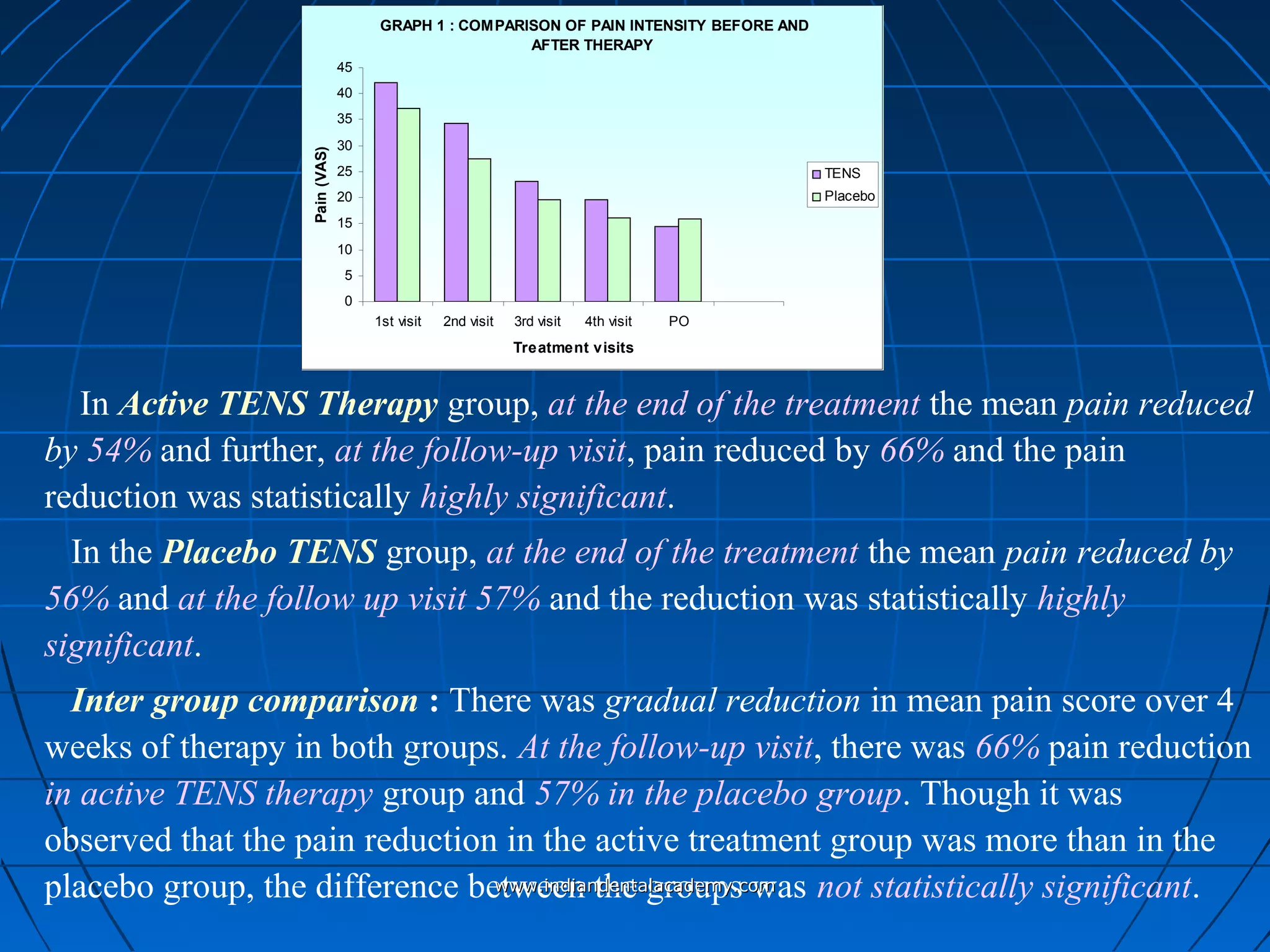
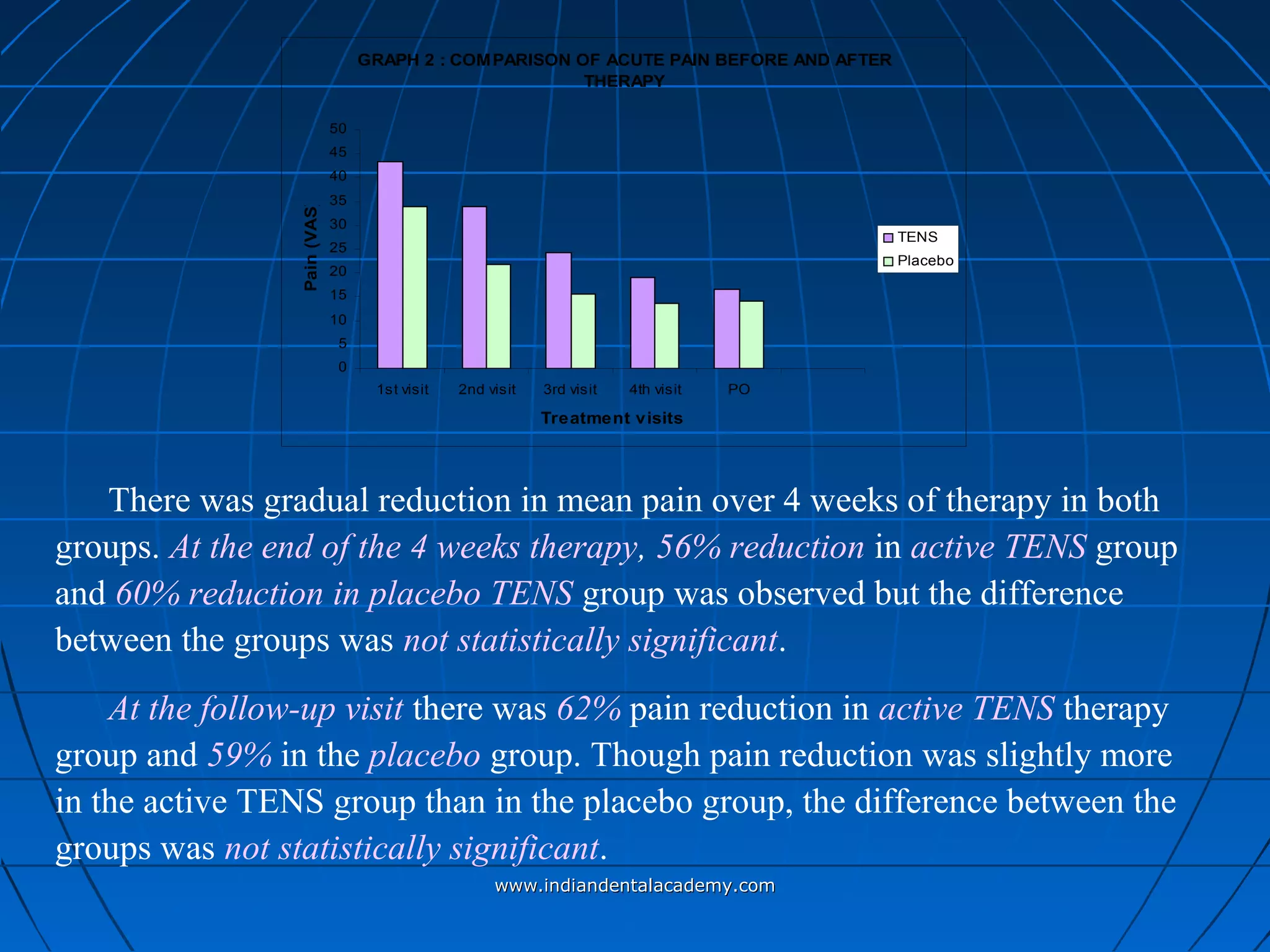
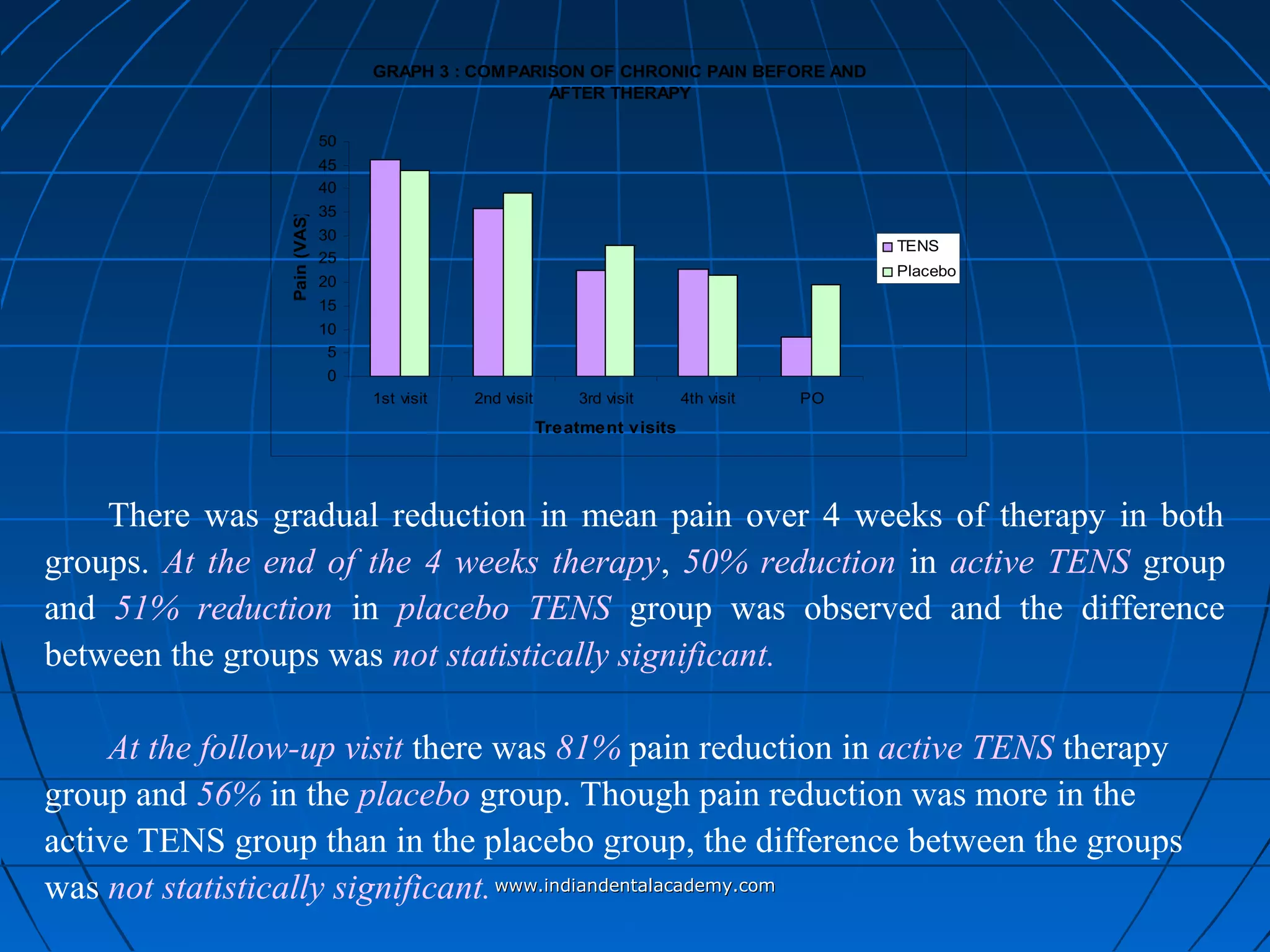
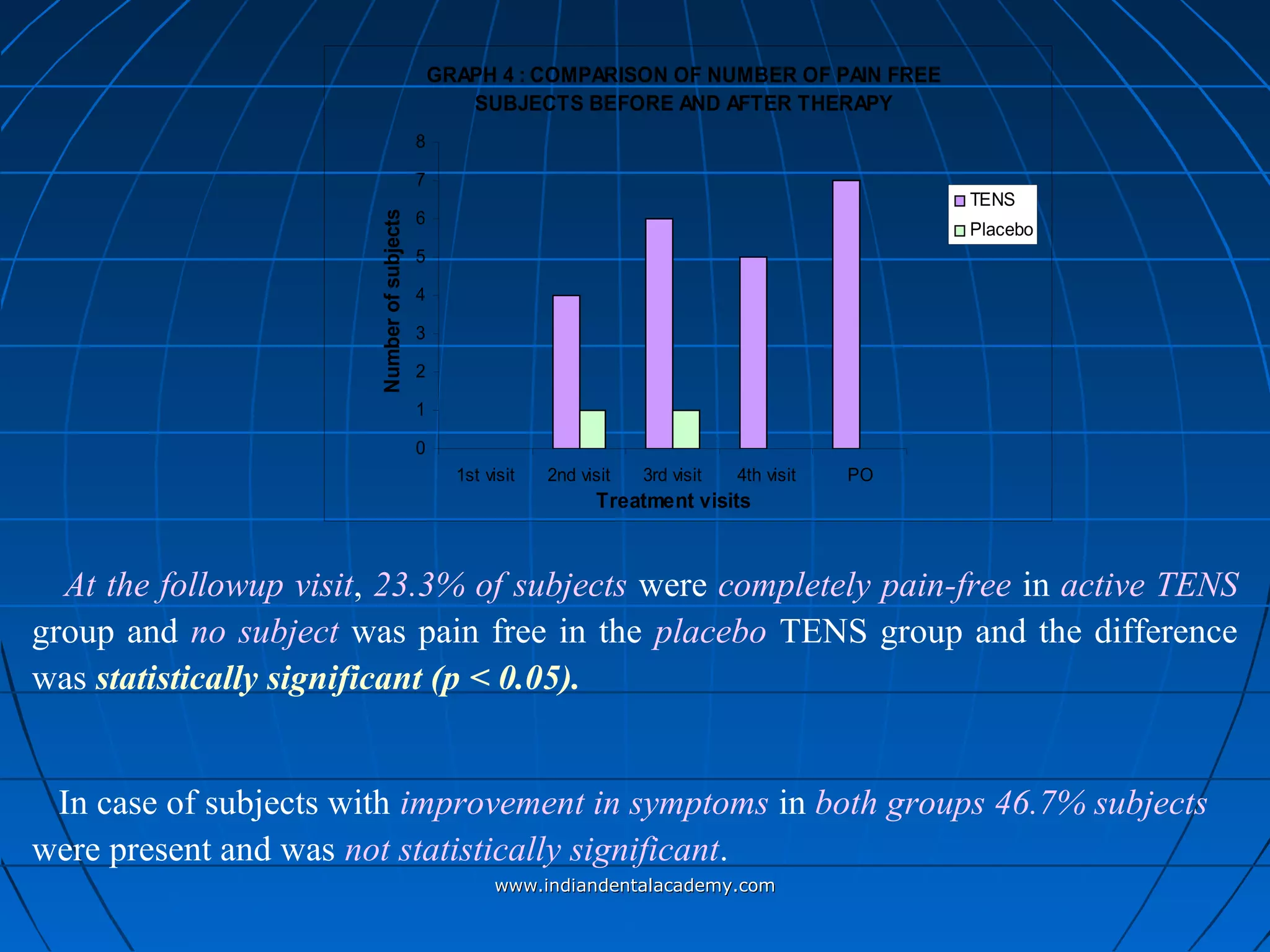
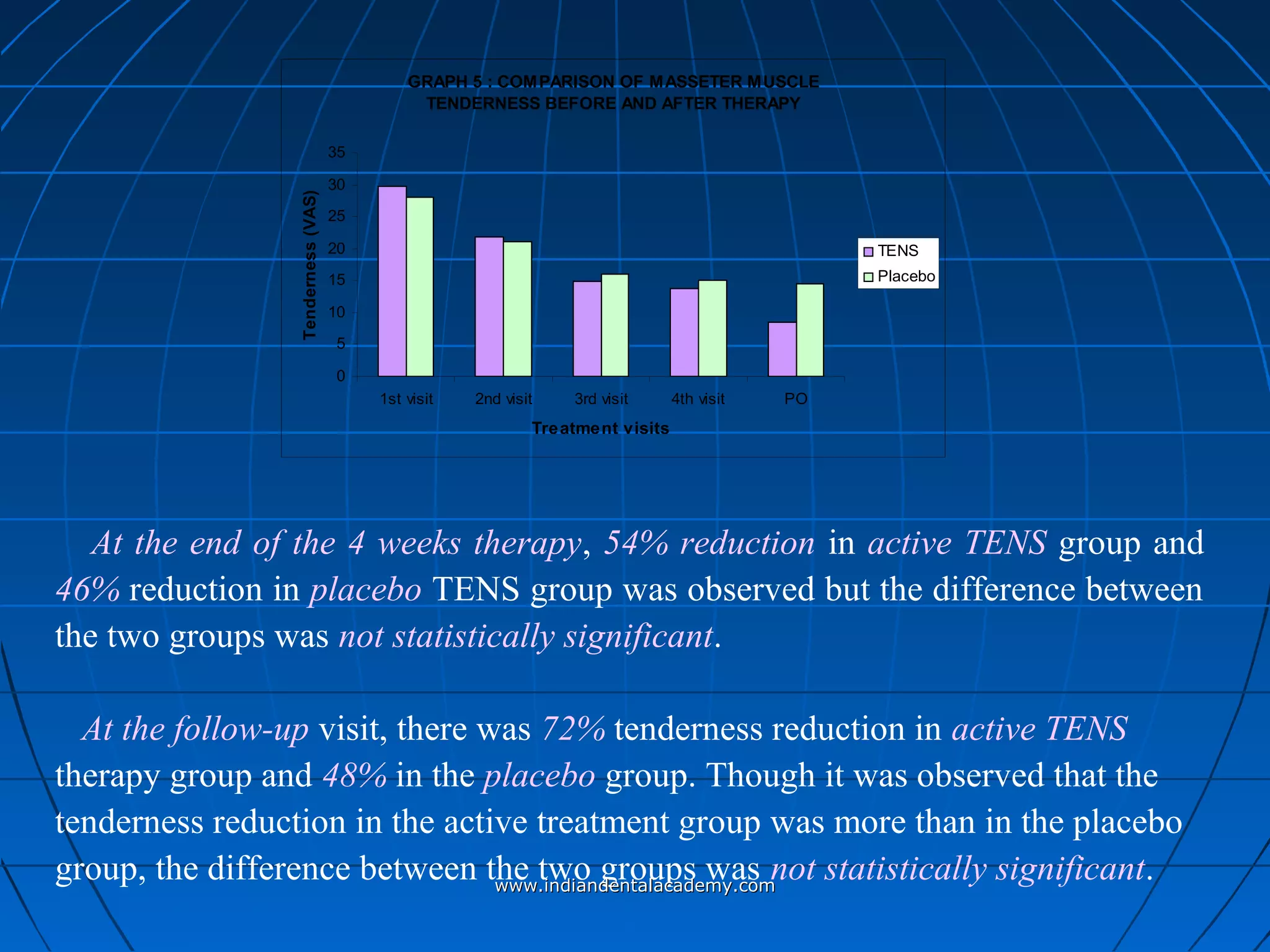
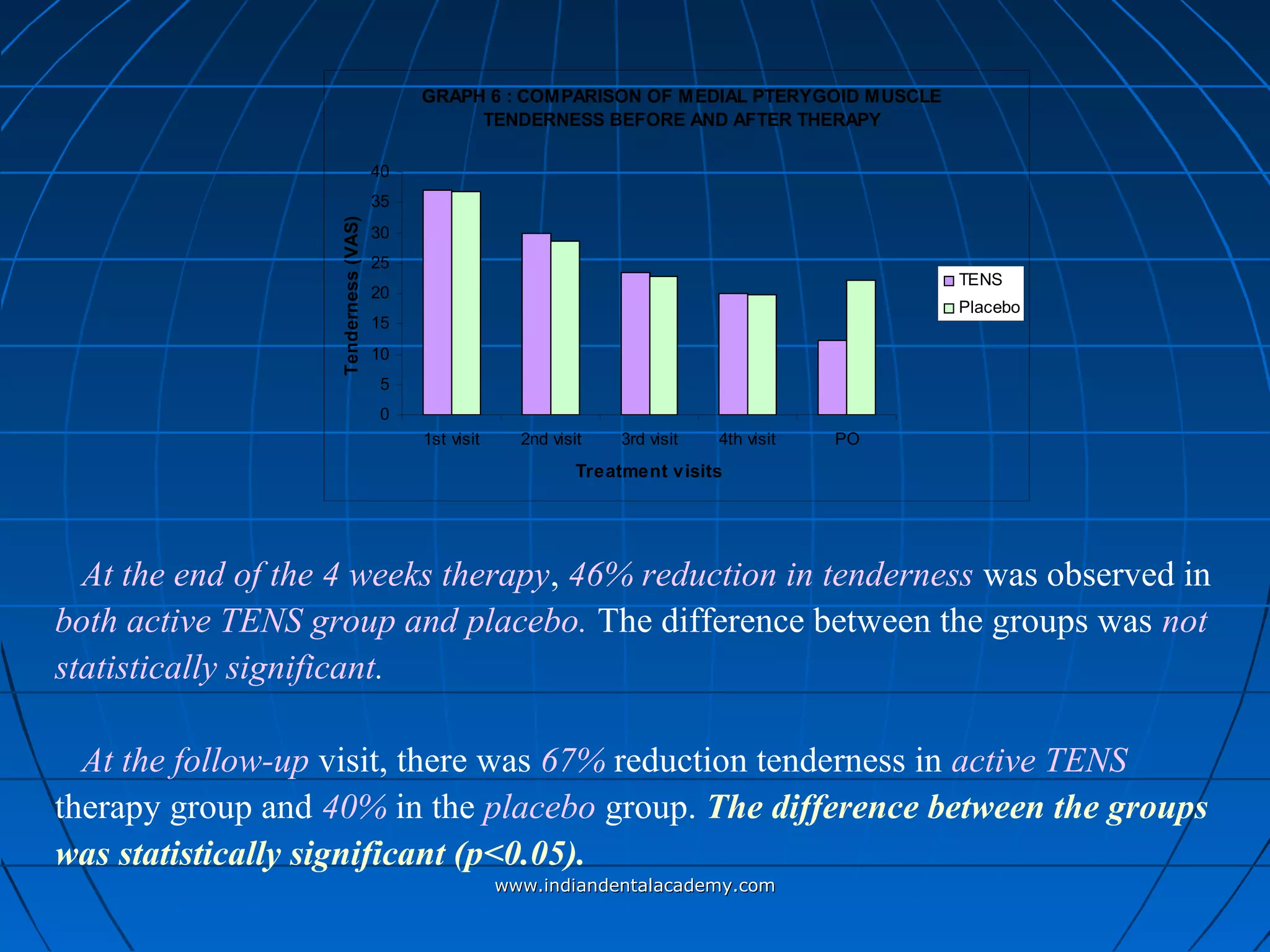
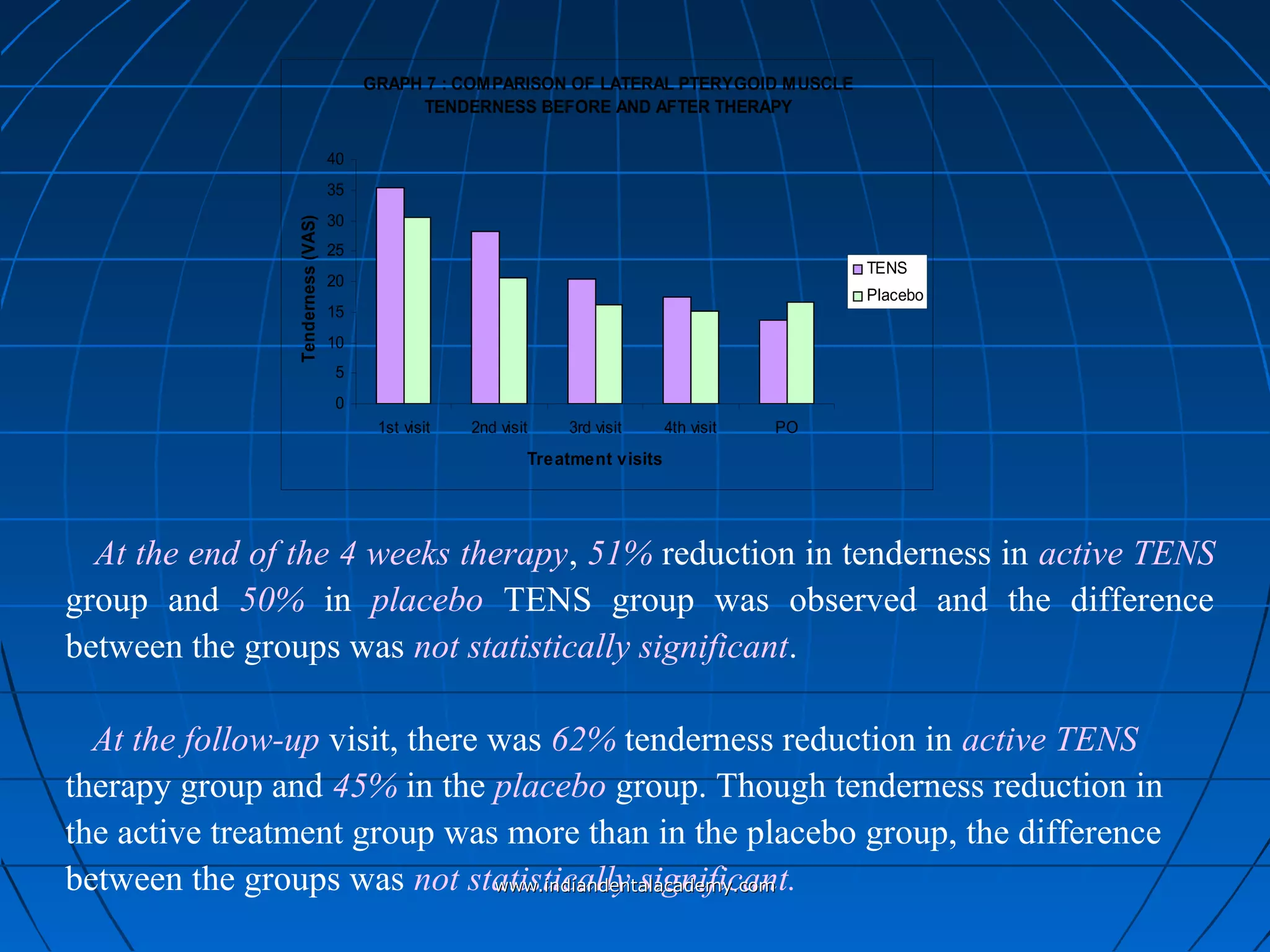
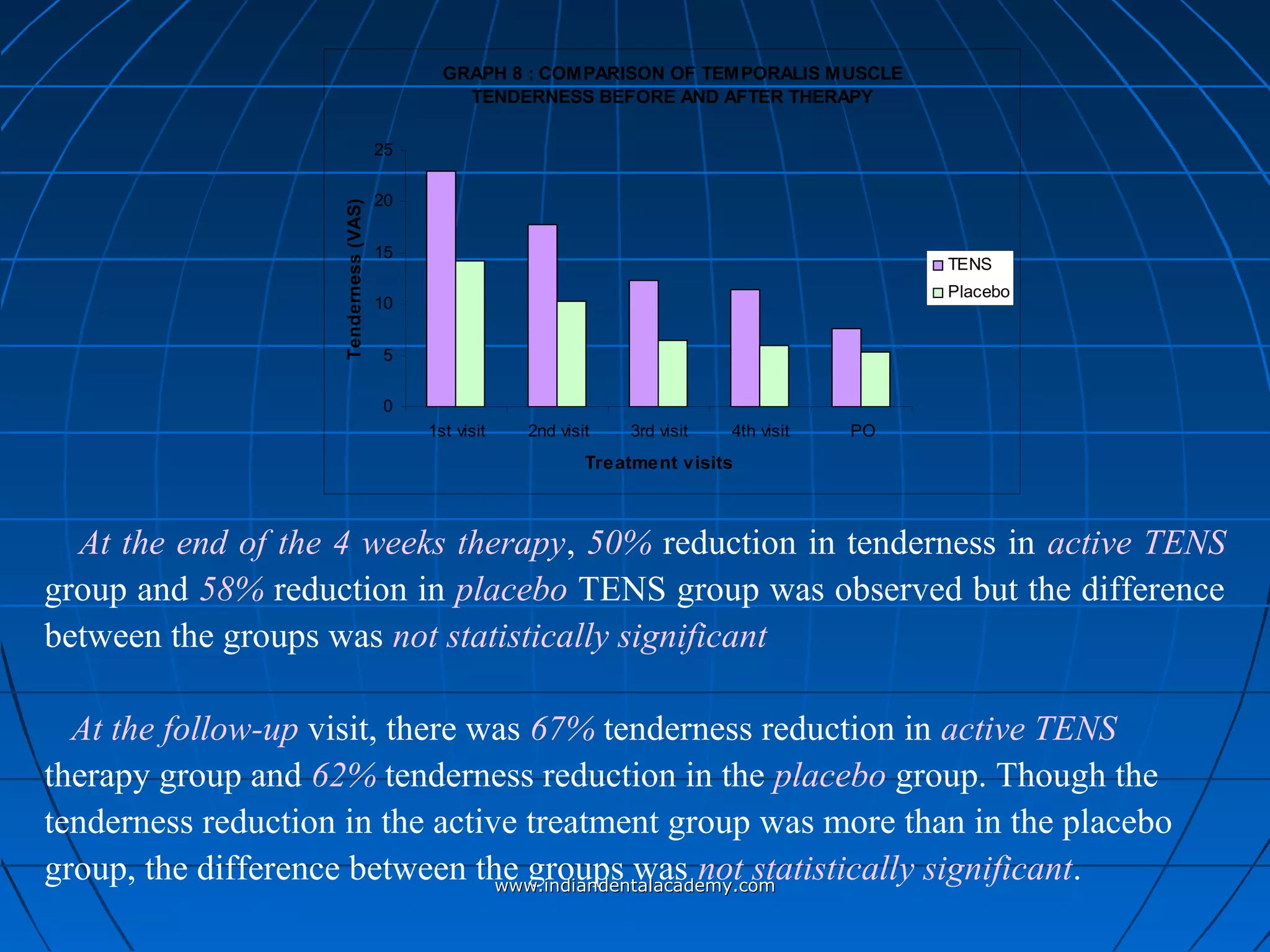
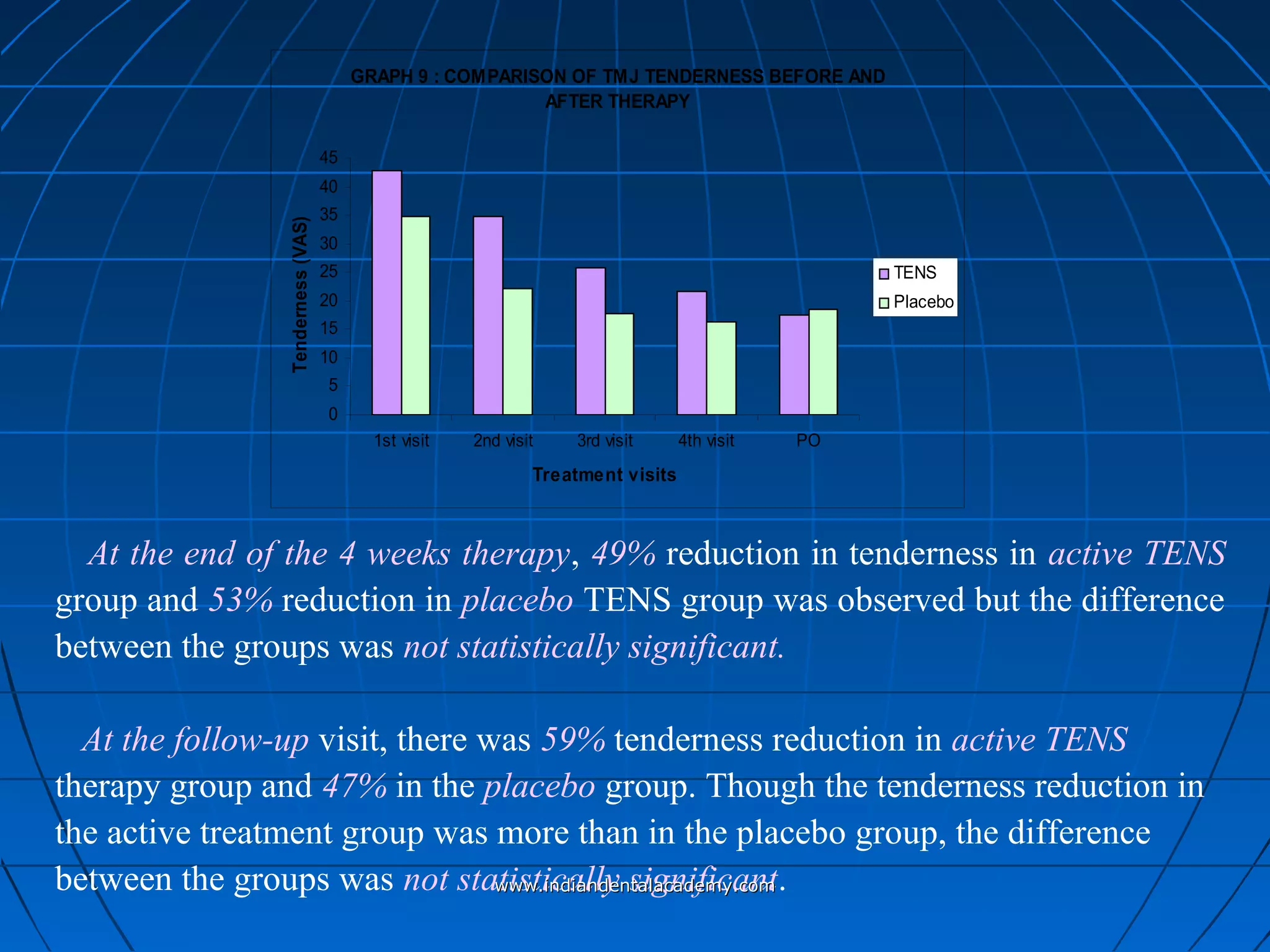
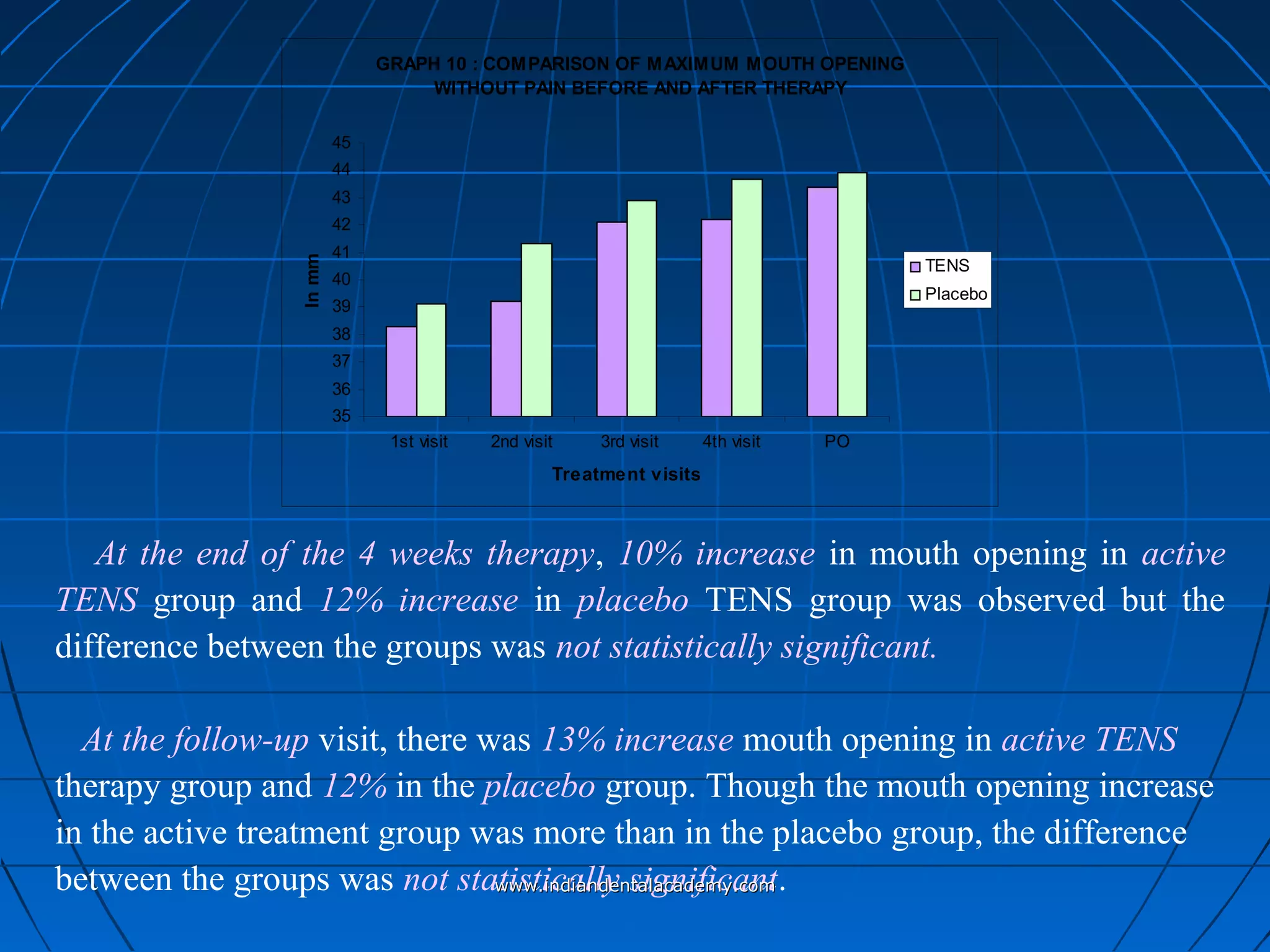
The document discusses a study evaluating the effectiveness of transcutaneous electrical nerve stimulation (TENS) therapy in managing temporomandibular disorders (TMD) pain. It highlights that while both active TENS and placebo TENS groups showed significant pain reduction over four weeks, the difference in effectiveness between the two was not statistically significant. The study concluded that TENS therapy is a potentially beneficial non-invasive option for TMD pain management, but further research is warranted to confirm these findings.