ENERGY CAN CHANGE FROM ONE FORM TO ANOTHER, BUT THE TOTAL AMOUNT OF ENERGY REMAINS CONSTANT. HEAT IS A FORM OF ENERGY THAT CORRESPONDS TO MECHANICAL WORK. THE TOTAL CHANGE IN ENTROPY OF A SYSTEM PLUS IT'S SURROUNDINGS WILL ALWAYS INCREASE FOR A SPONTANEOUS PROCESS. THE ENTROPY OF A PERFECT CRYSTAL OF ANY PURE SUBSTANCE APPROACHES ZERO AS THE TEMPERATURE APPROACHES ABSOLUTE ZERO.
![1
CLASS 11 CHEMISTRY
CHEMICAL THERMODYNAMICS
[PART 2]](https://image.slidesharecdn.com/therm2-250219053401-f44db038/75/THERMODYNAMICS-PART-2-CLASS-11-CHEMISTRY-1-2048.jpg)


![4
If we consider a system having internal energy, U1 and suppose heat Q
is supplied to the system. Thus, its energy increases from U1 to U1 + Q.
Further suppose that work [W] is done on the system. As a result, its
internal energy further increases and becomes equal to U2. Here, U2 is
the energy of the final state. Thus,
U2 = U1 + Q + W
Or, U2 – U1 = Q + W
Or, ΔU = Q + W
i.e. Change in internal energy = Heat added to the system + Work
done on the system. It is the mathematical form of first law of
thermodynamics.](https://image.slidesharecdn.com/therm2-250219053401-f44db038/85/THERMODYNAMICS-PART-2-CLASS-11-CHEMISTRY-4-320.jpg)



![8
4. It does not rule out the existence of a 100% efficient heat
engine which is in fact impossible.
Even though, various forms of energy can be completely
transformed into one another, heat is a typical form of energy,
which cannot be completely transformed into work.
Q. What violates the first law of thermodynamics?
A. A device that violates the first law of thermodynamics [by
creating energy] is called a Perpetual Motion Machine of the first
kind. The device supplies continuously energy without receiving it.
.](https://image.slidesharecdn.com/therm2-250219053401-f44db038/85/THERMODYNAMICS-PART-2-CLASS-11-CHEMISTRY-8-320.jpg)

![10
Q. By first law of thermodynamics prove that change in internal energy
is equal to heat absorbed or evolved at constant volume and constant
temperature.
A. According to first law of thermodynamics, ΔU = Q+W. Where, ΔU=
change in internal energy, Q= heat added to the system, W=work done by
the system.
If the process is such that work done by the system is only pressure-volume
work, then W = -PΔV. Thus,
ΔU = Q – PΔV, if the process takes place at constant volume, i.e, ΔV=0, then
PΔV=0 and thus ΔU = Qv. Hence, “Change in internal energy, ΔU is equal to
heat [Qv] absorbed or evolved at constant volume and constant temperature.](https://image.slidesharecdn.com/therm2-250219053401-f44db038/85/THERMODYNAMICS-PART-2-CLASS-11-CHEMISTRY-10-320.jpg)
![11
Q. What is the nature of internal energy of an ideal gas?
A. In an ideal gas, the intermolecular forces are assumed to
be absent and all the collisions are perfectly elastic. Thus,
the gas possesses only translational kinetic energy and
hence the internal energy of the ideal gas depends only on
the temperature.
Q. What is the physical significance of internal energy?
A. The physical significance of internal energy can be
understood in a better way by taking an example of everyday
life. The food taken by us gets converted into heat energy
[Q].
Contd.](https://image.slidesharecdn.com/therm2-250219053401-f44db038/85/THERMODYNAMICS-PART-2-CLASS-11-CHEMISTRY-11-320.jpg)
![12
A part of this energy is spent in doing work [-ve] and the rest
is stored in the body in the form of internal energy. During
growth period, a child takes more energy in the form of food
as compared to work done and the balance [Q – W] = ΔU is
added to the internal energy and the child grows in a healthy
manner.
However, after forty, any accumulation of extra energy is
dangerous, since it can lead to diabetes. Therefore, to keep
ΔU equal to zero, Q must be equal to work done. This work
may be external physical work or it may be internal work
such as internal movement of the heart and stomach, etc.
Contd.](https://image.slidesharecdn.com/therm2-250219053401-f44db038/85/THERMODYNAMICS-PART-2-CLASS-11-CHEMISTRY-12-320.jpg)
![13
Basal metabolism [the chemical process occuring in an organism
at rest], requires 300kJ of energy per hour. If no food is taken,
then 300kJ per hour consumption of energy will be supplied from
the reserve internal energy, which would cause a loss of weight.
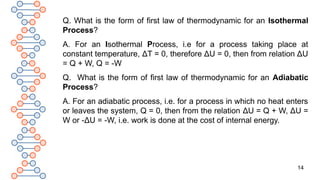
Q. What is the form of first law of thermodynamic for an
Isochoric Process?
A. For an isochoric process, i.e., for a process taking place at
constant volume, ΔV=zero. Therefore, PΔV=0. Hence, from
equation of the first law of thermodynamics, ΔU = Qv, where, Q is
the heat absorbed at constant volume. Thus, heat supplied to a
system under constant volume is used up in increasing internal
energy.](https://image.slidesharecdn.com/therm2-250219053401-f44db038/85/THERMODYNAMICS-PART-2-CLASS-11-CHEMISTRY-13-320.jpg)
![15
Q. What is the form of first law of thermodynamics for an
Isobaric Process?
A. For an isobaric process, i.e., for a process taking place at
constant pressure, ΔP=0. A system is considered, which
shows increase in volume from V1 to V2 at a constant pressure
P, during absorption of heat Q. The expansion of work or work
done by the system is W = -PΔV. From the equation, ΔU =
Q+W,or, Q=ΔU-W. Therefore, Qp=ΔU-[-PΔV] or, Qp = ΔU+PΔV
= U2 – U1 + P[V2 – V1]
= [U2 + PV2] - [U1 + P V1] = H2 – H1, where H2 and H1 are the
enthalpies of the system in final and initial state respectively.](https://image.slidesharecdn.com/therm2-250219053401-f44db038/85/THERMODYNAMICS-PART-2-CLASS-11-CHEMISTRY-15-320.jpg)
![16
Q. What is enthalpy?
A. Enthalpy is a measurement of energy in a thermodynamic
system. The quantity of enthalpy equals to the total content
of heat of a system, equivalent to the system’s internal
energy plus the product of volume and pressure.
When a process begins at constant pressure, the evolved
heat [either absorbed or released] equals the change in
enthalpy. Enthalpy change is the sum of internal energy
denoted by U and product of volume and pressure denoted
by PV, expressed in the following manner,
H = U + PV](https://image.slidesharecdn.com/therm2-250219053401-f44db038/85/THERMODYNAMICS-PART-2-CLASS-11-CHEMISTRY-16-320.jpg)
![17
Q. What are the characteristics of enthalpy?
A.1. Absolute value of enthalpy of a system cannot be
determined just like the internal energy.
2.The enthalpy of a system is a state function.
Therefore, the magnitude of enthalpy change [ΔH],
depends only on the enthalpies of the initial and final
states. Thus we can write, ΔH = Hfinal – Hinitial
3. Enthalpy is an extensive property.](https://image.slidesharecdn.com/therm2-250219053401-f44db038/85/THERMODYNAMICS-PART-2-CLASS-11-CHEMISTRY-17-320.jpg)
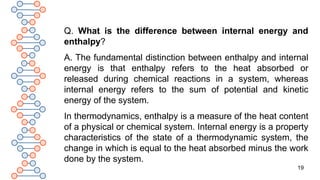
![18
Q. Why is enthalpy useful?
A. Enthalpy is important because it informs us how much
heat is in a system [energy]. Heat is important, since from it,
we can derive valuable work. An enthalpy shift shows us how
much heat was lost or obtained in terms of a chemical
reaction, enthalpy meaning the system’s heat energy.
Q. What is enthalpy of chemical reaction?
A. The bonds between atoms can dissolve, reform or both
during chemical reactions to either absorb or release energy.
The heat absorbed or emitted under constant pressure from
a device is referred to as enthalpy, and the reaction enthalpy
is the change in enthalpy arising from a chemical reaction.](https://image.slidesharecdn.com/therm2-250219053401-f44db038/85/THERMODYNAMICS-PART-2-CLASS-11-CHEMISTRY-18-320.jpg)
![20
Q. When does enthalpy become positive and when it is
negative?
A. If a reaction absorbs or uses more energy than it releases,
the reaction is endothermic, and enthalpy will be positive. On
the other hand, if a reaction releases more energy than it
absorbs, the reaction is exothermic and enthalpy will be
negative. The condition can be thought of as an amount of heat
leaving [or being substracted from] the reaction.](https://image.slidesharecdn.com/therm2-250219053401-f44db038/85/THERMODYNAMICS-PART-2-CLASS-11-CHEMISTRY-20-320.jpg)
![21
Q. What is the unit of enthalpy?
A. The unit of enthalpy is the same as energy, so in SI
unit, it is Joule[J]. In C.G.S unit, it is erg. One erg is equal
to 10-7
J.
Q. What is enthalpy of chemical reaction?
A. The amount of heat evolved or absorbed in a chemical
reaction when the number of moles of the reactants
aresented by the chemical equation have completely
reacted, is called the enthalpy of a chemical reaction.](https://image.slidesharecdn.com/therm2-250219053401-f44db038/85/THERMODYNAMICS-PART-2-CLASS-11-CHEMISTRY-21-320.jpg)
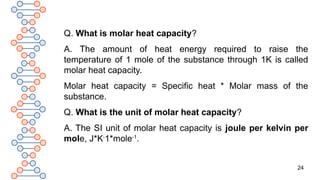
![22
Q. What is heat capacity?
A. Heat capacity or more accurately mean heat capacity
of a system, between any two temperatures, is defined
as the amount of heat required to raise the temperature
of the system from lower to higher temperature divided
by the temperature difference.
Thus, if Q is the amount of heat supplied to a system
and as a result, the temperature of the system rises from
T1 to T2, then the heat capacity C of the system is given
by, C = Q/[T2 – T1] = Q/ΔT.](https://image.slidesharecdn.com/therm2-250219053401-f44db038/85/THERMODYNAMICS-PART-2-CLASS-11-CHEMISTRY-22-320.jpg)


![26
3. The SI unit of heat capacity is joule per kelvin [J/K].
The SI unit of specific heat capacity is joule per kelvin per
kilogram[J/Kg-K]].
4. The formula of heat capacity = Q/ΔT
The formula of specific heat capacity = Q/mΔT
Where, ‘Q’ is the amount of heat
ΔT refers to the temperature
‘m’ stands for mass](https://image.slidesharecdn.com/therm2-250219053401-f44db038/85/THERMODYNAMICS-PART-2-CLASS-11-CHEMISTRY-26-320.jpg)
![27
Q. What is calorimetry?
A. Generally, calorimetry refers to an experimental
technique that we use for the measurement of enthalpy
[ΔH] and internal energy [ΔU].
Calorimetry techniques are based on thermometric
methods carried out in a vessel called calorimeter which is
immersed in a known volume of liquid. The heat evolved in
the process is generally calculated with the help of known
heat capacities of the liquid and and the calorimeter by
measuring the temperature differences. Two different
conditions under which these measurements are made are:
1) At constant pressure [Qp], 2) At constant volume [Qv].](https://image.slidesharecdn.com/therm2-250219053401-f44db038/85/THERMODYNAMICS-PART-2-CLASS-11-CHEMISTRY-27-320.jpg)


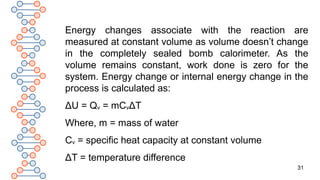
![30
Q. How do we measure the change in internal energy?
A. Internal energy change is the energy change at
constant volume. A bomb calorimeter is generally used for
the measurement of internal energy change. In this
technique, a steel vessel [commonly called bomb] is
immersed in a water bath in order to ensure that no heat
is lost to the surrounding. A combustible substance is
burnt in oxygen gas supplied in the bomb. The heat
evolved is absorbed by the around the bomb and the
change in temperature is measured.
Contd.](https://image.slidesharecdn.com/therm2-250219053401-f44db038/85/THERMODYNAMICS-PART-2-CLASS-11-CHEMISTRY-30-320.jpg)

![33
Q. Explain Hess’s law of constant heat summation with an
example.
A. We consider the following two paths for the preparation of
methylene chloride.
Path : CH4[g] + 2Cl2[g] → CH2Cl2[g] + 2HCl[g]
ΔH1 = -202.3 kJ
Path 2 : CH4[g] + Cl2[g] → CH3Cl[g] + HCl[g]
ΔH2 = -98.3 kJ [2]
Path 3 : CH3Cl[g] + + Cl2[g] → CH2Cl2[g] + HCl[g]
ΔH3 = -104.0 kJ [3]
Contd.](https://image.slidesharecdn.com/therm2-250219053401-f44db038/85/THERMODYNAMICS-PART-2-CLASS-11-CHEMISTRY-33-320.jpg)
![34
Adding two steps: [2] + [3]
CH4[g] + 2Cl2[g] → CH2Cl2[g] + 2HCl[g]
ΔH = -202.3 kJ
Thus, whether we follow path 1 or path 2, the enthalpy
change of the reaction is same.
ΔH1 = ΔH2 + ΔH3 = -202.3 kJ](https://image.slidesharecdn.com/therm2-250219053401-f44db038/85/THERMODYNAMICS-PART-2-CLASS-11-CHEMISTRY-34-320.jpg)
![36
Q. What is enthalpy of formation?
A. The standard enthalpy of formation [ΔHf] is defined as
the change in enthalpy when one mole of the compound
is formed from its constituent elements in their standard
states under standard conditions i.e. at 1atm pressure
and 25°C. For example, although oxygen can exist as
ozone (O3), atomic oxygen [O], and molecular oxygen
[O2], O2 is the most stable state.
Formation of methane from carbon and hydrogen:
C[graphite,s] + 2H2[g] → CH4[g], ΔHf = -74.81kJmol-1](https://image.slidesharecdn.com/therm2-250219053401-f44db038/85/THERMODYNAMICS-PART-2-CLASS-11-CHEMISTRY-36-320.jpg)
![37
Q. What is enthalpy of combustion?
A. Enthalpy of combustion is defined as the enthalpy change when
one mole of a compound is completely burnt in oxygen with all the
reactants and products in their standard state under standard
conditions [1atm pressure and 25°C].
Q. What is enthalpy of atomization?
A. The enthalpy of atomization is the change in enthalpy that
accompanies the total separation of all atoms in a chemical
substance either a Chemical Element or a Chemical Compound. It
means enthalpy of atomization is the energy that break one Mole of
bond into Atoms. Enthalpy of atomization is denoted by the symbol
Ha.
e.g H2[g] → 2H, ΔHa = 435kJ/mole](https://image.slidesharecdn.com/therm2-250219053401-f44db038/85/THERMODYNAMICS-PART-2-CLASS-11-CHEMISTRY-37-320.jpg)


![40
Q. What is enthalpy of ionization?
A. Ionization enthalpy is defined as the minimum amount of
energy that is required to remove the most loosely bounded
electrons that is electron present in the outermost shell from an
isolated gaseous atom. The unit of ionization enthalpy is electron
volts [eV] per atom.
Q. What is enthalpy of dilution?
A. Enthalpy of dilution, also known as the heat of dilution, can be
defined as the change in enthalpy that is associated with the
dilution of a specific component of a solution when the pressure
is kept constant. The most common units of enthalpy of dilution
is Joules/mole and kilojoules/mole.](https://image.slidesharecdn.com/therm2-250219053401-f44db038/85/THERMODYNAMICS-PART-2-CLASS-11-CHEMISTRY-40-320.jpg)
