BASIC PRINCIPLES IN ORGANIC CHEMISTRY INCLUDE: THE TETRAVALENCE OF CARBON[CARBON'S ABILITY TO FORM FOUR BONDS], ISOMERISM[MOLECULES WITH THE SAME FORMULA BUT DIFFERENT STRUCTURES], FUNCTIONAL GROUPS [SPECIFIC ATOMS OR GROUPS OF ATOMS THAT GIVE A MOLECULE , IT'S CHARACTERISTICS PROPERTIES], STRUCTURAL REPRESENTATION OF ORGANIC COMPOUNDS, CLASSIFICATION OF ORGANIC COMPOUNDS BASED ON FUNCTIONAL GROUPS, AND UNDERSTANDING OF REACTION MECHANISMS.
COMMON PURIFICATION TECHNIQUES INCLUDE : DISTILLATION, CRYSTALLIZATION, AND CHROMATOGRAPHY.
QUALITATIVE ANALYSIS INCLUDE COMBUSTION ANALYSIS AND CHEMICAL TESTS. QUANTITATIVE ANALYSIS INCLUDE TITRATION AND SPECTROSCOPY.
![1
CLASS 11 CHEMISTRY
ORGANIC CHEMISTRY:
SOME BASIC PRINCIPLES
&
TECHNIQUES
[PART 1]](https://image.slidesharecdn.com/org1-250304040023-5042b1b5/75/ORGANIC-CHEMISTRY-SOME-BASIC-PRINCIPLES-AND-TECHNIQUES-1-2048.jpg)




![9
Q. What are the classification of open-chain
compounds?
A. Open-chain compounds are classified as straight-
chain compounds and branched-chain compounds.
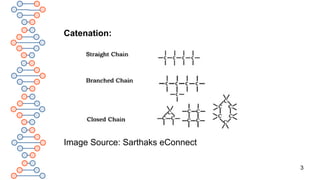
1. Straight-chain compounds: In straight-chain
compounds, the carbon skeleton is in the form of a
straight chain.
Example: n-Butane [CH3-CH2-CH2-CH3]
Contd.](https://image.slidesharecdn.com/org1-250304040023-5042b1b5/85/ORGANIC-CHEMISTRY-SOME-BASIC-PRINCIPLES-AND-TECHNIQUES-9-320.jpg)


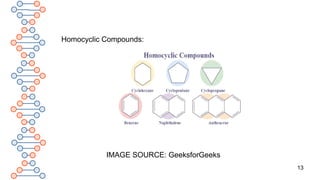
![15
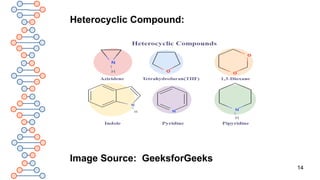
Q. How are Heterocyclic compounds are classified?
A. Heterocyclic compounds are classified as:
1. Hetero-alicyclic compounds
2. Hetero-aromatic compounds
Hetero-alicyclic compounds: Alicyclic compounds which
contain one heteroatom in the ring are called hetero-
alicyclic compounds, e.g. Tetrahydrofuran[THF].
Hetero-aromatic compounds: Aromatic compounds
which contain at least one heteroatom in the ring are
called hetero-aromatic compounds, e.g. Furan.](https://image.slidesharecdn.com/org1-250304040023-5042b1b5/85/ORGANIC-CHEMISTRY-SOME-BASIC-PRINCIPLES-AND-TECHNIQUES-15-320.jpg)


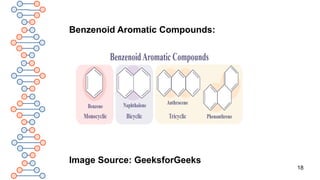
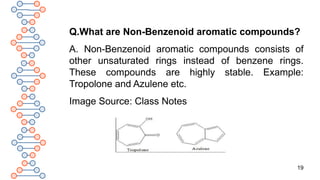




![24
5. Naming different substituents at equivalent
positions: If we find the presence of two different
substituents on the same position from the two ends, the
substituent first in the alphabetical order gets the lowest
number.
6. The Naming of complex Substituents: The complex
substituent is when the substituent on the parent chain
has a branched structure [i.e. complex structure]. We
name these substituents as a substituted alkyl group. It is
also important to note that the carbon atom of this
substituent gets the number 1. We write the name of this
substituent in the bracket.](https://image.slidesharecdn.com/org1-250304040023-5042b1b5/85/ORGANIC-CHEMISTRY-SOME-BASIC-PRINCIPLES-AND-TECHNIQUES-24-320.jpg)






![31
Similar to that of carbon, these elements may form
rings or chains that will have many derivatives. Rules
come in handy in naming the parent or main
compounds and their substituents. Hydrides
belonging to the group 13-17 of the periodic table get
the suffix -ane. For example, -Borane, Phosphane,
and Oxidane etc.
Therefore, substitutive nomenclature takes a parent
compound and identifies substituents which replaces
hydrogen on it with either prefixes [chloro-] or suffixes
[-ol, -one] etc.](https://image.slidesharecdn.com/org1-250304040023-5042b1b5/85/ORGANIC-CHEMISTRY-SOME-BASIC-PRINCIPLES-AND-TECHNIQUES-31-320.jpg)
![32
Q. What is additive nomenclature?
A. Additive nomenclature is the method of naming that
has been developed principally for coordination of
compounds, although it can be applied more widely.
An example of its application is penta-amine-chlorido-
cobaltchloride, used to describe the coordination
compound given by the formula [CoCl(NH3)5]Cl2.
The prefix ‘chloro’ corresponds to a chloride, whereas
the prefix ‘chlorido’ corresponds to the ligand.](https://image.slidesharecdn.com/org1-250304040023-5042b1b5/85/ORGANIC-CHEMISTRY-SOME-BASIC-PRINCIPLES-AND-TECHNIQUES-32-320.jpg)

![35
Q. What are the different steps involved in the
characterisation of an organic compound?
A. The characterisation of an organic compound involves a
series of steps:
1. Qualitative analysis [Detection of an element]
2. Quantitative analysis [Estimation of an element]
3. Determination of molecular mass
4. Calculation of empirical and molecular formulae
5. Elucidation of structure by various physical and chemical
methods.](https://image.slidesharecdn.com/org1-250304040023-5042b1b5/85/ORGANIC-CHEMISTRY-SOME-BASIC-PRINCIPLES-AND-TECHNIQUES-35-320.jpg)
![36
Q. What is qualitative analysis of of organic compound?
A. The qualitative analysis of of organic compound involves the
detection of various element, commonly present in it such as
carbon, hydrogen, oxygen, nitrogen, halogens, sulphur and
phosphorus.
Q. How do carbon and hydrogen could be determined in
organic compound?
A. Carbon and hydrogen are present in almost all the organic
compounds. Carbon and hydrogen are detected by heating the
organic compound with cupric oxide[CuO] strongly, where carbon is
oxidized to carbon dioxide and hydrogen to water. Carbon dioxide
is tested by lime water test, whereas water is tested by anhydrous
copper sulphate test.](https://image.slidesharecdn.com/org1-250304040023-5042b1b5/85/ORGANIC-CHEMISTRY-SOME-BASIC-PRINCIPLES-AND-TECHNIQUES-36-320.jpg)
![37
Q. Explain the procedure of detection of carbon and
hydrogen in an organic compound.
A. The given organic compound is heated with dry copper[II]
oxide or cupric oxide in a hard glass test tube when carbon
present is oxidised to carbon dioxide and hydrogen is oxidised to
water.
I] C + 2CuO → CO2 + 2Cu, ii] 2H + CuO → H2O + Cu
Carbon dioxide turns lime water milky while water condenses on
the cooler parts of the test tube and turns anhydrous copper
sulphate blue.
Ca[OH]2+CO2→CaCO3+CuSO4, CuSO4+5H2O→CuSO4.5CuSO4](https://image.slidesharecdn.com/org1-250304040023-5042b1b5/85/ORGANIC-CHEMISTRY-SOME-BASIC-PRINCIPLES-AND-TECHNIQUES-37-320.jpg)

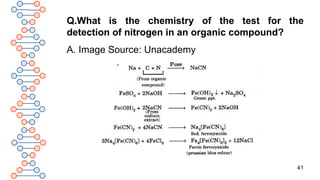
![39
Q. How is nitrogen detected in an organic compound?
A.1.The presence of nitrogen in an organic compound is
detected by fusing organic compounds with sodium metal to
give sodium cyanide[NaCN].
2.Sodium cyanide formed during fusion dissolves in distilled
water. Excess of sodium reacts with water to give sodium
hydroxide. Thus an alkaline solution of sodium cyanide is
obtained if the compound contains nitrogen.
3.The solution is filtered. The filtrate thus obtained is called
Lassaigne’s solution or sodium extract.
Na + C + N → NaCN
Contd.](https://image.slidesharecdn.com/org1-250304040023-5042b1b5/85/ORGANIC-CHEMISTRY-SOME-BASIC-PRINCIPLES-AND-TECHNIQUES-39-320.jpg)

![42
Q. How can the presence of both nitrogen and sulphur be
detected in an organic compound?
A. If both nitrogen and sulphur are present in an organic
compound, they may combine during fusion to form
thiocyanate[SCN-
] instead of cyanide ion[CN-
], due to
insufficient amount of sodium metal. Thus, sodium
sulphocyanide is formed instead of sodium cyanide. This
sodium sulphocyanide when reacts with ferric chloride gives
blood red colouration due to formation of blood red coloured
ferric sulphocyanide.
i) Na+C+N+S → NaCNS[sodium sulphocyanide]
Ii] 3NaCNS+FeCl3 → Fe(CNS)3 + 3NaCl](https://image.slidesharecdn.com/org1-250304040023-5042b1b5/85/ORGANIC-CHEMISTRY-SOME-BASIC-PRINCIPLES-AND-TECHNIQUES-42-320.jpg)
![43
Q.How is sulphur detected in an organic compound?
A.(1) Lassaigne’s test:
If sulphur is present in the organic compound, then on
fusion with sodium metal, sodium sulphide is formed. 2Na
+ S → Na2S
i] Sodium nitroprusside test : A small portion of the
Lassaigne’s filtrate is treated with a few drops of sodium
nitroprusside solution when a violet colouration is
obtained. This colour slowly fades on standing.
Na2S + Na2[Fe(CN)5(NO)] → Na4[Fe(CN)5(NOS)]
Contd.](https://image.slidesharecdn.com/org1-250304040023-5042b1b5/85/ORGANIC-CHEMISTRY-SOME-BASIC-PRINCIPLES-AND-TECHNIQUES-43-320.jpg)
![44
ii) Lead Acetate test: Another portion of Lassaigne’s
filtrate is acidified with dilute acetic acid and a few drops
of lead acetate solution are added to it. Formation of
black lead sulphide indicates the presence of sulphur in
the given compound.
Na2S + [CH3COOH]Pb → PbS + 2CH3COONa
Iii) Oxidation test: The given organic compound is fused
with a mixture of potassium nitrate and sodium
carbonate. If sulphur is present, it gets oxidized to
sulphate.The fused mass is then extracted with water
and filtered.
Contd.](https://image.slidesharecdn.com/org1-250304040023-5042b1b5/85/ORGANIC-CHEMISTRY-SOME-BASIC-PRINCIPLES-AND-TECHNIQUES-44-320.jpg)
![45
The filtrate is first acidified with dilute hydrochloric acid
and then treated with barium chloride solution when a
white precipitate insoluble in hydrochloric is obtained.
KNO3 → KNO2 + [O]
Na2CO3 + S + 3[O] → Na2SO4 + CO2
Na2SO4 + BaCl2 → 2NaCl + BaSO4](https://image.slidesharecdn.com/org1-250304040023-5042b1b5/85/ORGANIC-CHEMISTRY-SOME-BASIC-PRINCIPLES-AND-TECHNIQUES-45-320.jpg)
![46
Q. How is the presence of halogen is detected in an
organic compound?
A. Lassaigne’s test for halogen: The sodium fusion extract,
SFE can be used to detect the presence of chlorine, bromine
and iodine but not fluorine. To detect their presence, the SFE is
first acidified with HNO3 and then added with AgNO3 solution.
1] The formation of a curdy white precipitate that is soluble in
NH4OH, indicates the presence of chlorine in the organic
compound.
Cl-
+ AgNO3 → AgCl[white ppt] + [NO3]-
AgCl + 2NH4OH → [Ag(NH3)2]Cl [soluble complex] + 2H2O](https://image.slidesharecdn.com/org1-250304040023-5042b1b5/85/ORGANIC-CHEMISTRY-SOME-BASIC-PRINCIPLES-AND-TECHNIQUES-46-320.jpg)
![47
2] The formation of a pale yellow precipitate that is
partially soluble in NH4OH confirms the presence of
bromine.
Br-
+ AgNO3 → AgBr[pale yellow ppt.] + [NO3]-
3] Whereas the formation of a yellow precipitate
insoluble in NH4OH confirms the presence of iodine in
the organic compound.
I-
+ AgNO3 → AgI[yellow ppt.] + [NO3]-
It is not possible to detect the presence of fluorine,
since the solubility of AgF is more and thus no
precipitate is formed.](https://image.slidesharecdn.com/org1-250304040023-5042b1b5/85/ORGANIC-CHEMISTRY-SOME-BASIC-PRINCIPLES-AND-TECHNIQUES-47-320.jpg)

![49
Q. Write down the summary of Lassaigane’s test.
A. SFE → Sodium fusion extract
1. SFE + FeSO4 + FeCl3 + HCl → a] If a prussian blue colour is
formed → Nitrogen is confirmed.
b] If blood red colouration is observed → b] Both nitrogen and
sulphur are confirmed.
2. SFE + Sodium nitroprusside → a] If a violet colouration is
observed, sulphur is confirmed.
SFE + CH3COOH + Pb[CH3COO]2 → b] If a black precipitate is
formed, sulphur is confirmed.
Contd.](https://image.slidesharecdn.com/org1-250304040023-5042b1b5/85/ORGANIC-CHEMISTRY-SOME-BASIC-PRINCIPLES-AND-TECHNIQUES-49-320.jpg)
![50
3. SFE + HNO3 + AgNO3 → a] If a white ppt, soluble in
NH4OH is formed → Chlorine is confirmed.
SFE + HNO3 + AgNO3 → b] If a pale yellow ppt,
partially soluble in NH4OH is formed → Bromine is
confirmed.
SFE + HNO3 + AgNO3 → c] If a yellow ppt, insoluble
in NH4OH is formed → Iodine is confirmed.](https://image.slidesharecdn.com/org1-250304040023-5042b1b5/85/ORGANIC-CHEMISTRY-SOME-BASIC-PRINCIPLES-AND-TECHNIQUES-50-320.jpg)
