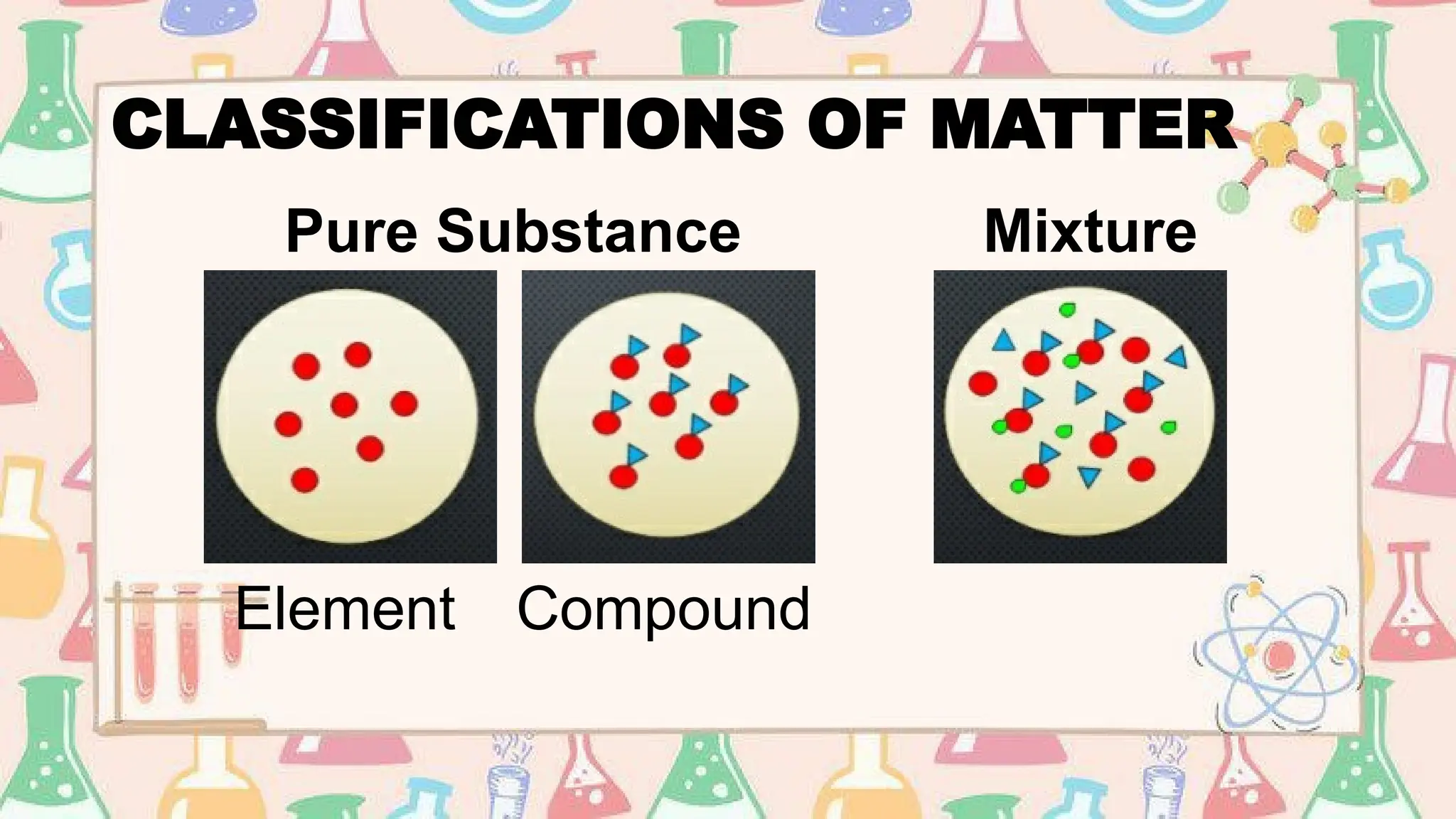
The document provides an extensive overview of atomic theory, subatomic particles, and the periodic table's history and structure. It covers key scientific developments from ancient theories to modern atomic models, details about elements, including their properties and organization in the periodic table, and interactive activities for students to enhance learning. The document also discusses concepts such as isotopes, atomic numbers, and average atomic mass, offering various assessments to gauge understanding.