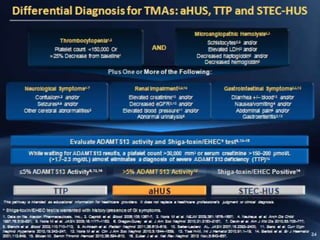
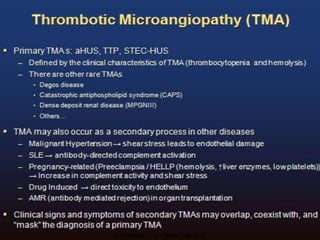
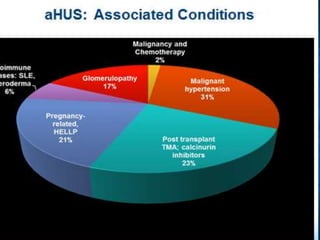
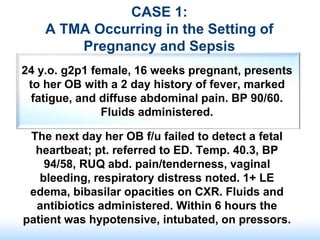
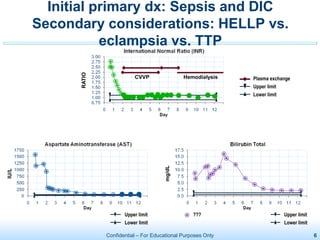
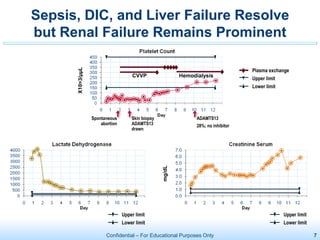
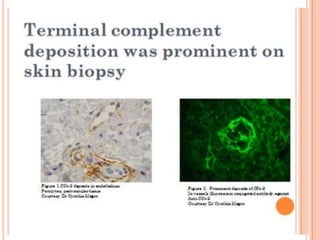
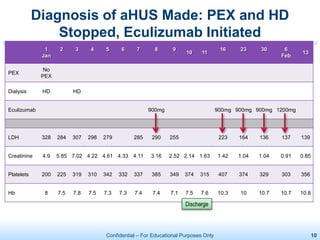
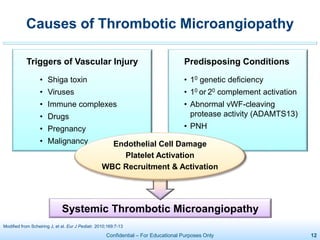
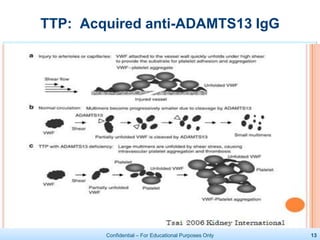
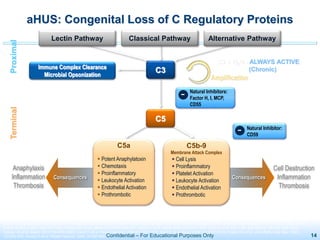
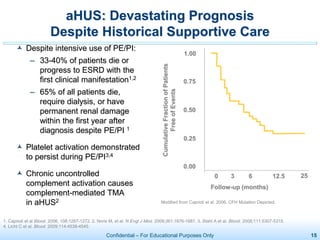
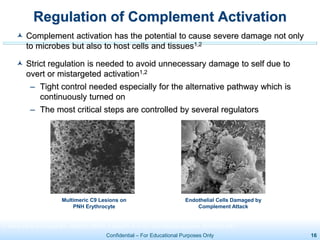
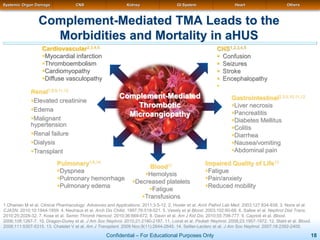
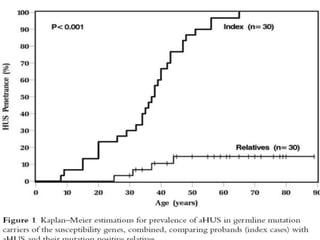
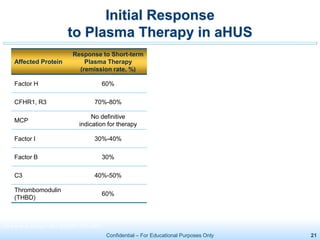
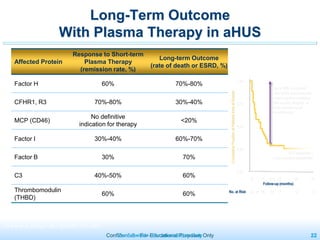
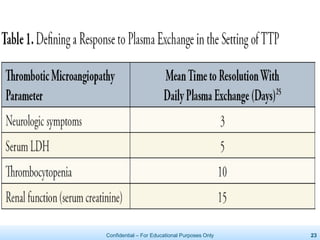
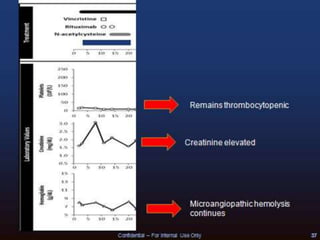
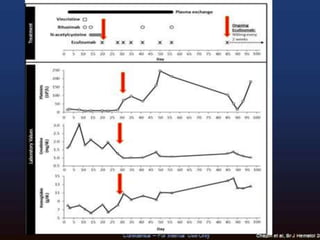
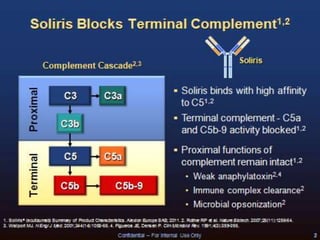
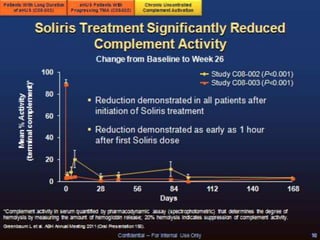
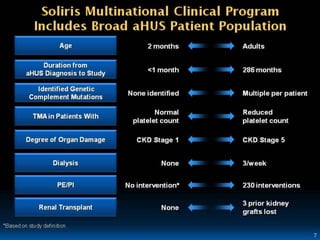
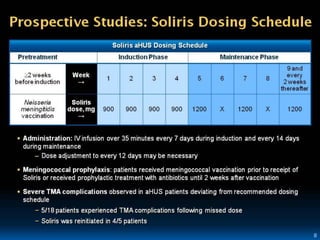
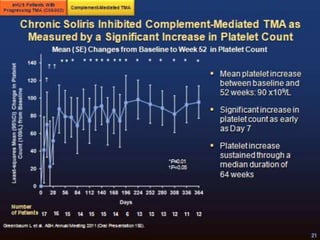
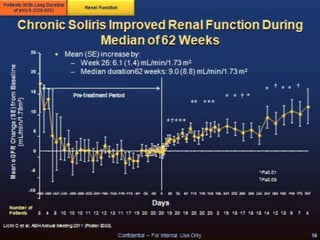
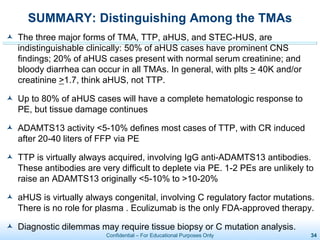
The document discusses thrombotic microangiopathies (TMAs) such as thrombotic thrombocytopenic purpura (TTP) and atypical hemolytic uremic syndrome (aHUS). TMAs are caused by endothelial cell damage and platelet activation leading to thrombosis. While plasma therapy can induce remission in some patients with aHUS, genetic mutations affecting complement regulatory factors often result in poor long-term outcomes of end-stage renal disease or death despite treatment.