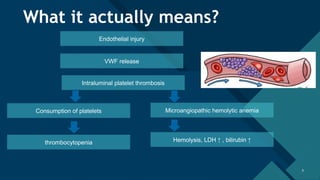
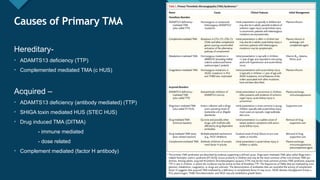
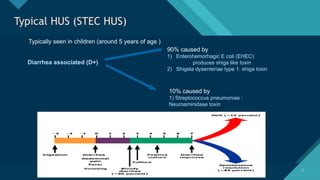
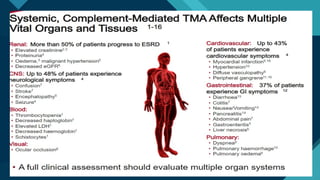
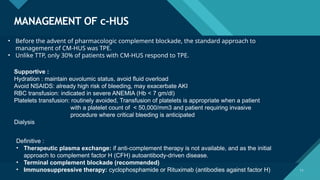
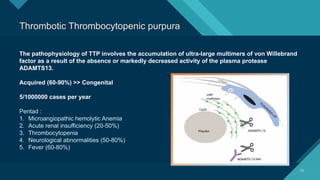
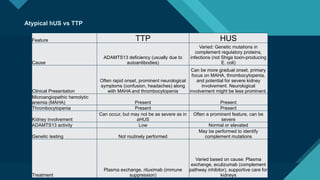
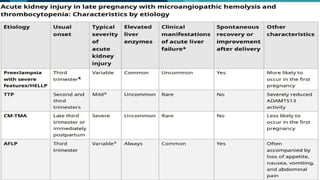
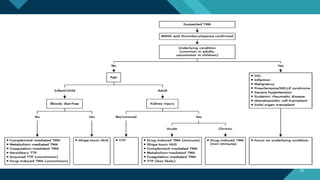
Thrombotic microangiopathy (TMA) is a pathological process characterized by endothelial injury, microvascular thrombosis, microangiopathic hemolytic anemia (MAHA), thrombocytopenia, and organ dysfunction. It includes primary forms like thrombotic thrombocytopenic purpura (TTP), caused by ADAMTS13 deficiency, and atypical hemolytic uremic syndrome (aHUS), driven by complement dysregulation, as well as secondary forms associated with infections, autoimmune diseases (SLE, antiphospholipid syndrome), malignancies, pregnancy, and drugs. Clinically, TMA presents with schistocytes on peripheral smear, hemolysis (↑ LDH, ↓ haptoglobin), thrombocytopenia, and variable organ involvement (renal failure in aHUS, neurological symptoms in TTP). Early diagnosis and targeted therapy (plasma exchange for TTP, complement inhibition for aHUS) are crucial to prevent life-threatening complications.