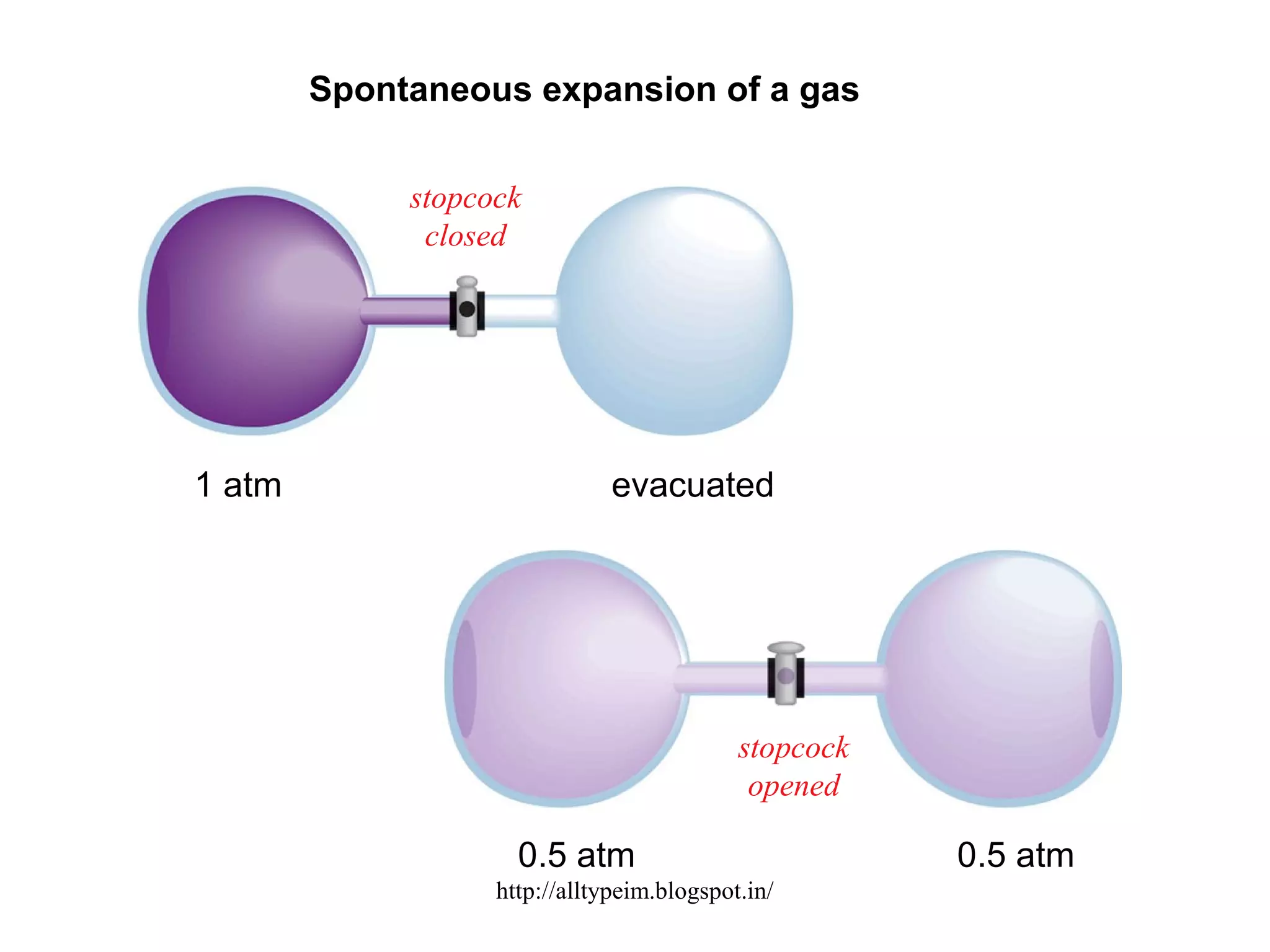
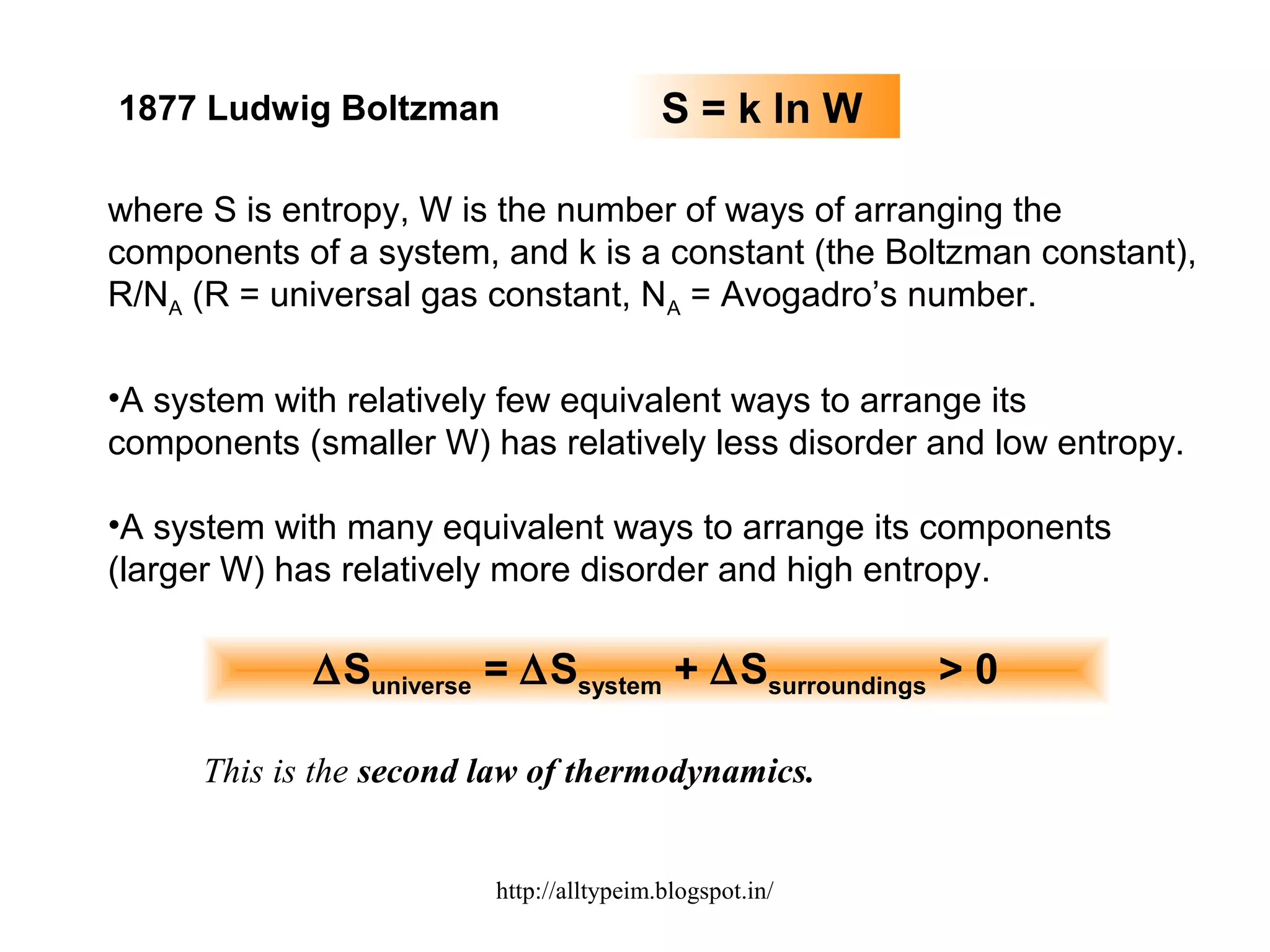
1) The document discusses entropy and the three laws of thermodynamics. It defines entropy as a measure of disorder and explains how the second law of thermodynamics relates entropy to the spontaneity of processes in the universe.
2) Equations are provided relating the change in entropy to the temperature and state of a system. More disordered states like liquids and gases have higher entropy than ordered solids or crystals.
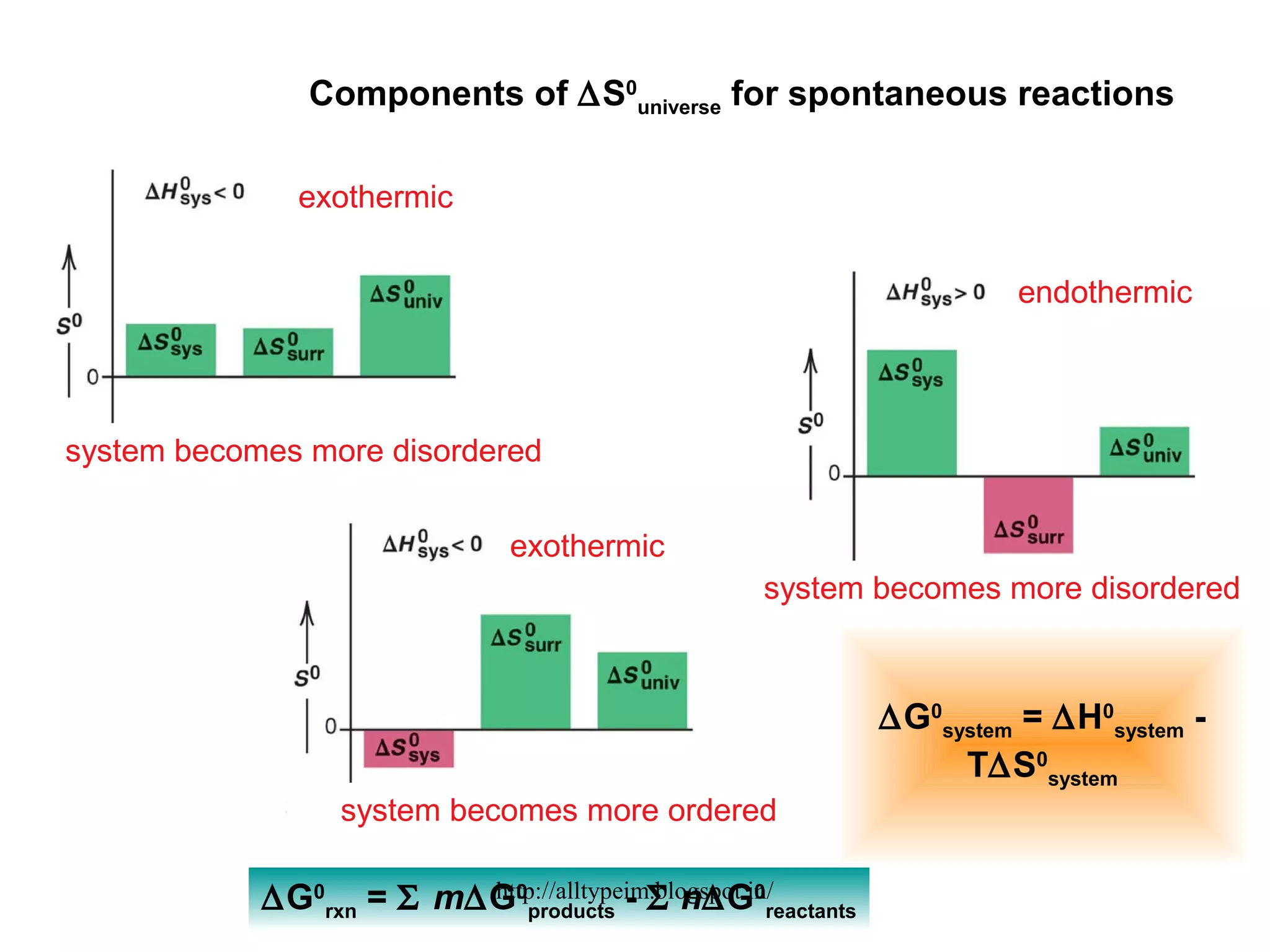
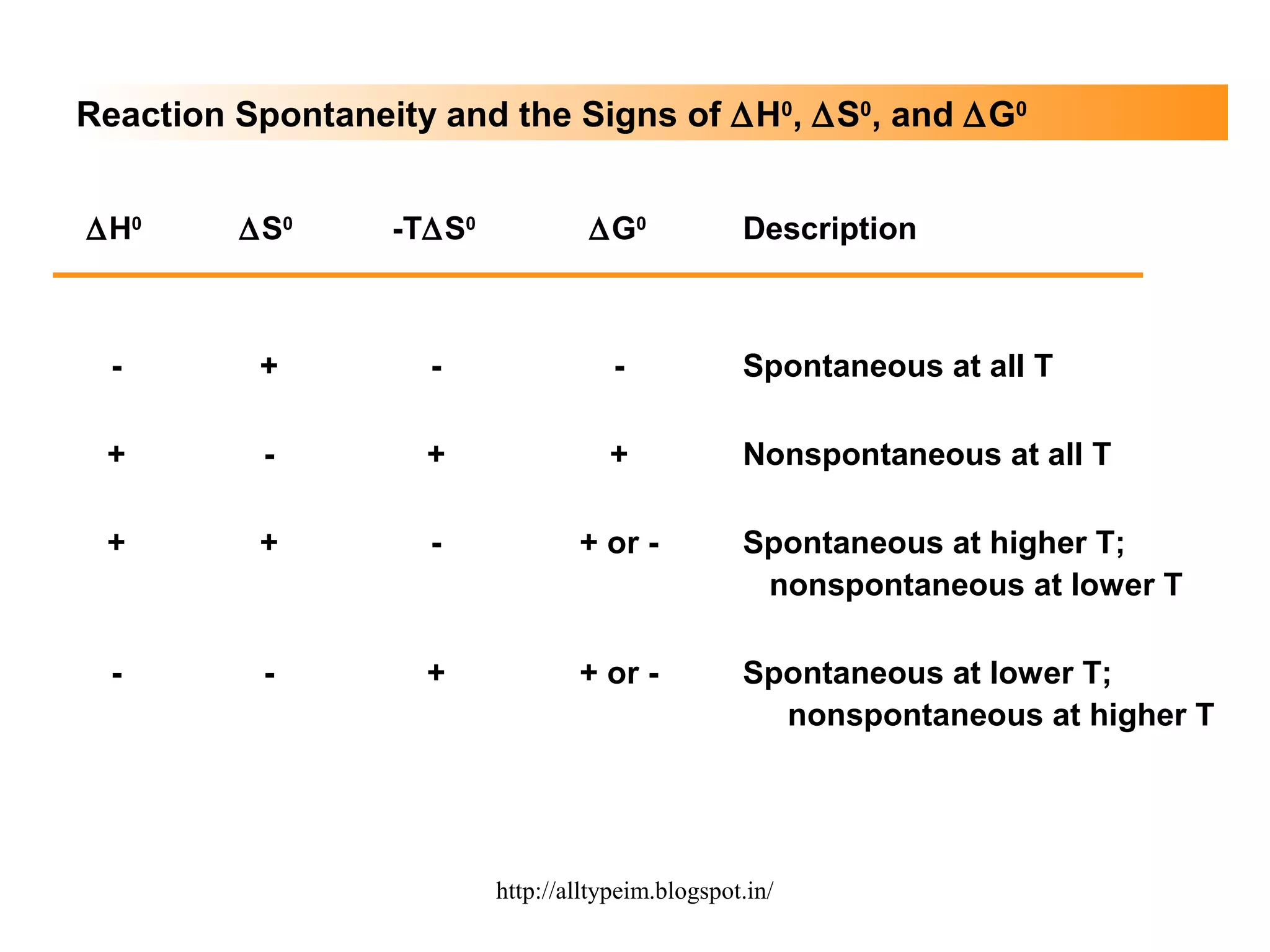
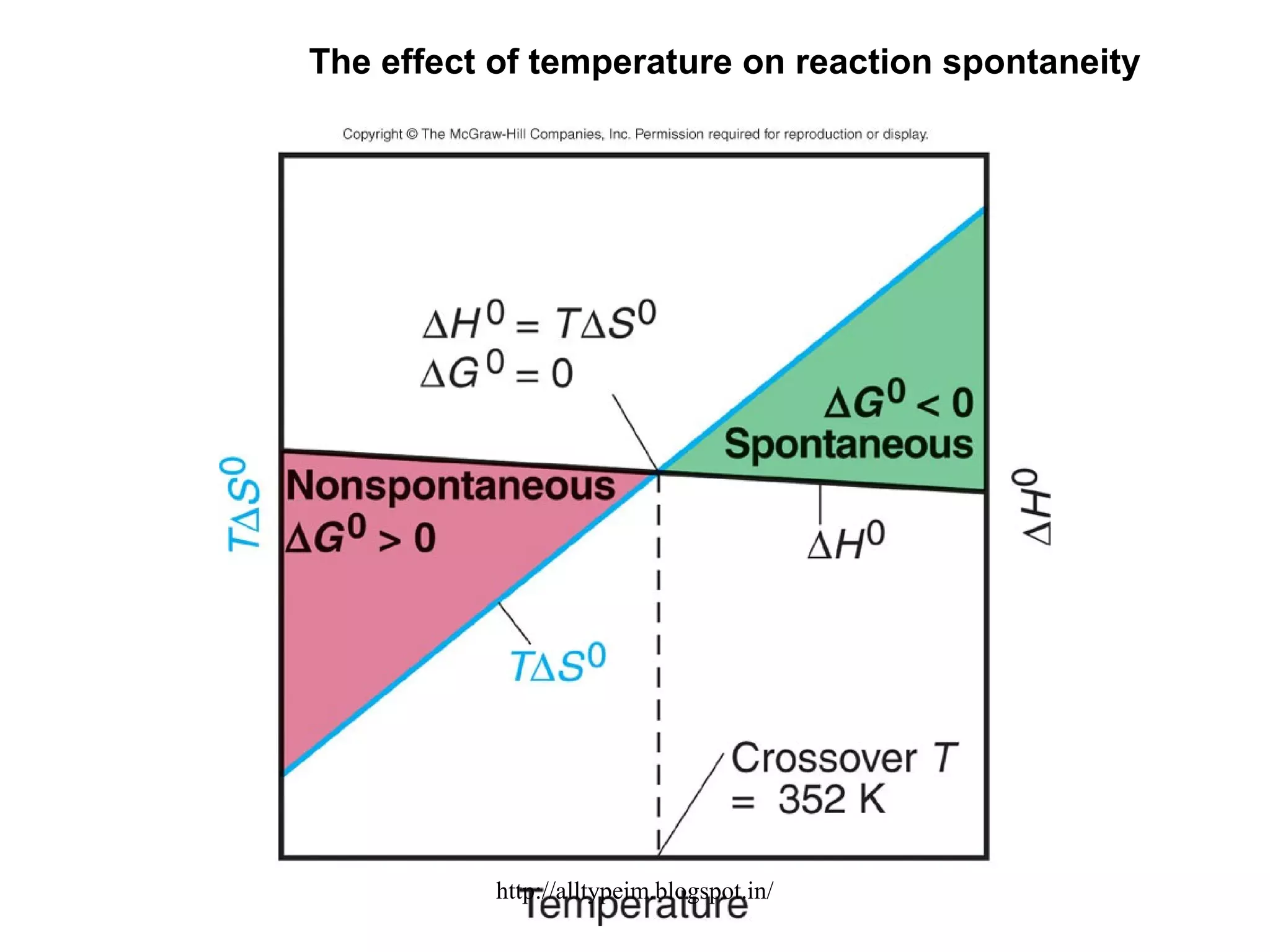
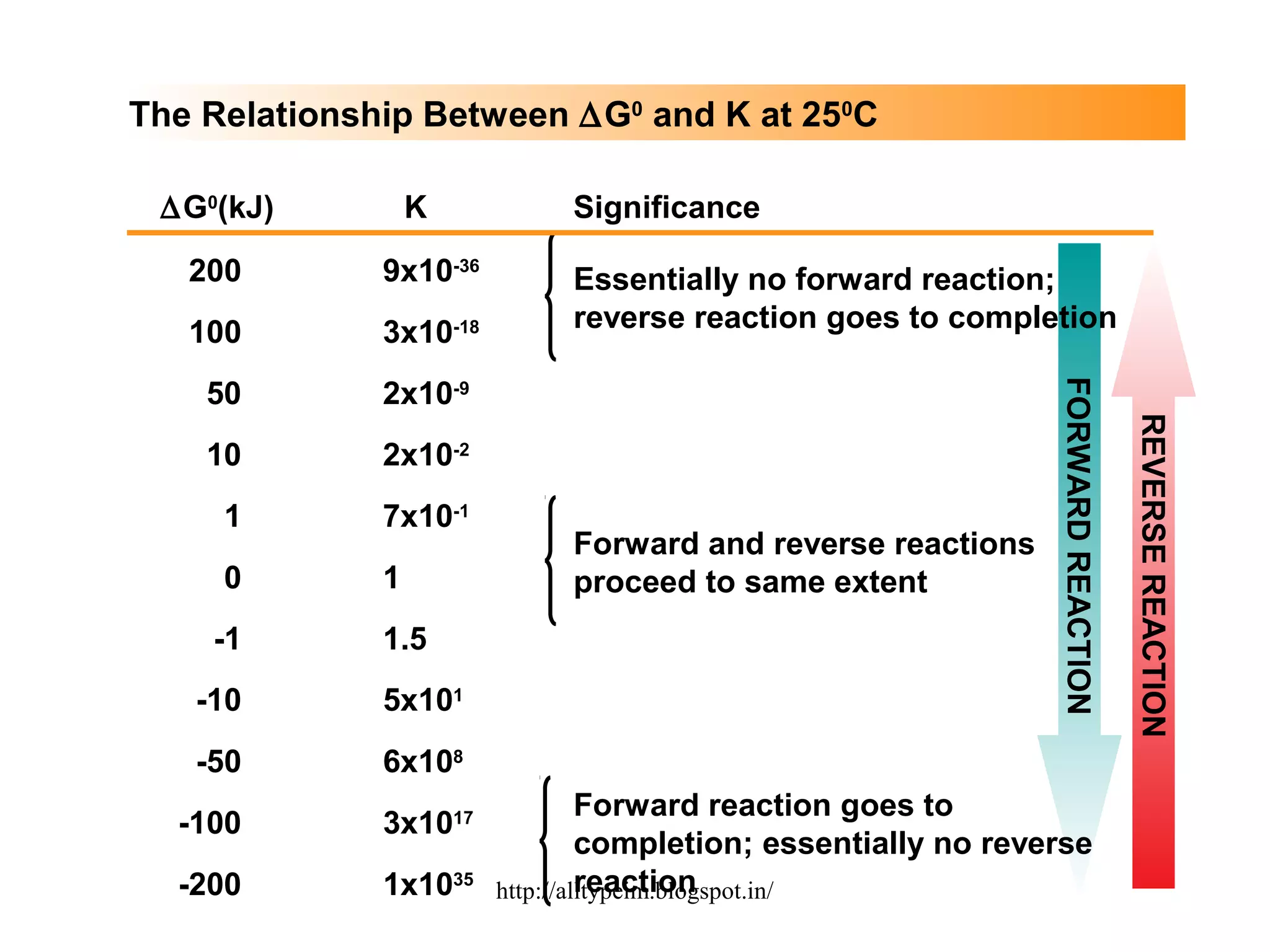
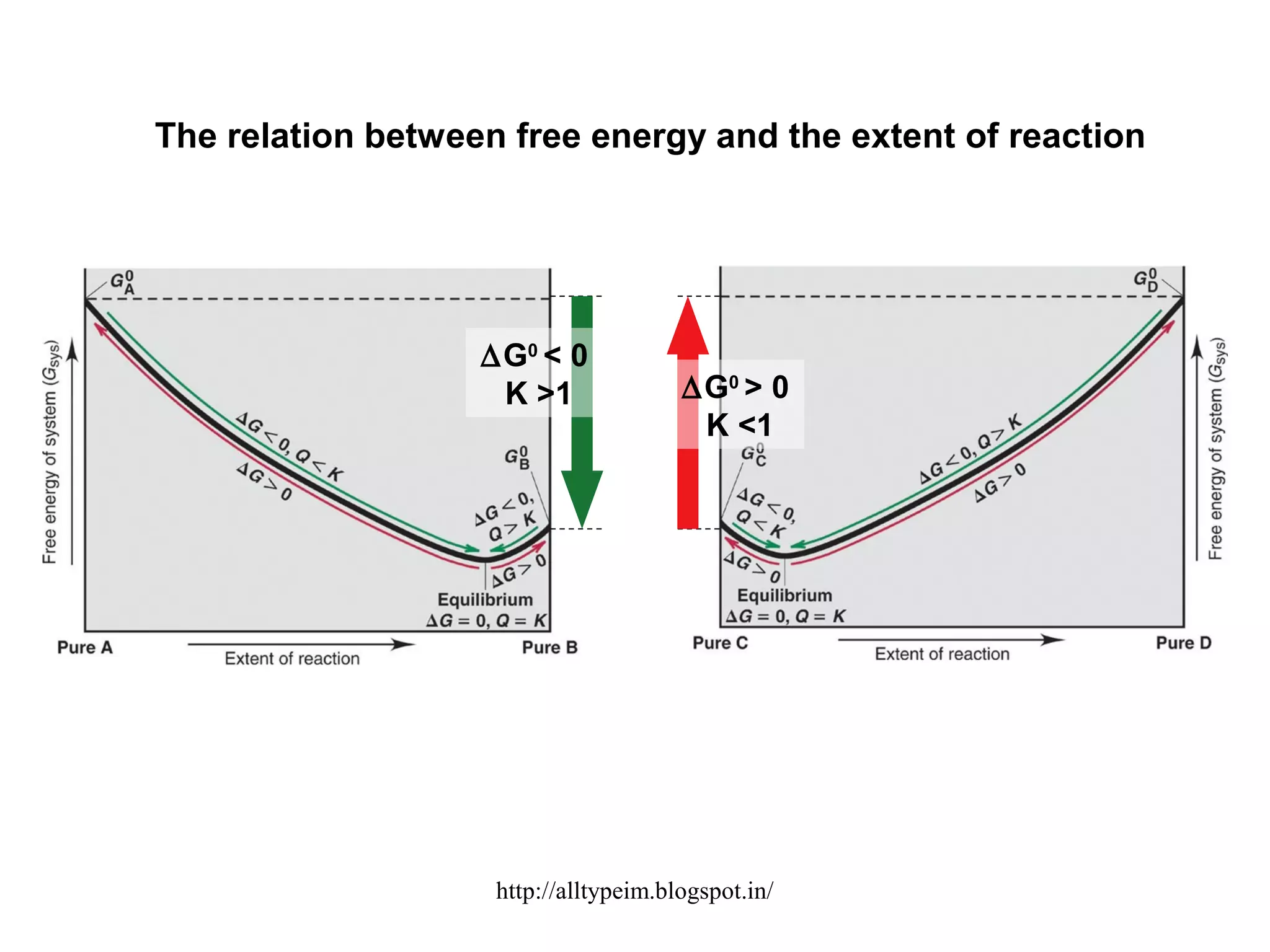
3) Graphs and diagrams show how temperature and the signs of change in enthalpy, entropy, and free energy determine whether chemical reactions proceed spontaneously. Entropy drives reactions at higher temperatures while enthalpy dominates at lower temperatures.