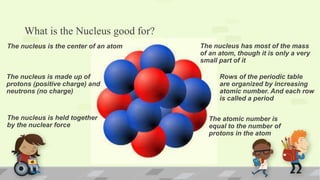
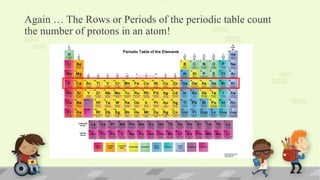
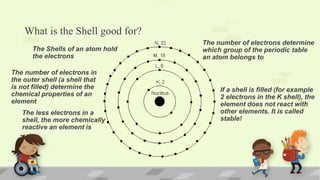
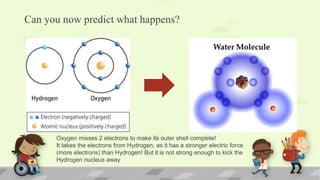
The document explains the periodic table, its purpose, and its historical significance, notably mentioning Dmitri Mendeleev's creation in 1869. It details the organization of elements by atomic number, the structure of atoms, and the properties that define chemical reactivity. Unique facts about elements, such as carbon's ability to form many compounds and the origins of the name Argentina, are also highlighted.