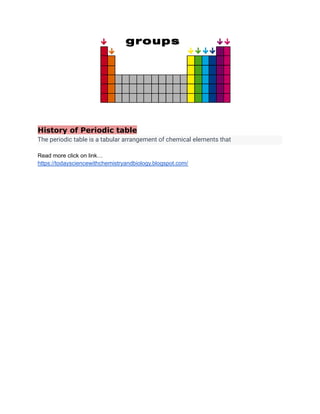
The periodic table is a systematic arrangement of chemical elements organized by atomic number and electron configuration, highlighting relationships and periodic trends among elements. It consists of horizontal rows called periods and vertical columns known as groups, with similar properties shared by elements in each group. The historical development and significance of the periodic table contribute to its importance as a tool in understanding chemical behavior and properties.