Embed presentation
Downloaded 578 times



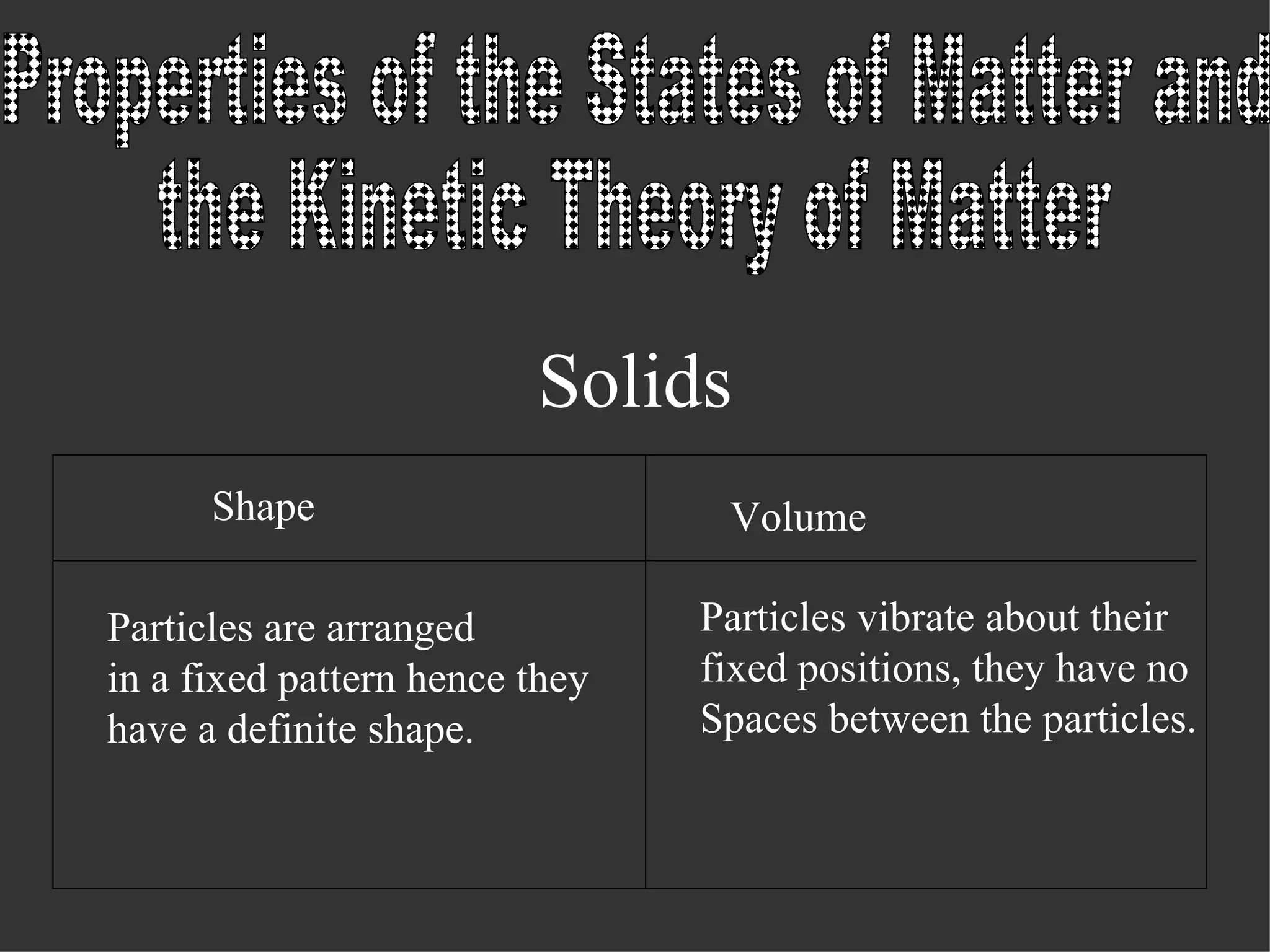
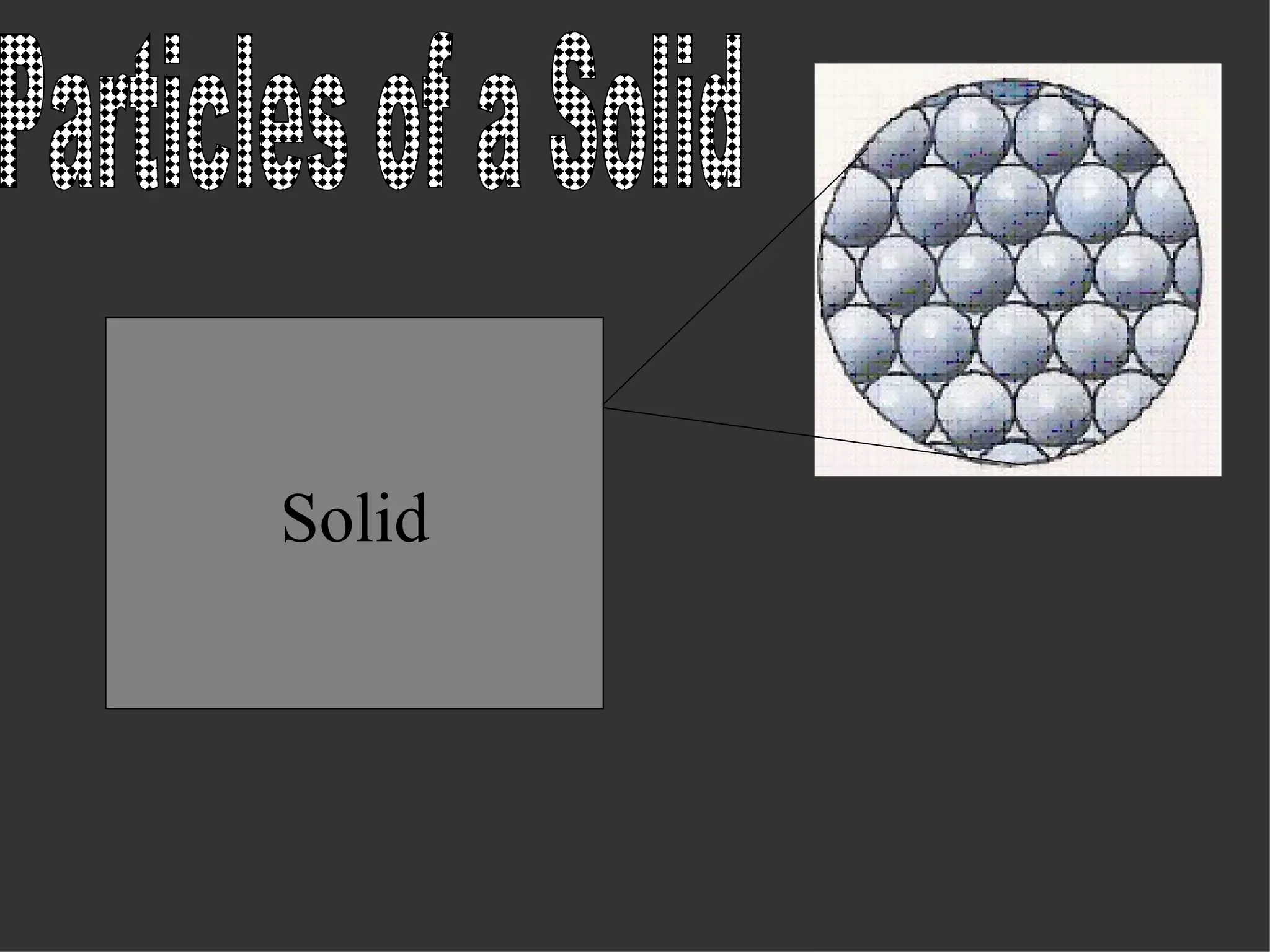





The document discusses the three common states of matter - solids, liquids, and gases - and explains their properties based on the kinetic theory of matter. It states that solids have a fixed shape and volume as their particles vibrate in a fixed position with no spaces between them. Liquids take the shape of their container but have a definite volume, as their particles are arranged in clusters with small intermolecular spaces. Gases occupy the shape of their container and have no definite volume, as their widely-spaced particles move randomly throughout.








