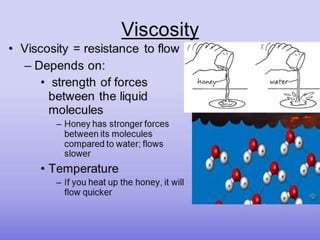
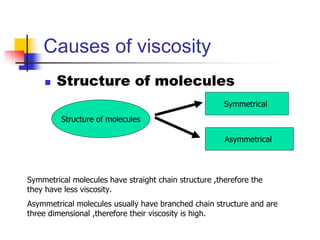
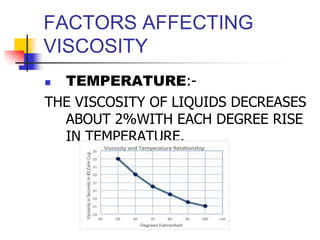
Viscosity is the resistance of a fluid to flow. Liquids with higher viscosity, like honey, do not flow easily due to molecular interactions that cause friction. Viscosity is inversely proportional to fluidity. Factors that affect viscosity include temperature, chemical composition, presence of colloidal particles, and suspended materials. Viscosity decreases with increasing temperature and is greater for larger/asymmetrical molecules versus smaller/symmetrical molecules. Hyperviscosity syndrome occurs when plasma viscosity increases significantly, which can cause circulatory and bleeding issues. Treatment involves plasmapheresis to reduce plasma protein levels and decrease viscosity.