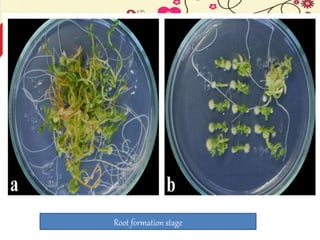
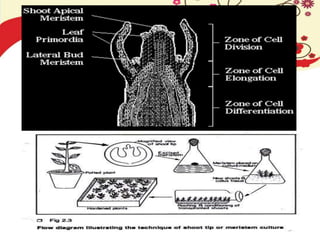
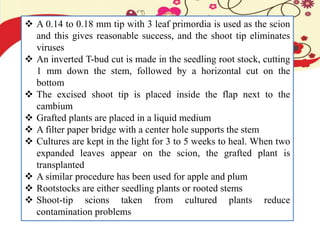
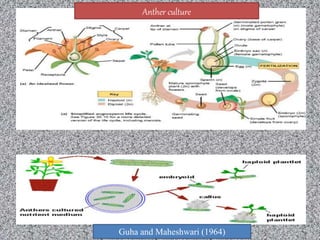
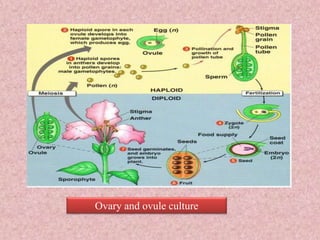
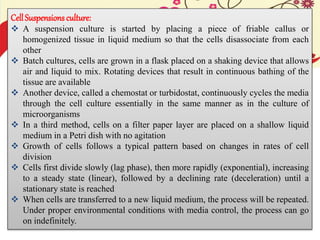
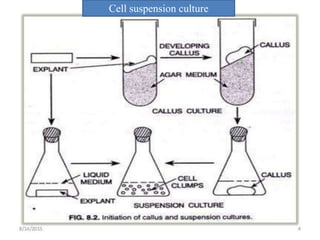
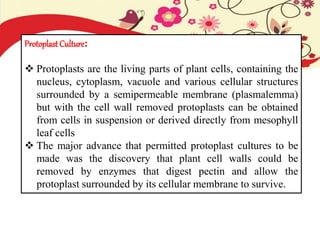
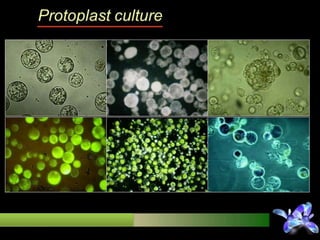
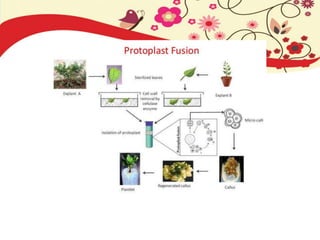
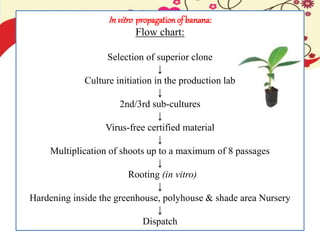
This document discusses techniques for in vitro clonal propagation of fruit crops. It covers the basics of micropropagation, which involves four stages: establishment, shoot multiplication, root formation, and acclimatization. Various tissue culture techniques are described that can be used for clonal propagation, including meristem culture, shoot tip micrografting, anther culture, embryo culture, ovary/ovule culture, callus culture, cell suspension culture, and protoplast culture. Requirements for facilities, media preparation, and procedures for each stage of micropropagation are provided. The document aims to inform the reader about the various in vitro techniques that can be used for commercial clonal propagation of fruit crops.