THE LAWS OF THERMODYNAMICS DESCRIBE HOW THE ENERGY IN A SYSTEM CHANGES AND WHETHER THE SYSTEM CAN PERFORM USEFUL WORK ON IT'S SURROUNDINGS. THERMODYNAMICS IS USED TO ANALYZE THERMAL SYSTEMS SUCH AS HEATING AND AIR-CONDITIONING SYSTEMS, REFRIGERATORS, AND WATER HEATERS.



![6
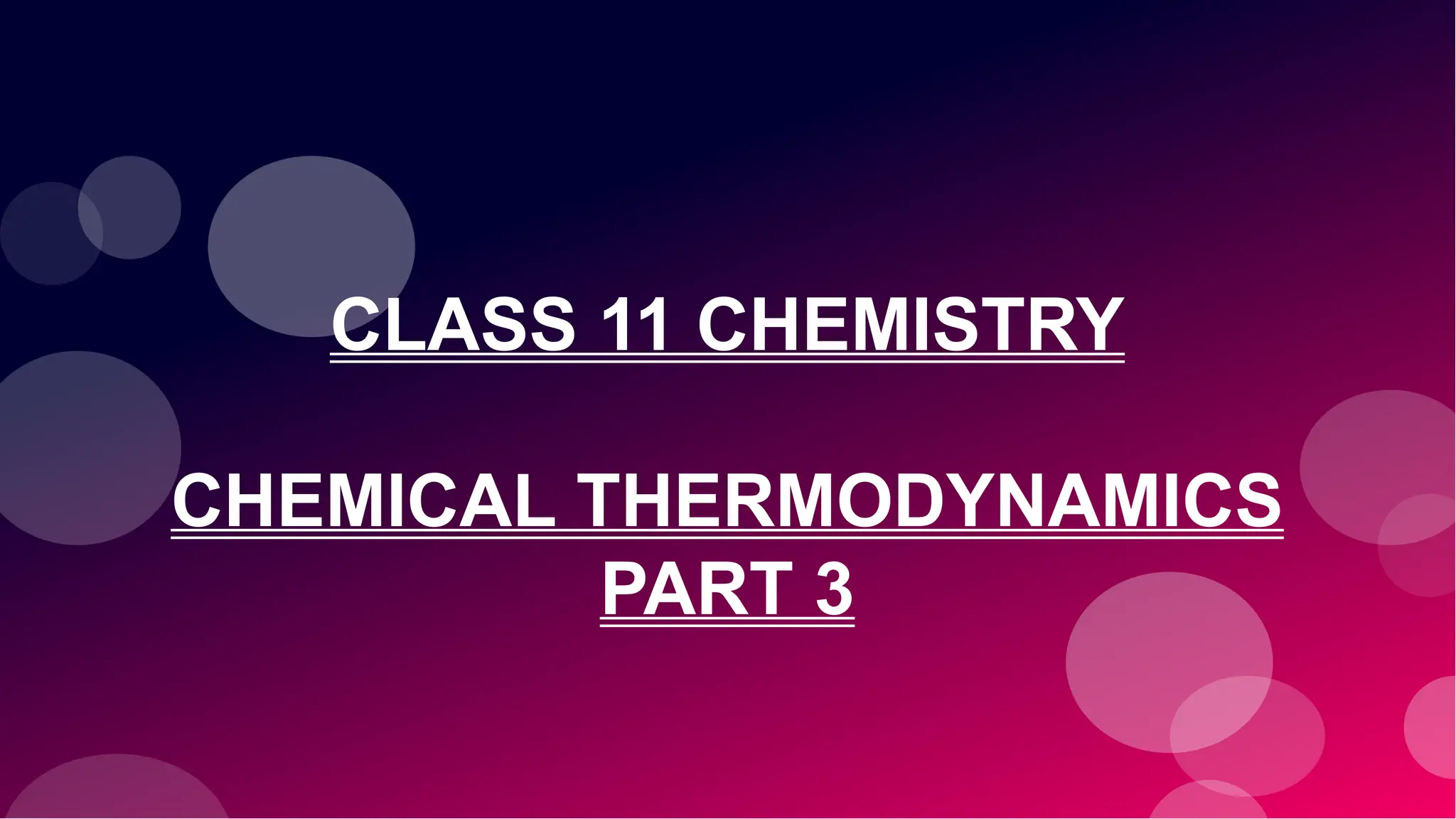
Q. What is entropy?
A. Entropy is a Greek word with no equivalent in common
language. Entropy stands for ‘trope’ [a Greek word
meaning change], the suffix ‘en’ is used to identify it with
energy.
Entropy is defined as: ‘the property of a substance
which measures the disorder or randomness of a
system.’](https://image.slidesharecdn.com/therm3a-250219054445-0147ea61/85/THERMODYNAMICS-PART-3-CLASS-11-CHEMISTRY-6-320.jpg)



![10
6. Value of ΔS is zero for a cyclic or reversible
process.
7. Total entropy of the universe remains unaltered in
a reversible process but increases in an irreversible
process [ΔS > 0].
8. Entropy of the system increases up to equilibrium
state and attains maximum value after the
establishment of equilibrium.
9. The value of entropy depends on the independent
variables used to define the state of the system.](https://image.slidesharecdn.com/therm3a-250219054445-0147ea61/85/THERMODYNAMICS-PART-3-CLASS-11-CHEMISTRY-10-320.jpg)
![11
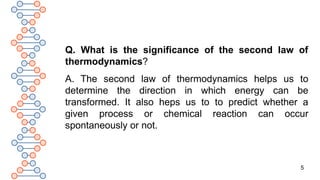
Q. What is ‘Entropy Change’?
A. Entropy is a state function like enthalpy and internal energy.
So, it depends upon the initial and final states of a system. Thus,
entropy change, ΔS can be mathematically represented as:
ΔS = S[final state] – S[initial state], when a system undergoes a change
from initial to final state.
For any chemical process, ΔS = S[products] – S[reactants]
For a reversible process, ΔS = qrev / T at equilibrium, where qrev is
the amount of heat supplied at temperature T in a reversible
process.](https://image.slidesharecdn.com/therm3a-250219054445-0147ea61/85/THERMODYNAMICS-PART-3-CLASS-11-CHEMISTRY-11-320.jpg)
![13
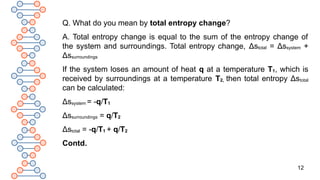
1.If Δstotal is positive, the process is spontaneous.
2.If Δstotal is negative, the process is non-spontaneous.
3.If Δstotal is zero, the process is at equilibrium.
Q. What is entropy of phase transition?
A. The change of one state[solid, liquid, gas] to another is
called phase transition. It occurs at definite temperatures
such as melting point [solid to liquid], boiling point [liquid to
vapour], etc. Phase transitions are accompanied by
absorption or evolution of heat. The entropy change for
these transitions may be calculated as: ΔS = q/T, where q is
the heat evolved or absorbed during transition and T is the
temperature.](https://image.slidesharecdn.com/therm3a-250219054445-0147ea61/85/THERMODYNAMICS-PART-3-CLASS-11-CHEMISTRY-13-320.jpg)

![15
Q. What is entropy of vaporisation?
A. One mole of a liquid can be changed into vapours at its boiling
point [Tb] by supplying latent heat of vaporization. The change in
entropy of vaporization, ΔSv is given by:
ΔSv = ΔHv / Tv, where, ΔSv = Entropy of vaporisation per mole, Tv =
boiling point in K.
For water, ΔHv = 40.73 kJ mol-1
, Tv = 373k
Therefore, ΔSv = 40730/373 = 109JK-1
mol-1](https://image.slidesharecdn.com/therm3a-250219054445-0147ea61/85/THERMODYNAMICS-PART-3-CLASS-11-CHEMISTRY-15-320.jpg)
![16
Q. What is entropy of sublimation?
A. It is the entropy change when one mole of solid
changes into vapour at a particular temperature. For
example, for the reaction, I2[s] → I2[g], the entropy change
is given as: ΔSsub = ΔHsub / T
ΔS[sub] = SI2[g] – SI2[s],
ΔHsub = Enthalpy of sublimation at the temperature T in
Kelvin,
ΔHsub = ΔHfus + ΔHvap](https://image.slidesharecdn.com/therm3a-250219054445-0147ea61/85/THERMODYNAMICS-PART-3-CLASS-11-CHEMISTRY-16-320.jpg)



![20
Q. What happens to the entropy when an egg is boiled
hard?
A. On hard boiling of an egg, entropy increases due to
denaturation of egg protein which results in change of
structure of protein from helical form [more ordered] to
random coiled form [less ordered].
Q. What happens to the entropy when the rubber band
is stretched?
A. On stretching a rubber band, the long flexible
macromolecules get uncoiled. These uncoiled molecules
are arranged in a more specific manner resulting in
discrease in disorder or entropy.](https://image.slidesharecdn.com/therm3a-250219054445-0147ea61/85/THERMODYNAMICS-PART-3-CLASS-11-CHEMISTRY-20-320.jpg)





![26
Examples of spontaneous processes which take
place on initiation:
1. Lightning of candle involving burning of wax.
2. Heating of calcium carbonate to give calcium oxide
and carbon dioxide.
CaCO3[s] → CaO[s] + CO2[g]
3. Combination of hydrogen and oxygen to form water
when initiated by passing an electric spark.
H2[g] + ½ O2 [g] → H2O[g]](https://image.slidesharecdn.com/therm3a-250219054445-0147ea61/85/THERMODYNAMICS-PART-3-CLASS-11-CHEMISTRY-26-320.jpg)




![31
Q. How free energy is related to enthalpy and entropy?
A. The equation for Gibb’s free energy shows the free
energy of a system [G] at any moment. It is defined as the
enthalpy [H] of the system minus the product of the
temperature [T] times the entropy of the system [S]. Gibb’s
free energy is usually expressed in kilojoules per mole
[kJmol-1
].
G = H - TS
Enthalpy and entropy are both thermodynamic properties
of the system.
Contd.](https://image.slidesharecdn.com/therm3a-250219054445-0147ea61/85/THERMODYNAMICS-PART-3-CLASS-11-CHEMISTRY-31-320.jpg)
![32
Enthalpy is defined as the sum of the internal energy
[E] of the system plus the product of the pressure [P]
and volume [V], H = E + PV
Entropy is the molecular disorder or randomness of
the system. It measures the thermal energy per unit
of temperature that is unavailable for doing useful
work.](https://image.slidesharecdn.com/therm3a-250219054445-0147ea61/85/THERMODYNAMICS-PART-3-CLASS-11-CHEMISTRY-32-320.jpg)
![33
Q. Discuss the effect of temperature on free energy for
exothermic reaction.
A. The conditions will depend upon the thermodynamic
equation ΔG = ΔH – TΔS. Here, G is the free energy, H is the
enthalpy, T is the temperature and S is the entropy. If a
reaction is exothermic [H is negative] and the entropy S is
positive [more disorder], the free energy change is always
negative. On increasing the temperature, whether Gibb’s free
energy will increase or decrease, will depend upon the entropy
of the given reaction. If the value of ΔS is positive, then the
value of -TΔS will become more negative. On increasing the
temperature, the value of free energy becomes very small.](https://image.slidesharecdn.com/therm3a-250219054445-0147ea61/85/THERMODYNAMICS-PART-3-CLASS-11-CHEMISTRY-33-320.jpg)




![39
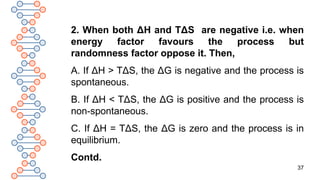
4. When ΔH is positive and TΔS is negative i.e.
when energy factor as well as randomness factor
opposes the process, ΔG will be highly positive and
the process will be non-spontaneous.
To sum up, if [ΔG]T,P < 0, the process is spontaneous.
if [ΔG]T,P = 0, the process is in equilibrium state.
if [ΔG]T,P > 0, the process is non-spontaneous.
Hence, only that process can occur spontaneously
which results in decrease in free energy, G.](https://image.slidesharecdn.com/therm3a-250219054445-0147ea61/85/THERMODYNAMICS-PART-3-CLASS-11-CHEMISTRY-39-320.jpg)
