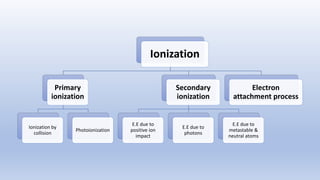
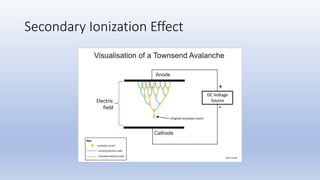
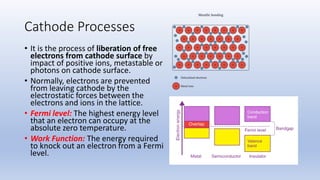
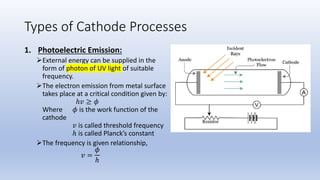
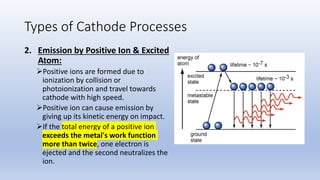
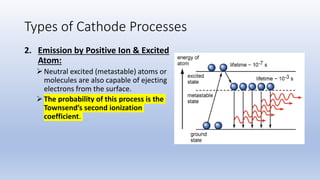
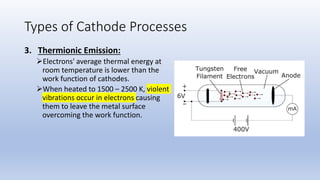
The document discusses the ionization process in gases as an insulating medium, detailing primary and secondary ionization processes, types of cathode processes, and theories explaining breakdown mechanisms. Key processes for electron emission include photoelectric emission, ion impact emission, thermionic emission, and field emission. The cathode's role is crucial in initiating and sustaining electric discharges by providing the necessary electrons.