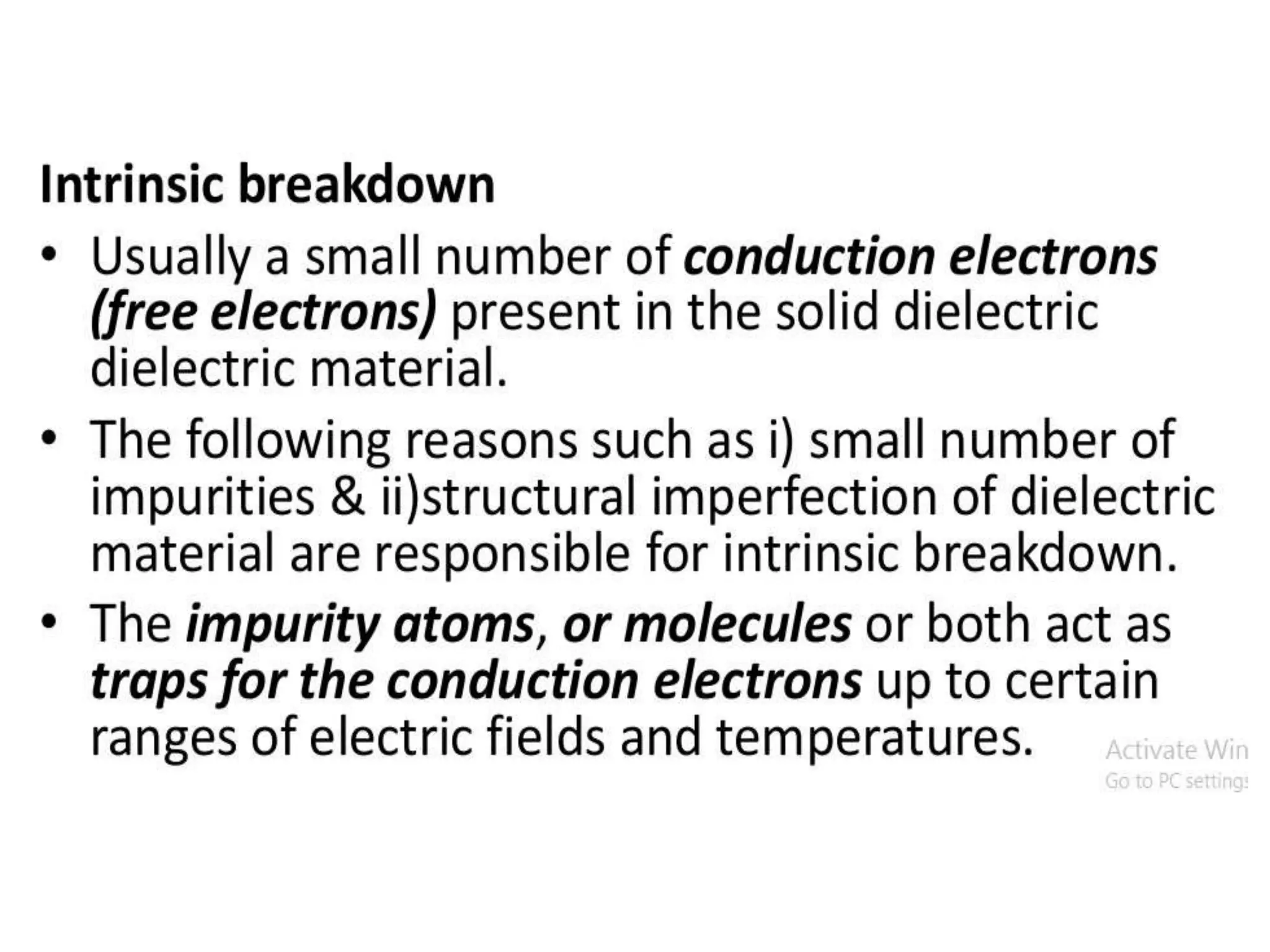
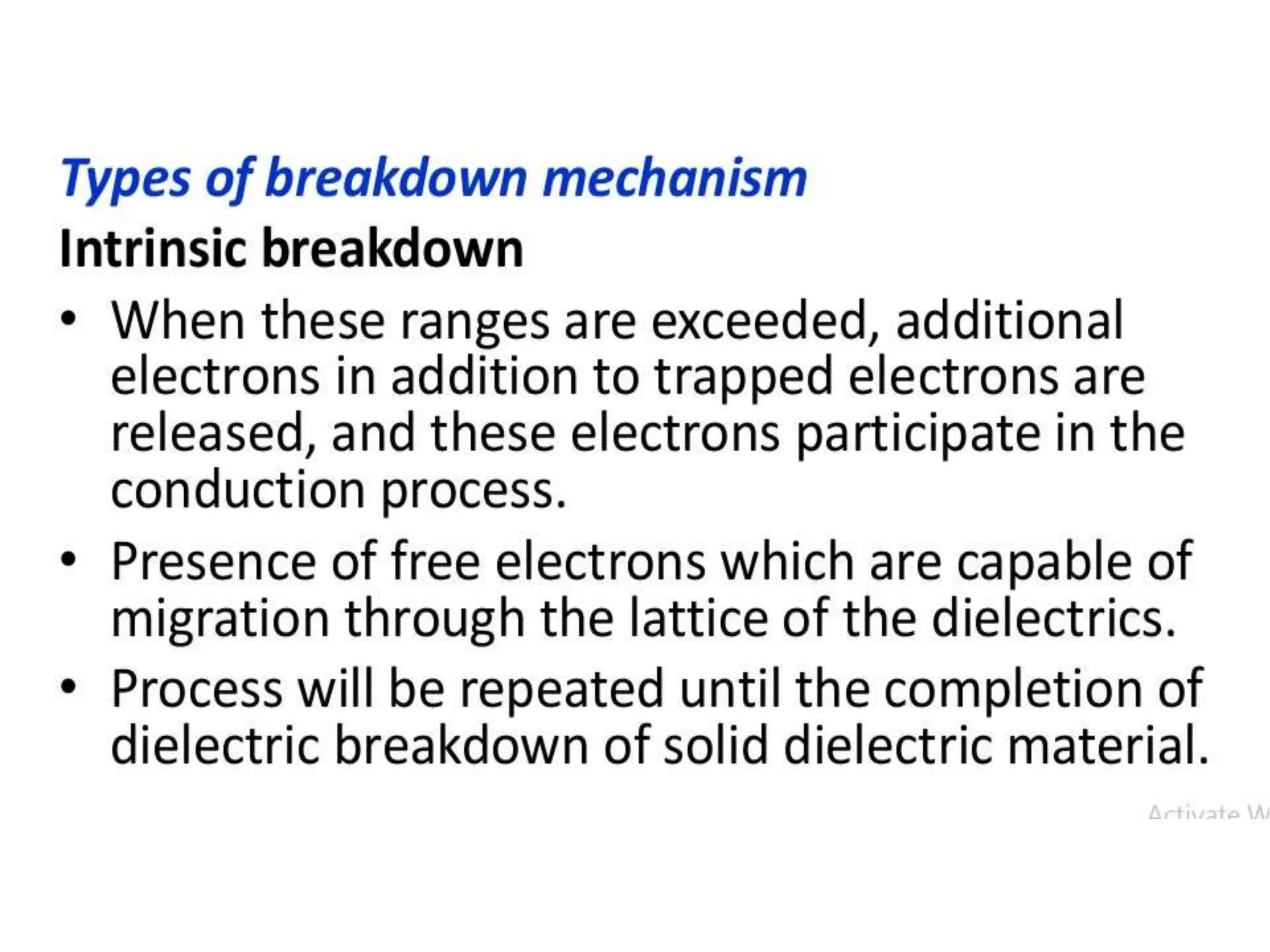
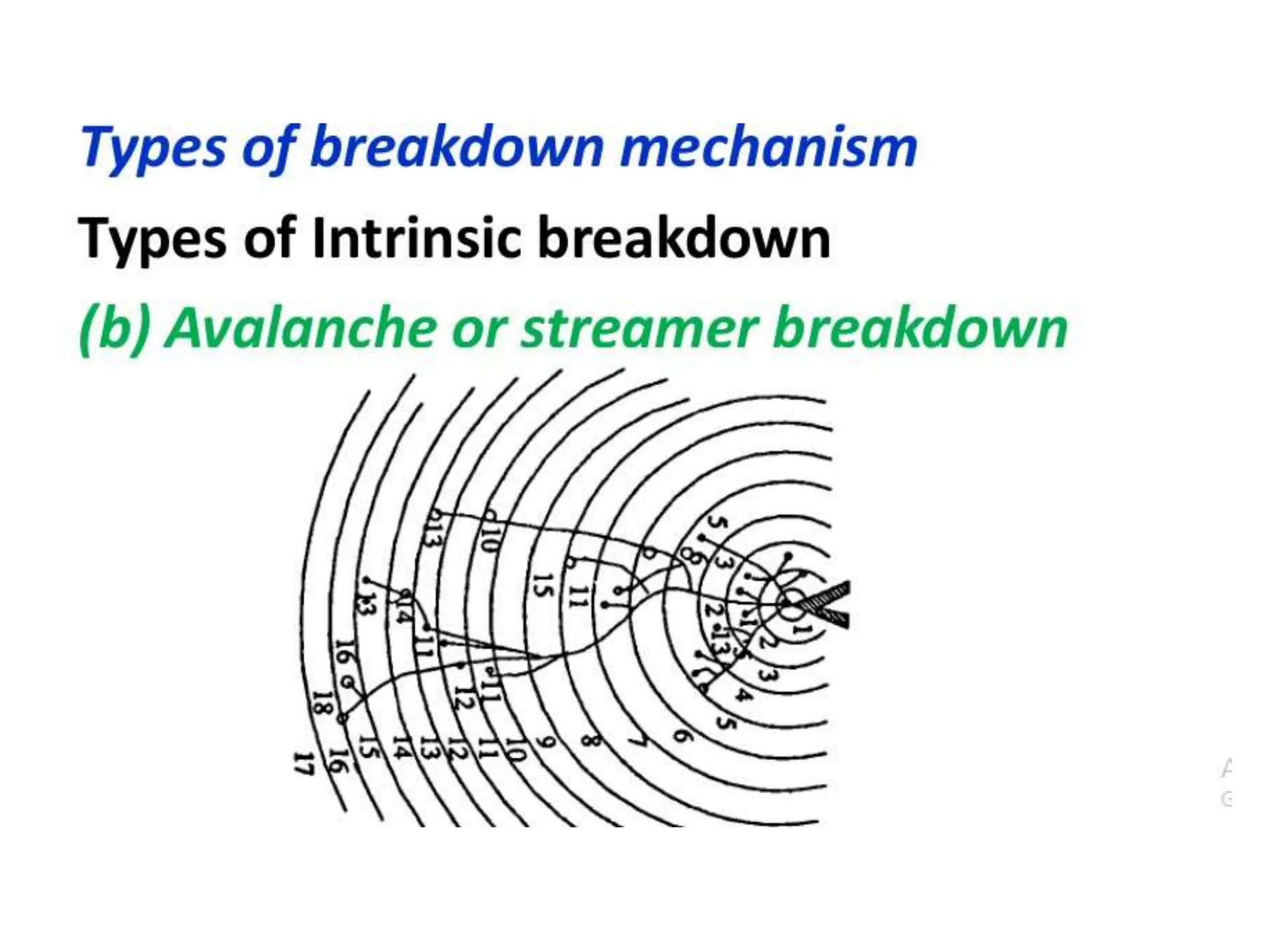
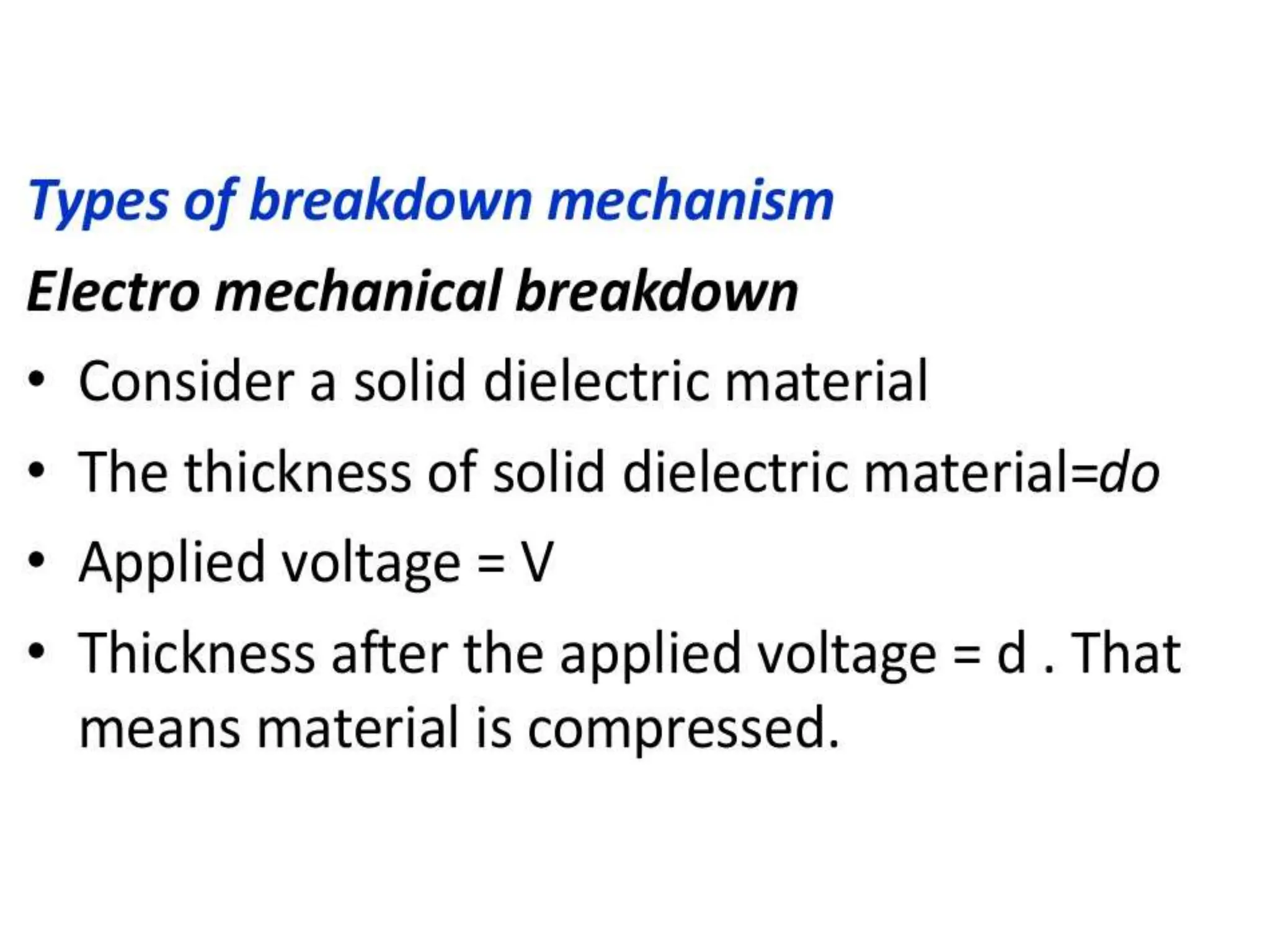
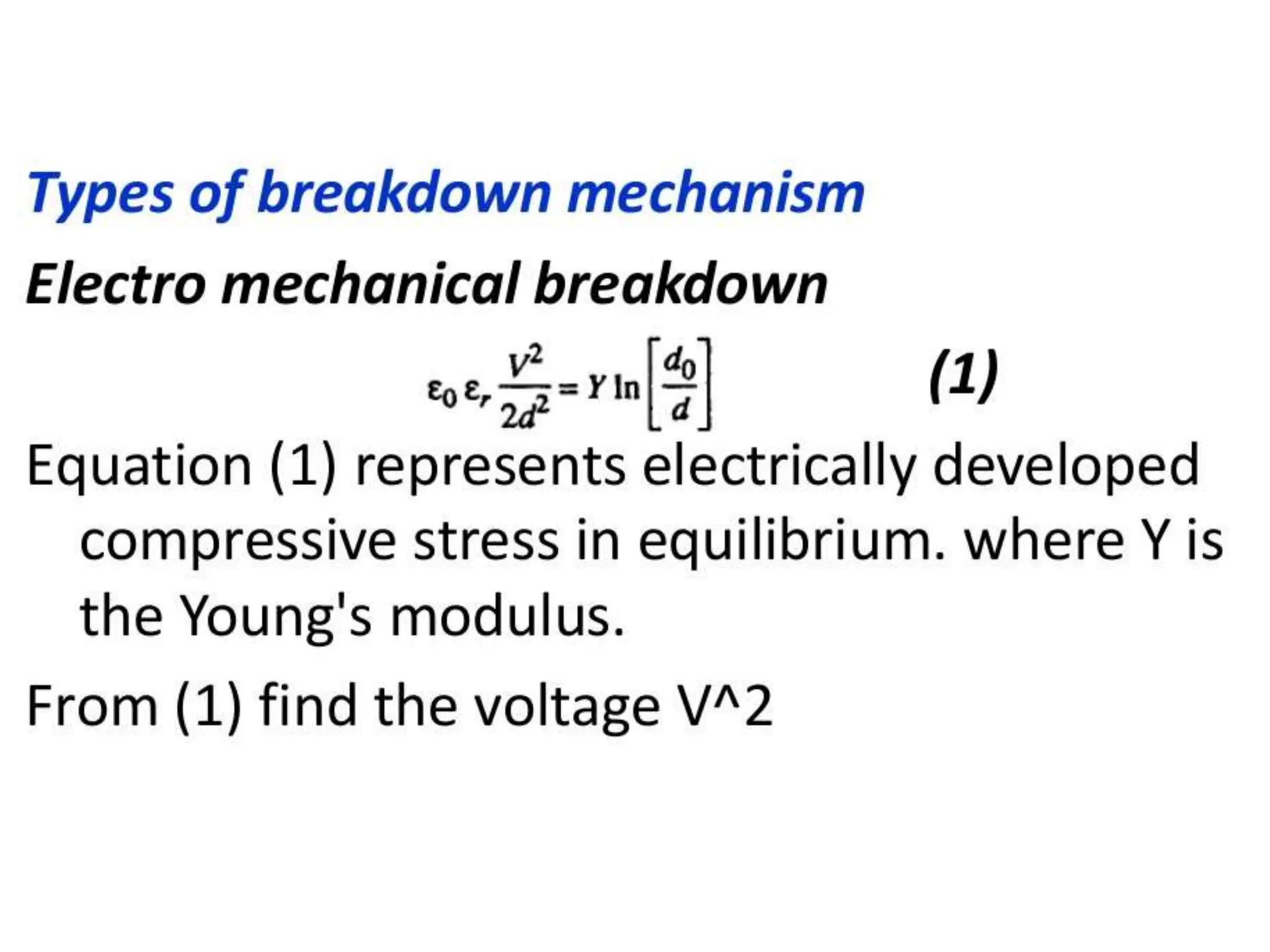
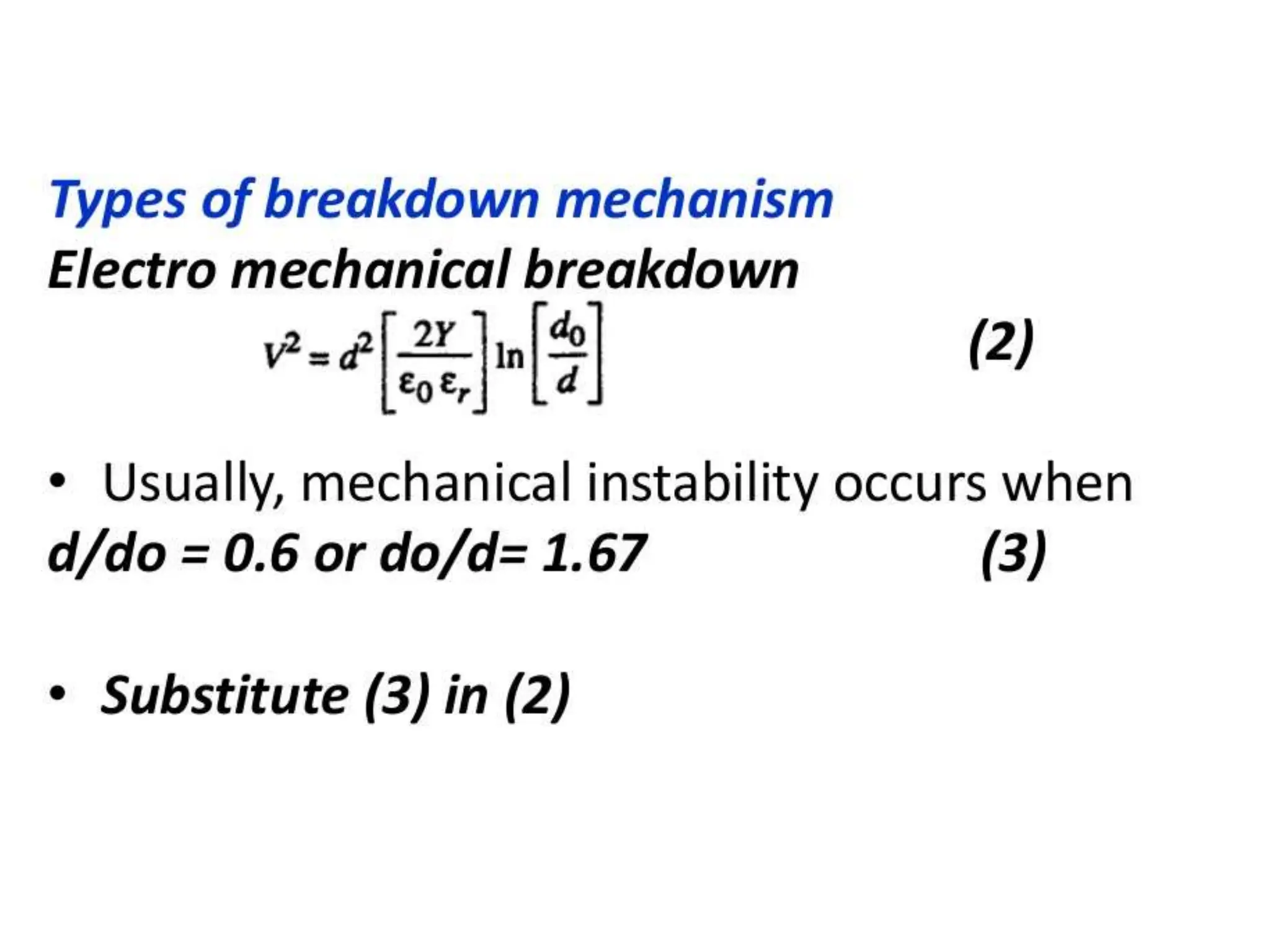
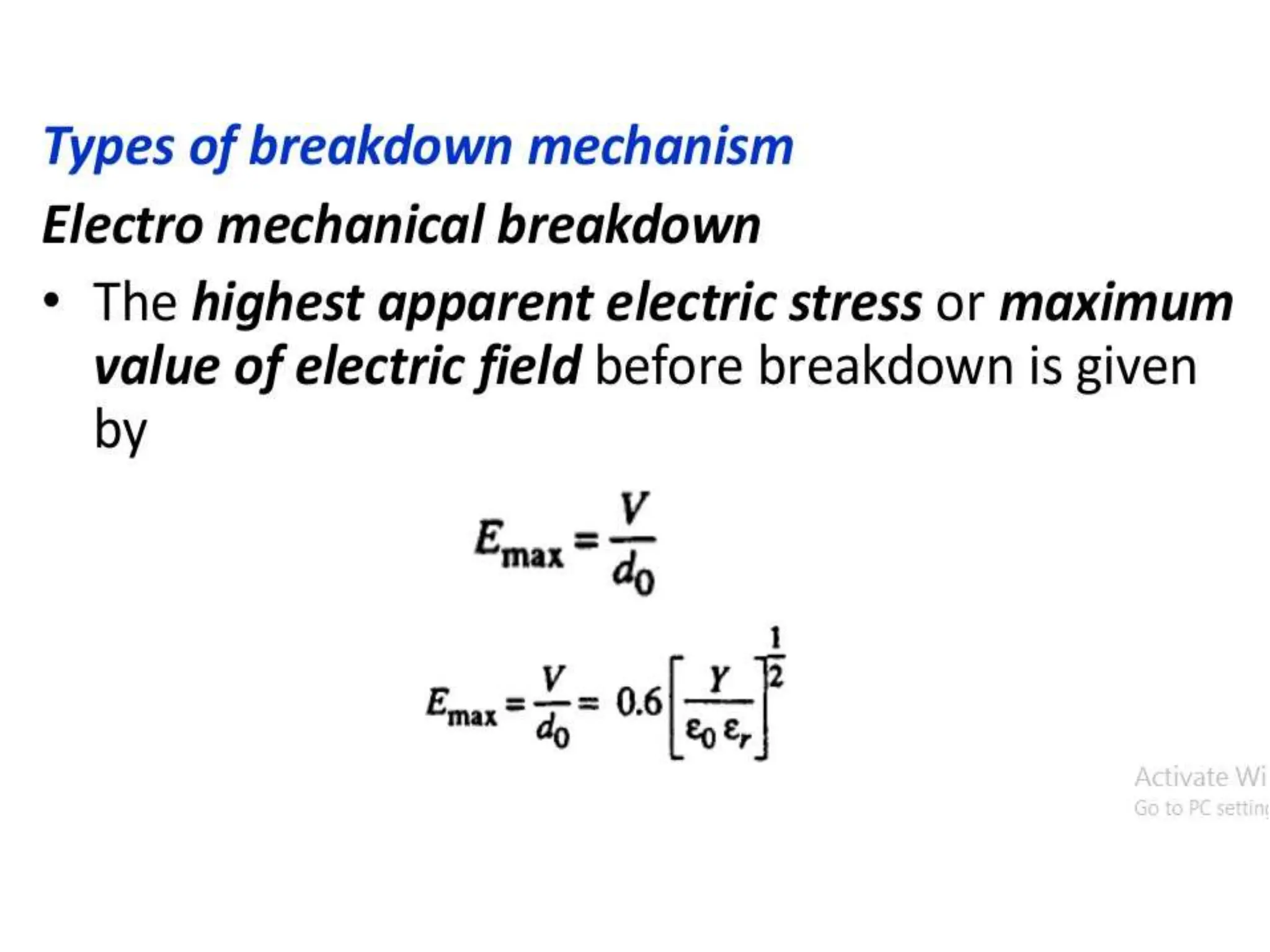
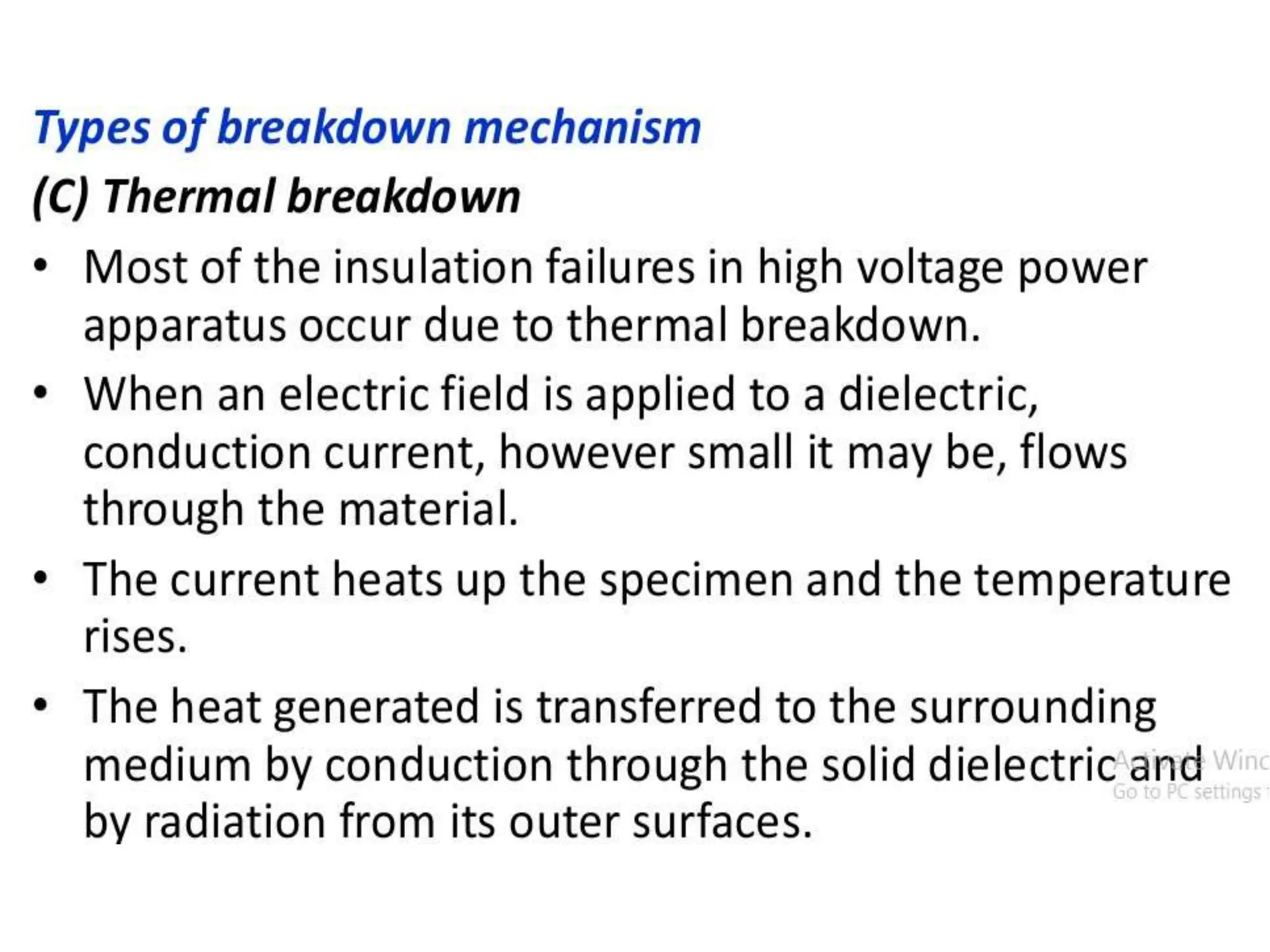
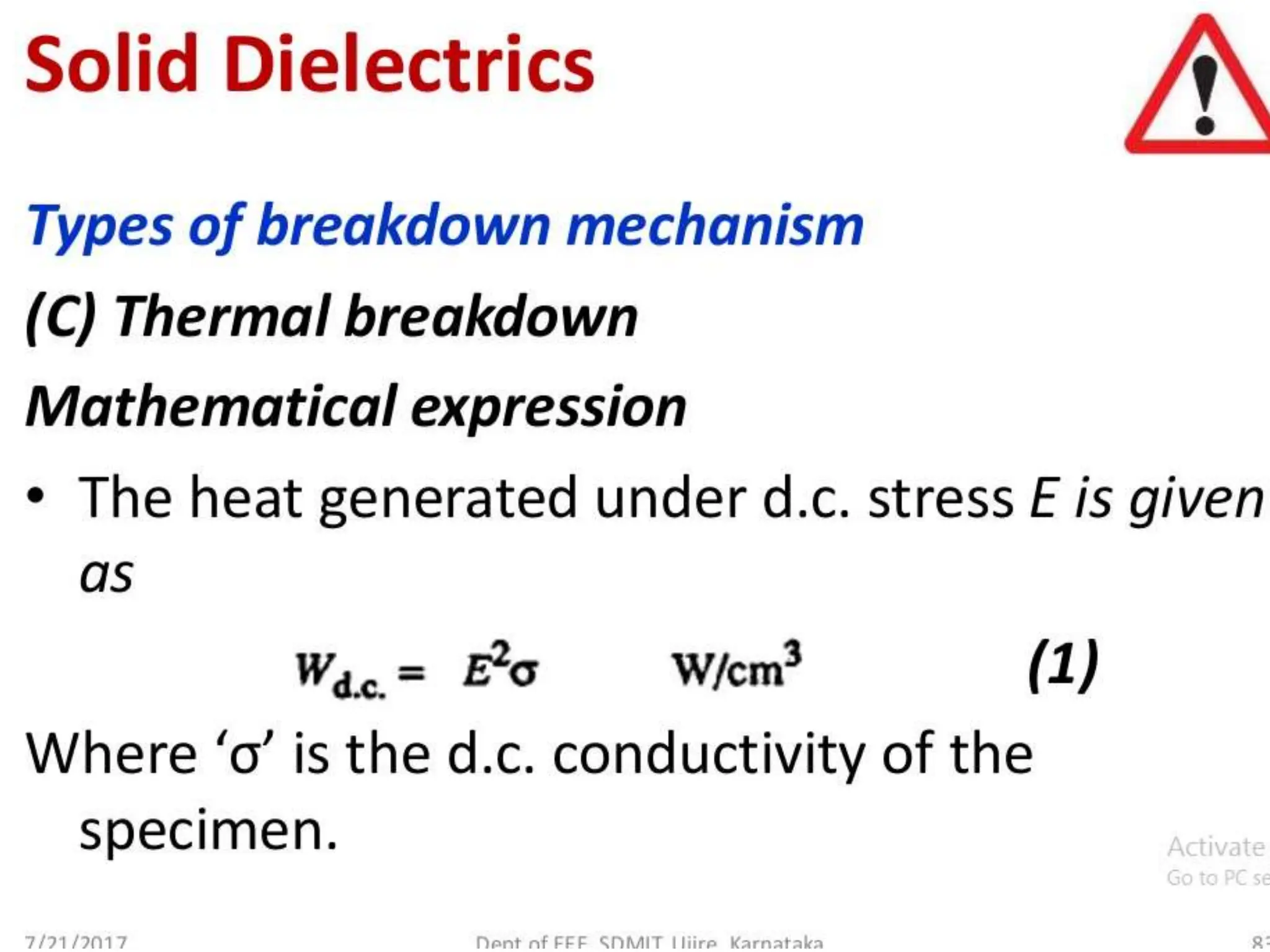
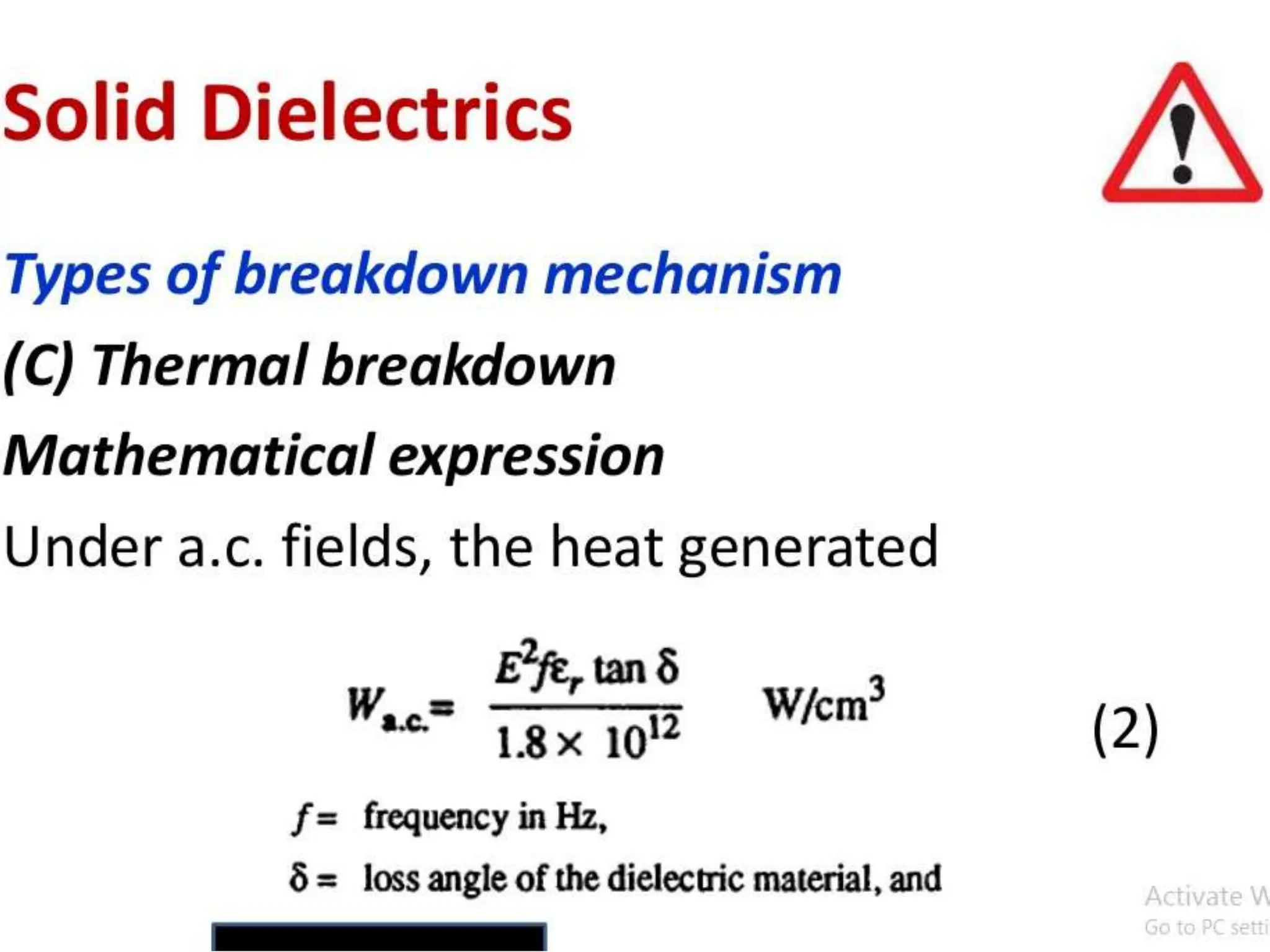
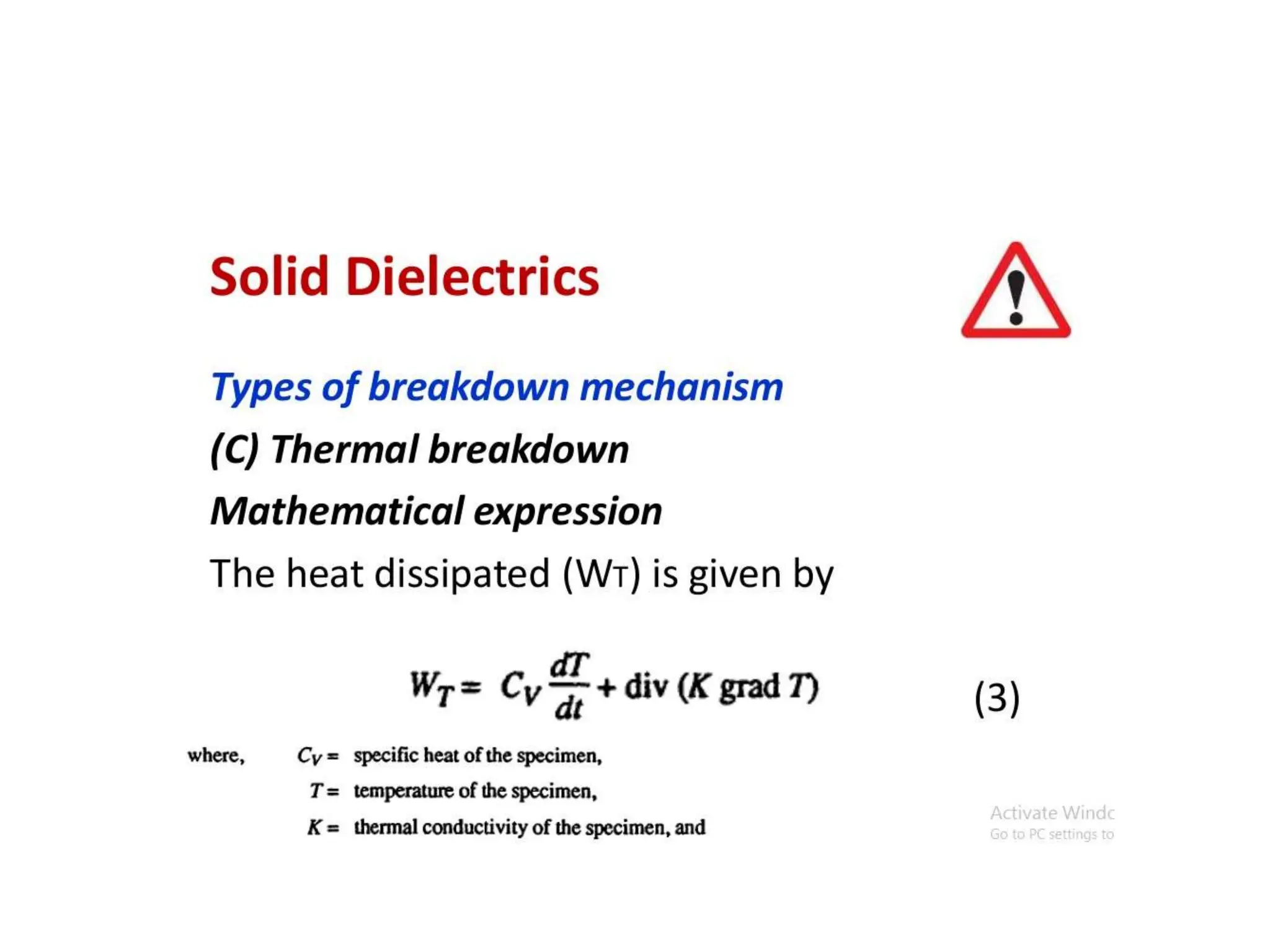
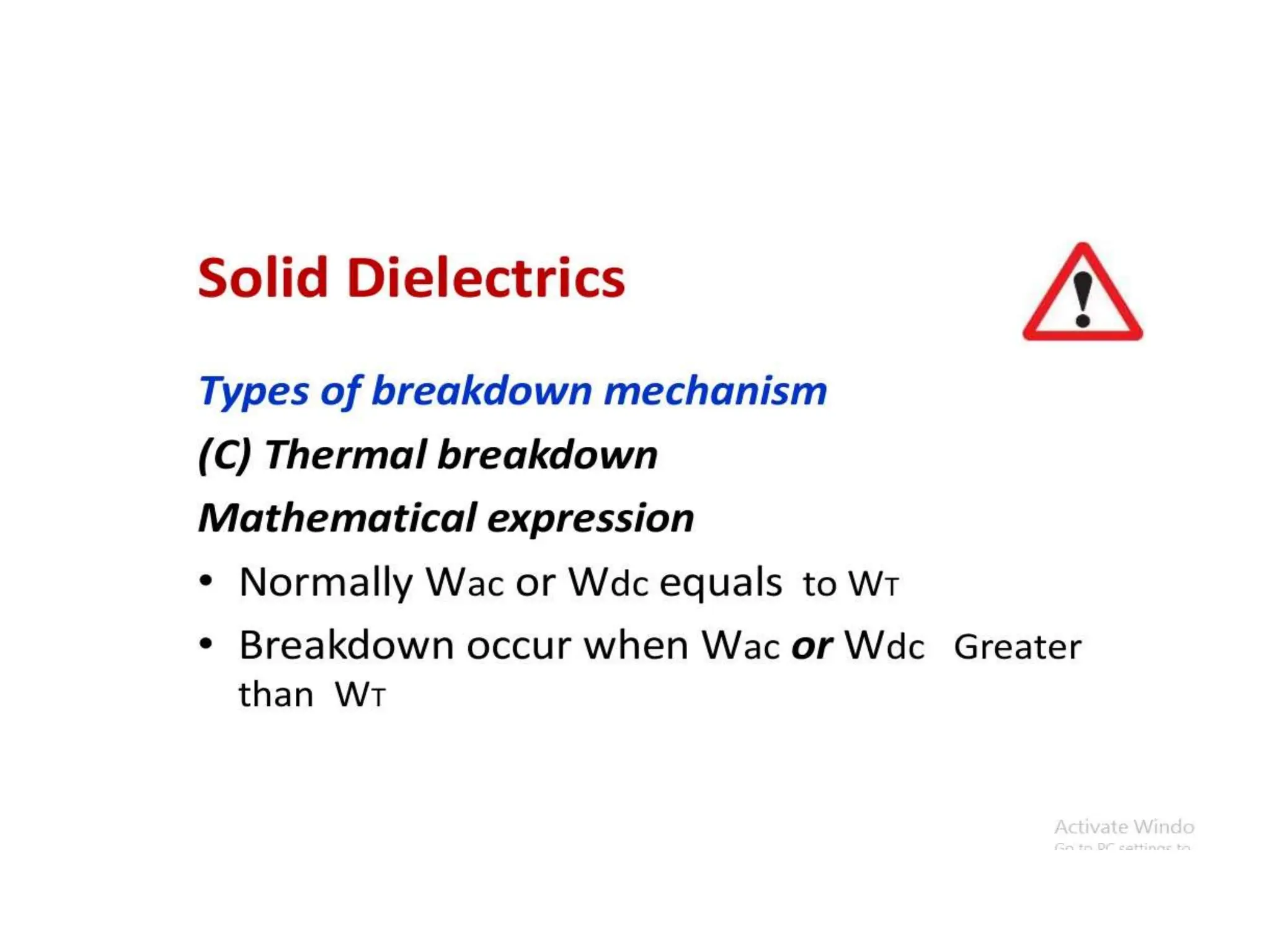
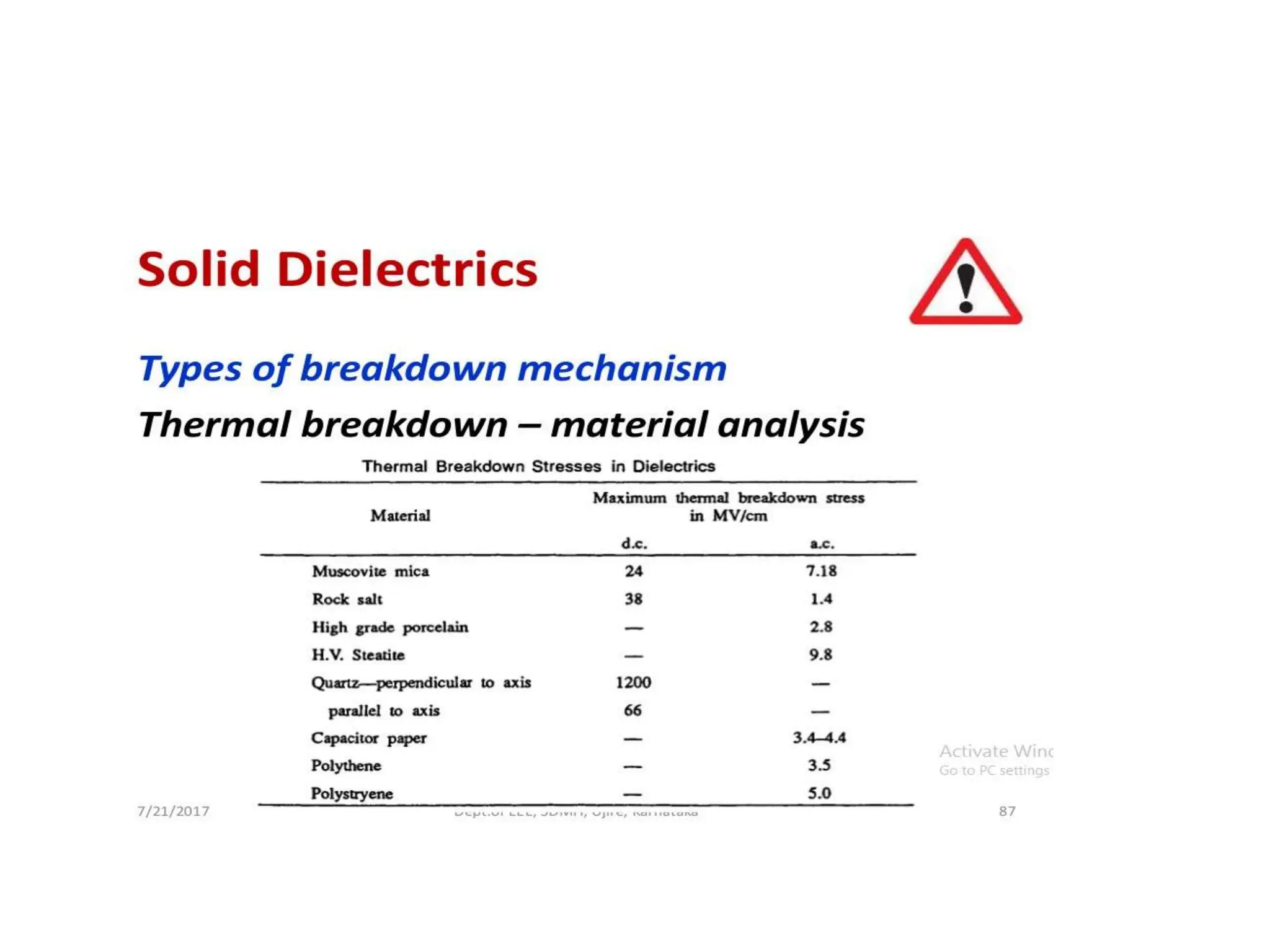
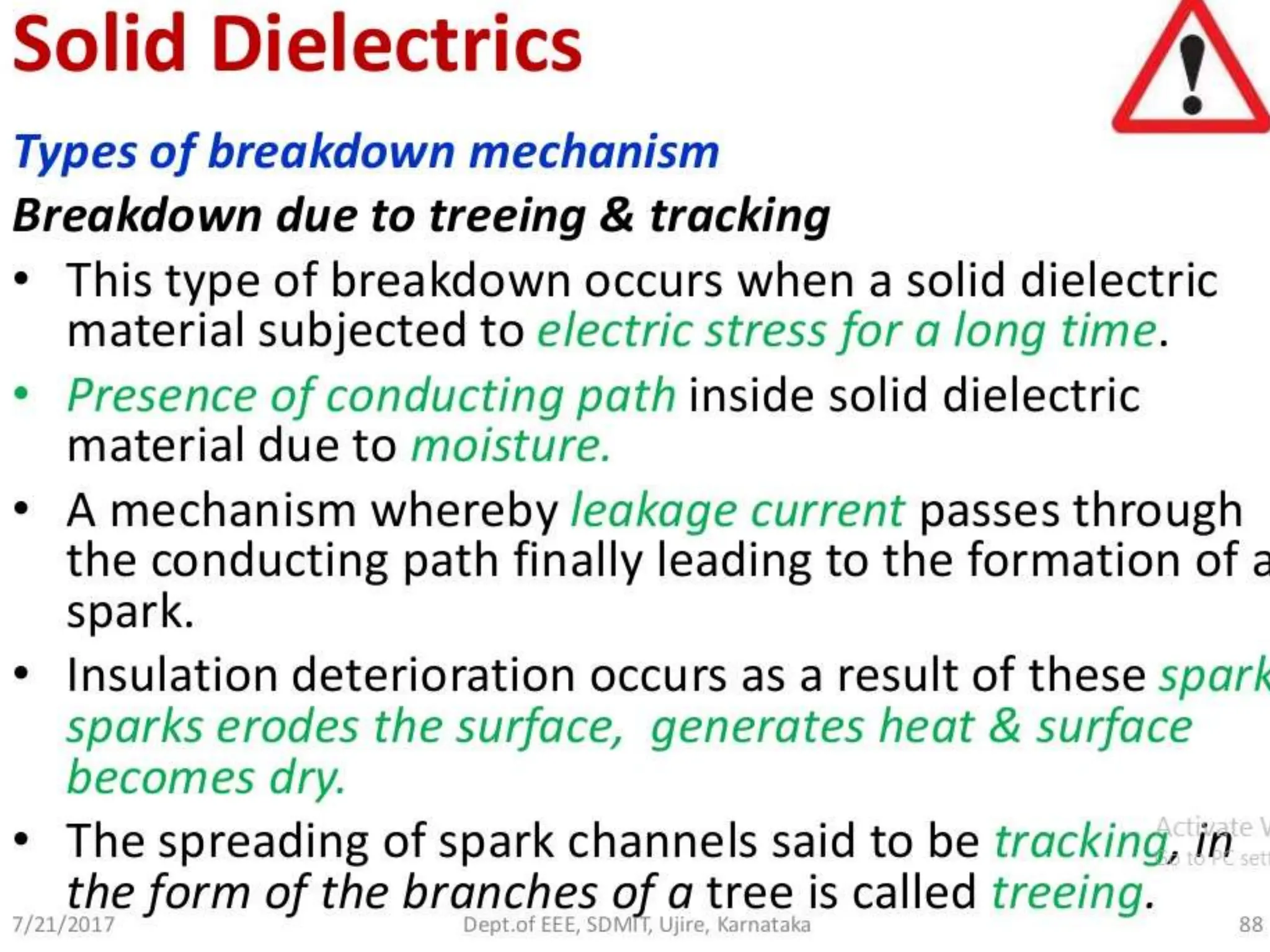
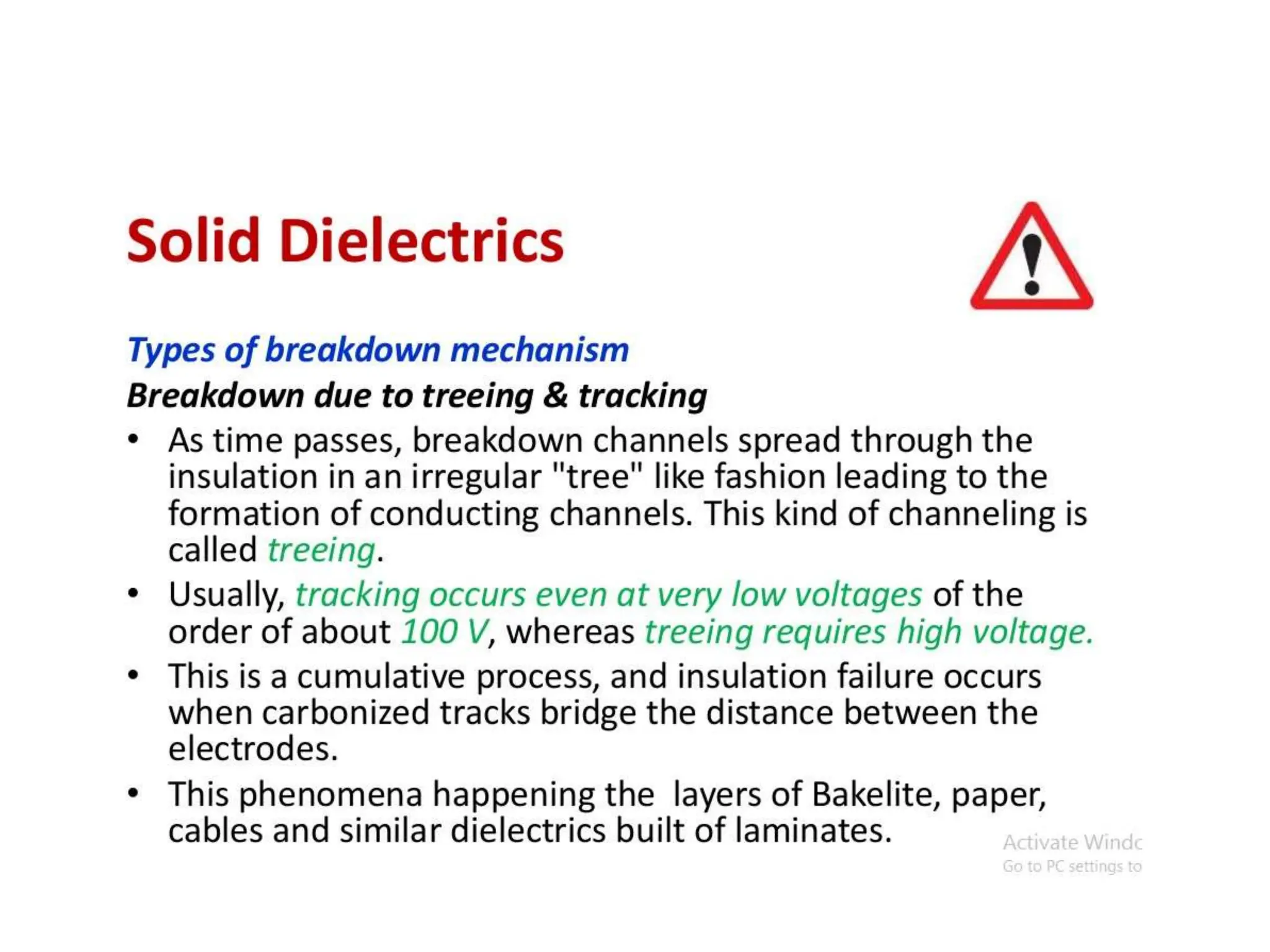
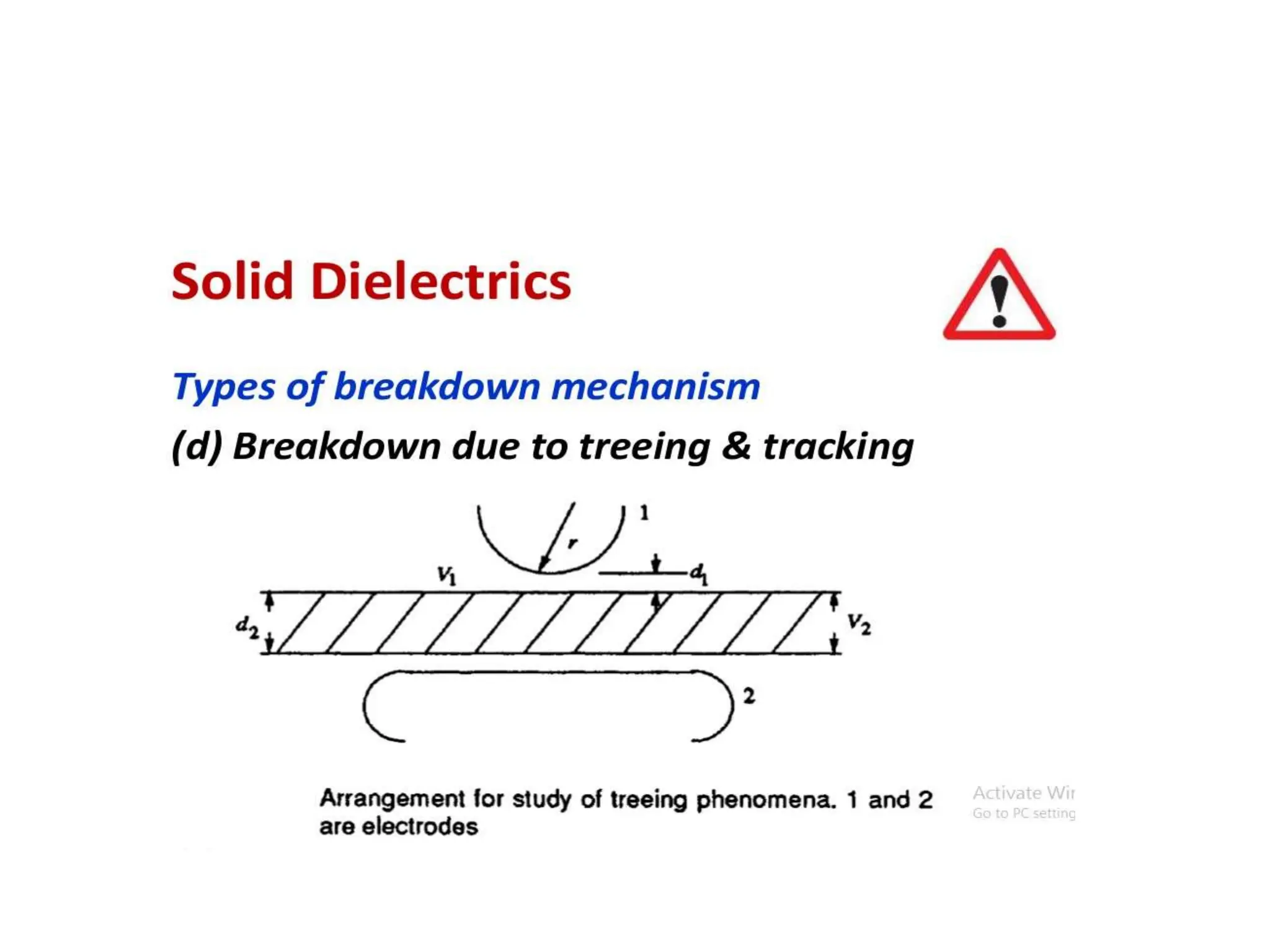
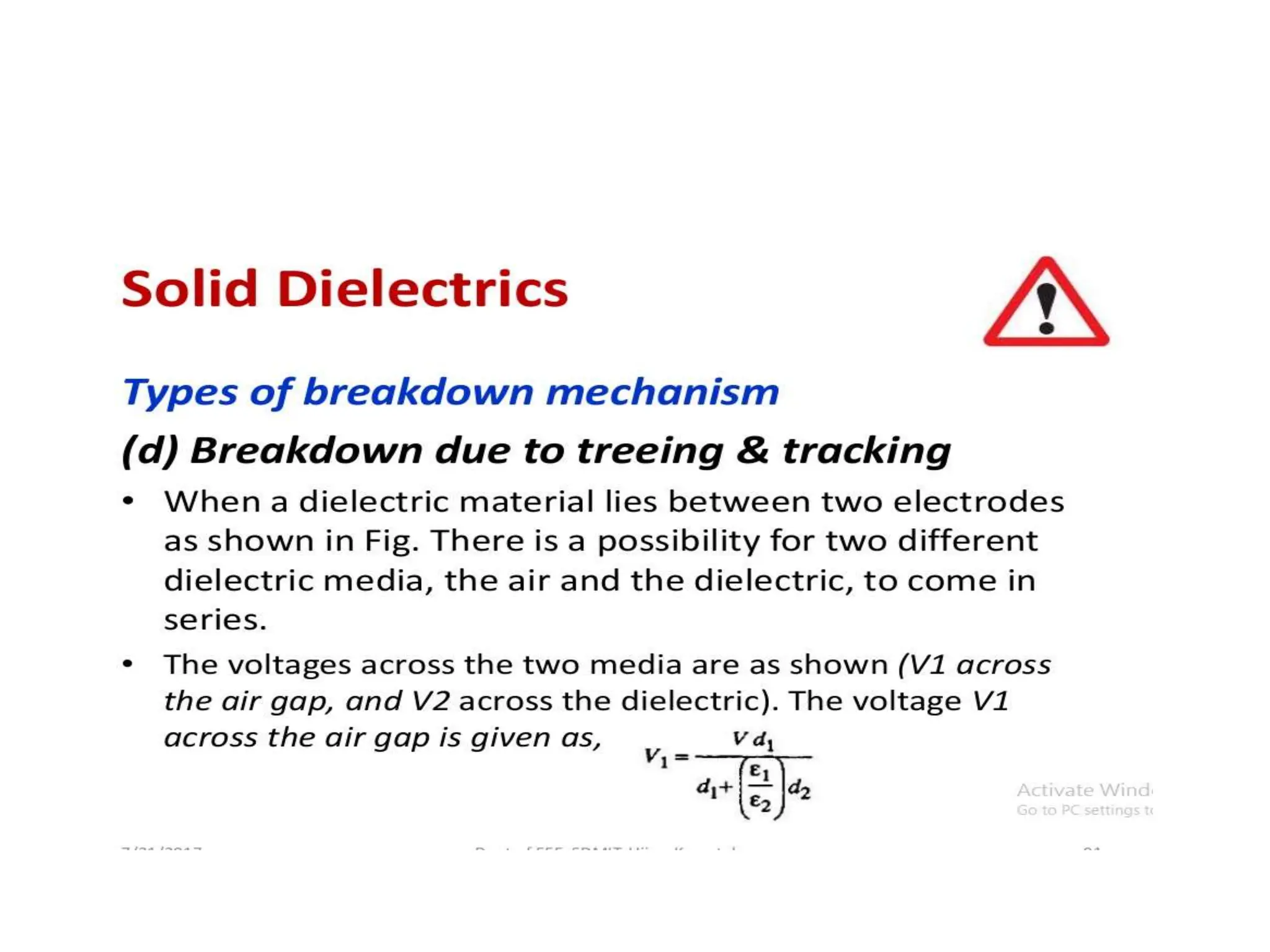
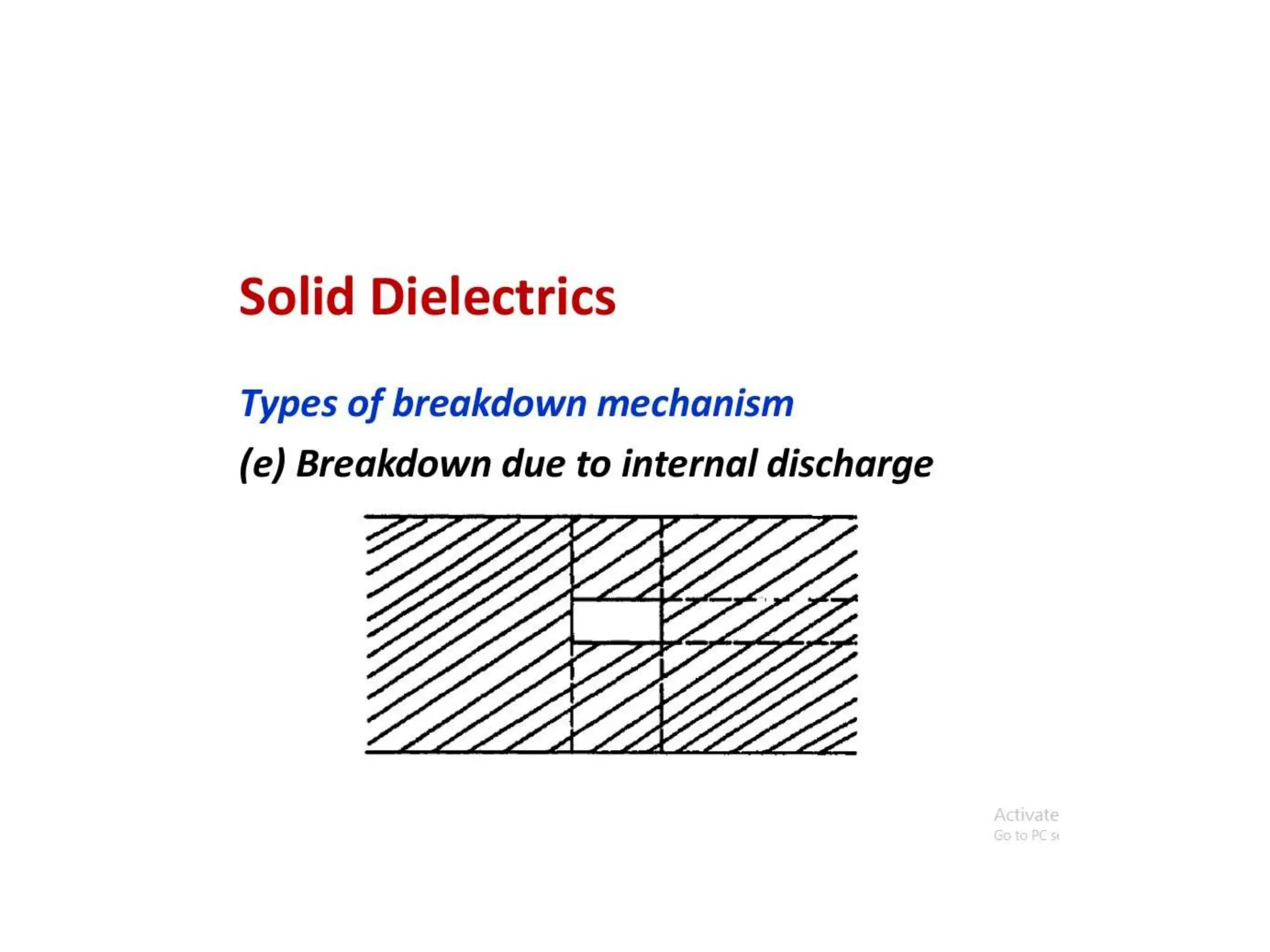
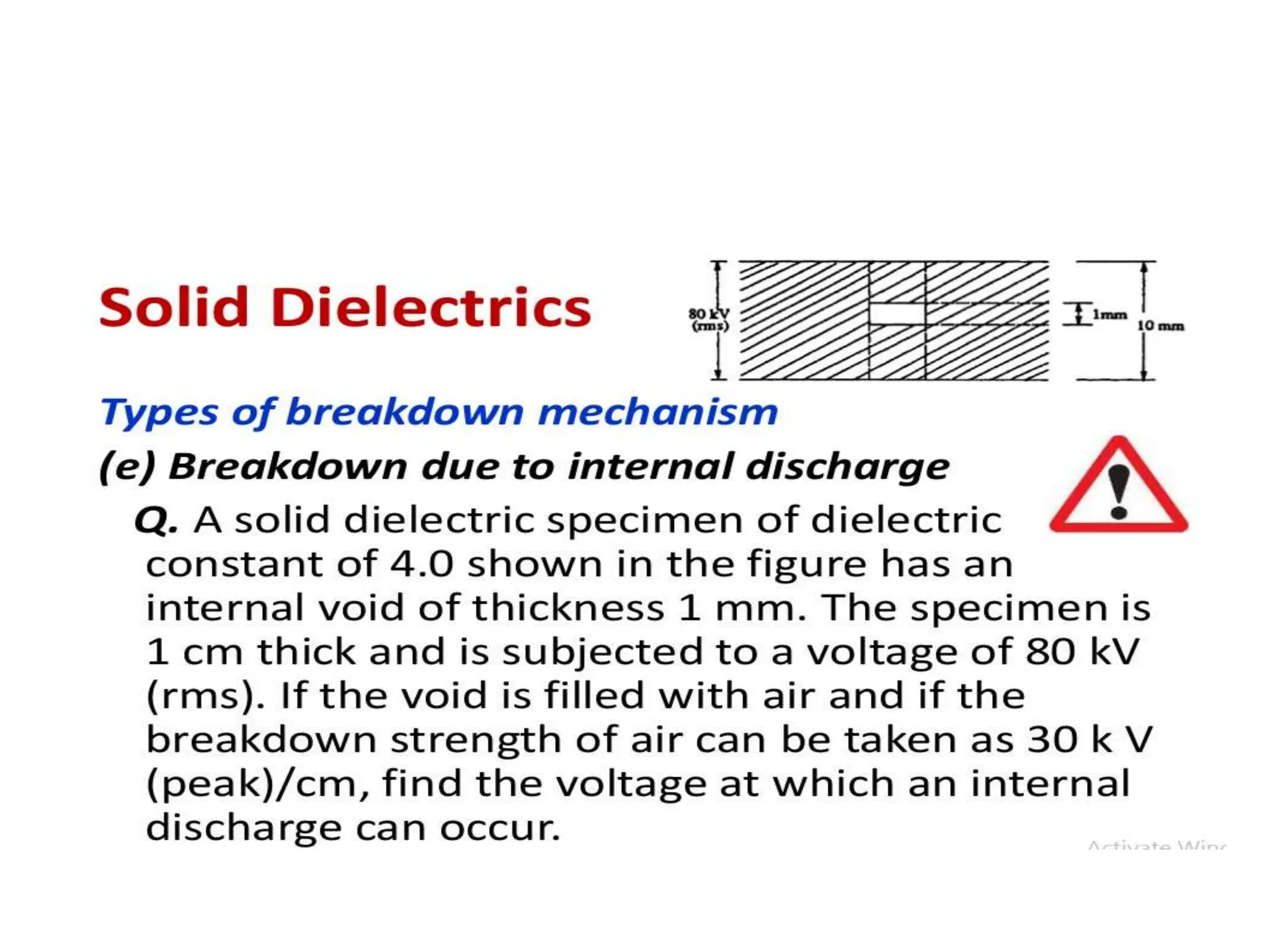
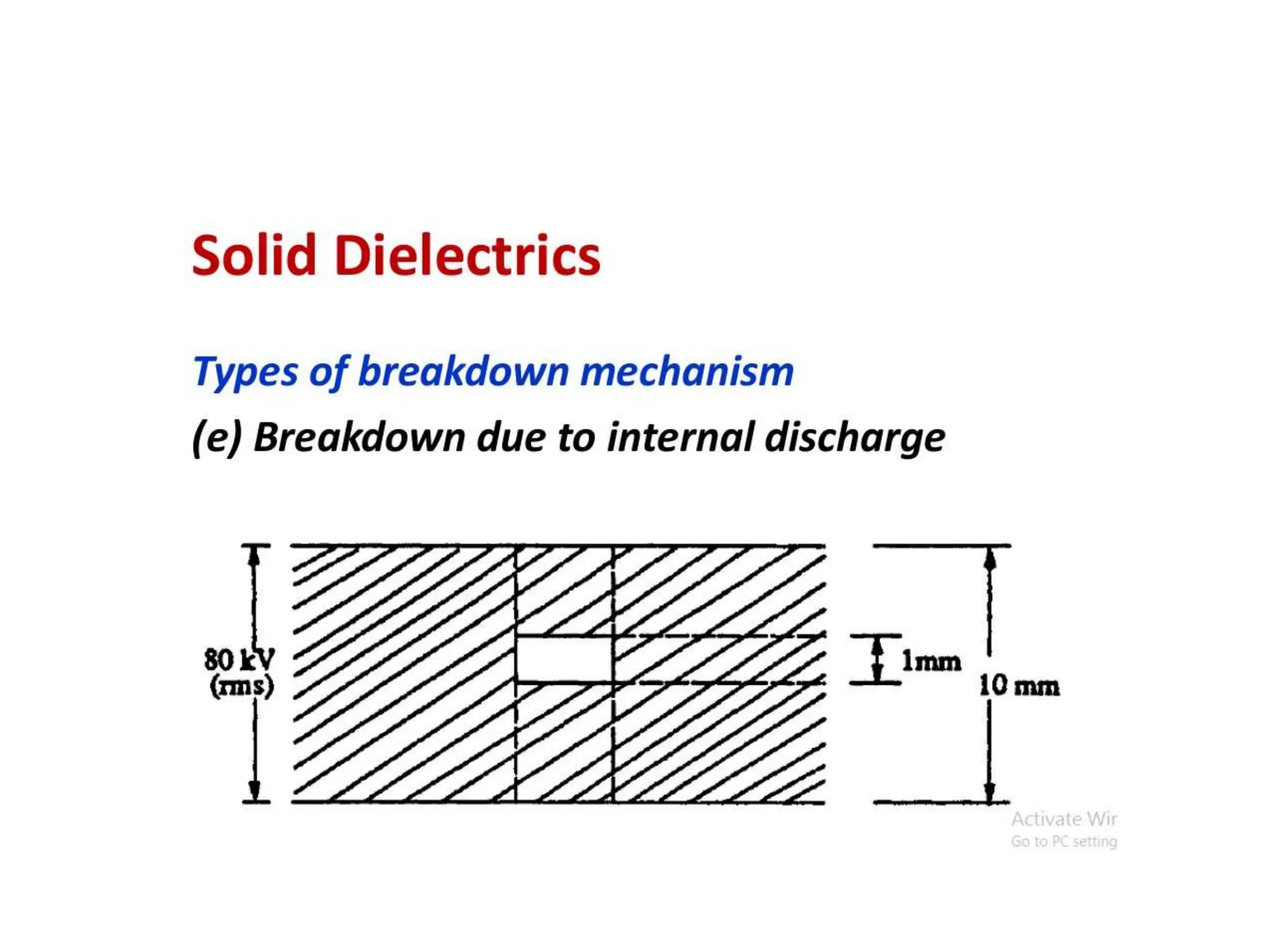
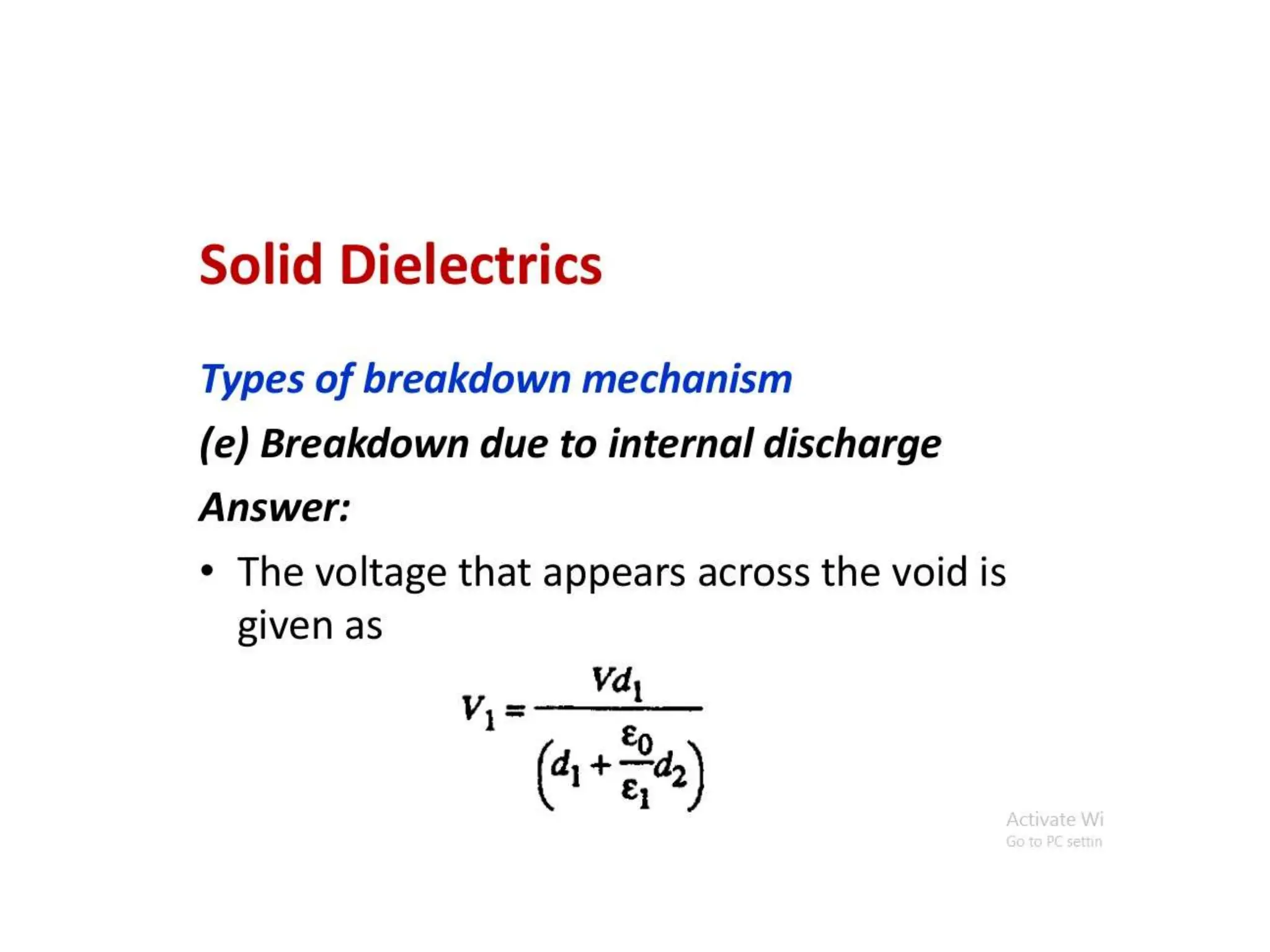
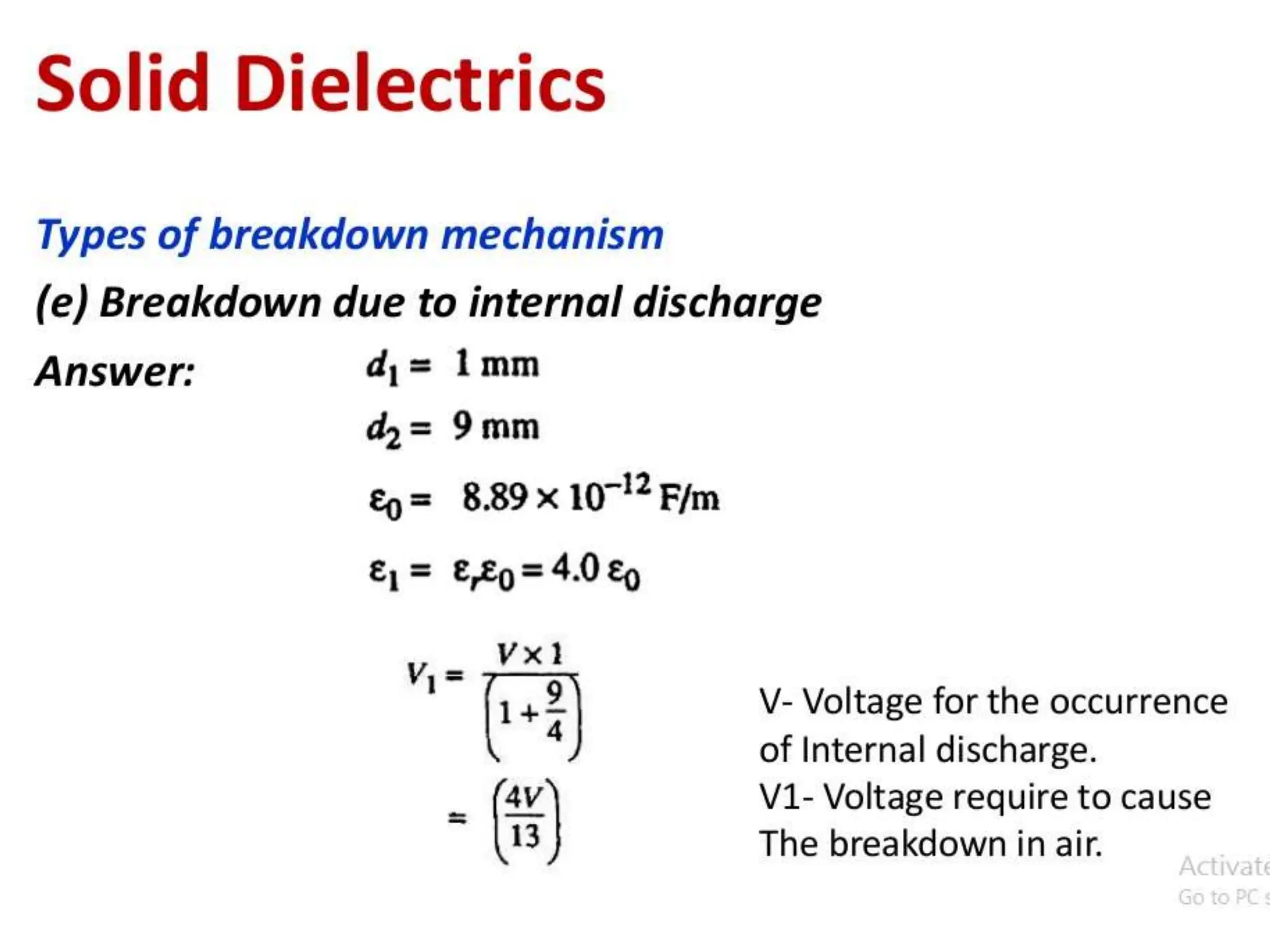
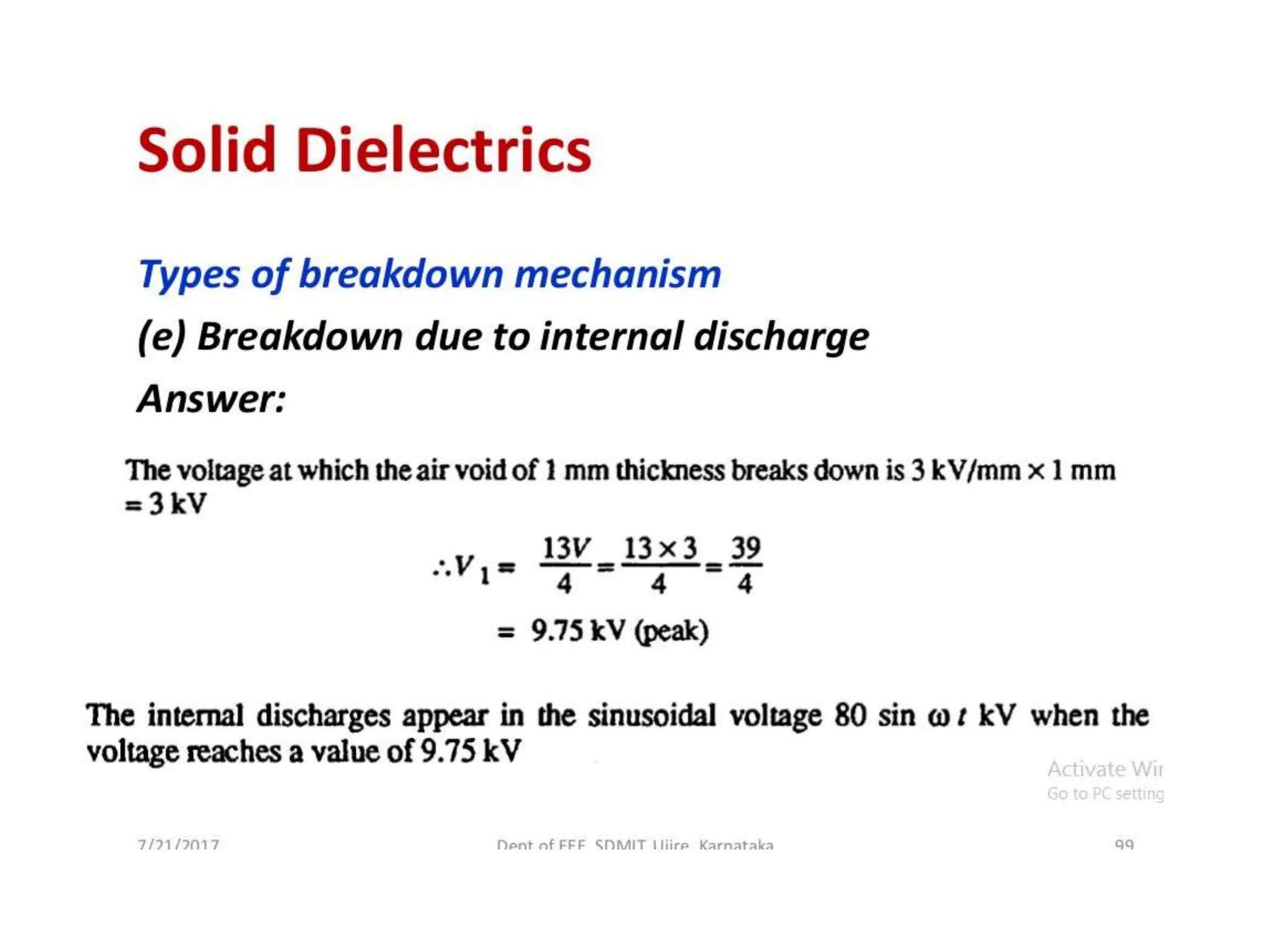
The document summarizes key topics related to breakdown in dielectric materials. It discusses intrinsic breakdown, avalanche breakdown, thermal breakdown, and electro-mechanical breakdown in solid dielectrics. It also covers suspended particle theory, electronic breakdown, cavity breakdown theory, and electroconvection breakdown in liquid dielectrics. Finally, it examines ionization processes including collisional ionization, Townsend's theory of gas breakdown, and streamer theory of non-uniform field breakdown in gases.